Abstract
Objective
A retrospective study was carried out in patients with giant cell tumor of bone to compare the functional and radiographic outcome of curettage and bone grafting using a novel CT based selection strategy to that of patients of a similar age treated with anatomic/standard curettage and bone grafting.
Methods
Curettage and bone grafting after CT classification was performed in 31 patients and curettage and bone grafting without CT classification in 20. The surgical approach for curettage in the CT classified group of patients was through the site of the cortical break, irrespective of the standard approach to the particular region of bone involved. The aim of this approach was to achieve wide excision of the possibly involved soft tissue.
Results
At similar duration of follow up (72 months) in patients with a similar mean age (33 years), Musculoskeletal Tumor Society (MSTS) scores for CT classified patients were similar to those of patients who had undergone standard curettage. However, the postoperative recurrence rate in the CT classified group was significantly less (12.9%) than in the non‐CT classified group.
Conclusion
A CT based selection strategy is a valid preoperative tool for evaluation of giant cell tumor. Further, for curettage these lesions are better approached through the site of cortical break, irrespective of standard approaches, so that adequate soft tissue clearance can be achieved.
Keywords: Giant cell tumor of bone, Tomography, Treatment outcome, X‐ray computed
Introduction
Giant cell tumor (GCT) of bone is rare and unpredictable1. The areas of bone most often involved are the distal femur, proximal tibia, proximal humerus, and distal radius2. Treatments range from surgical curettage to wide resection and varying oncological and functional results have so far been reported1. In large series, the postoperative recurrence rate has been reported to vary from 8% to 50%3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. Most local recurrences occur within 3 years1. There is no uniformly accepted treatment protocol for this tumor14. Because the cause of GCT remains unknown and there is no uniform accepted standard for treating this tumor, investigators continue to focus on these issues.
The purpose of this article is twofold. First, it serves to emphasize the importance of surgically approaching GCT of bone through the site of cortical break (with the aim of addressing possible soft tissue extension). Secondly, we describe a CT based selection strategy for curettage and bone grafting in patients with GCT of bone. To our knowledge, this is the first study reporting the use of a CT based classification of GCT and comparing the results of curettage and bone grafting with and without the use of this CT based classification system with more than 3 years mean follow up.
Our research questions were as follows: (1) In patients with GCT of bone, does a CT based selection strategy for curettage and bone grafting and an approach to the lesion through the site of cortical break result in a lower postoperative recurrence rate than that in patients of similar mean age and follow up duration treated by standard curettage and bone grafting? (2) Does the CT based selection strategy for curettage and bone grafting in patients with GCT of bone affect the long term functional outcome?
Materials and Methods
Of 51 patients with primary GCT of bone treated at a single center from May 1996 to April 2008, we retrospectively reviewed 31 patients (group A) in whom curettage and bone grafting had been performed using novel selection criteria and surgical approaches. The hospital records, including data from preoperative studies, operative reports, and postoperative visits, were reviewed in all patients. The patients included 12 men and 19 women with a mean age of 33 years (range, 18–42 years). They were followed clinically and radiographically for a minimum of 24 months (mean, 72 months; range, 24–164 months). The center had specific Institutional Review Board approval for the study and all patients gave their informed consent before their inclusion in the study.
The tumors, all of which were primary at presentation, were in the distal femur (n = 14), proximal tibia (n = 9), proximal humerus (n = 3), distal radius (n = 2), calcaneus (n = 1), metatarsal (n = 1) and talus (n = 1). At the time of diagnosis, all patients had experienced pain for at least 1 month.
The GCTs were evaluated by preoperative clinical, radiological, MRI, and CT examinations and CT guided core biopsies (Fig. 1a,b). Based on the CT findings, they were classified into the following classes. Class I tumors were intraosseous with no cortical breaks. Class II tumors were extraosseous lesions with cortical breaks confined to one surface and not exceeding one third of the bone's circumference. Class III tumors were extraosseous lesions that had broken through the cortex at more than one surface or extended into more than one third of the bone's circumference. (Fig. 2a–c). The patients in classes I and II (31 patients) were selected for curettage and bone grafting. Patients with class III lesions and recurrent GCT's were treated by wide resection and reconstruction.
Figure 1.
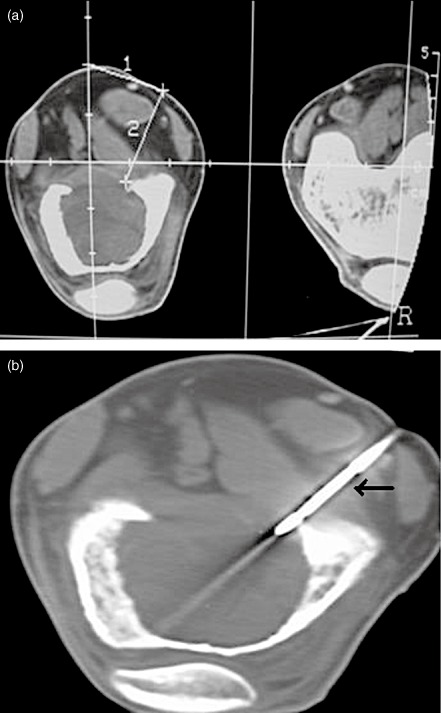
The GCTs were evaluated by preoperative CT examinations and CT guided core biopsies. Computerized tomography pictures of a GCT of the distal femur showing (a) localization of tumor and measurements and (b) the track of the core biopsy needle (black arrow).
Figure 2.
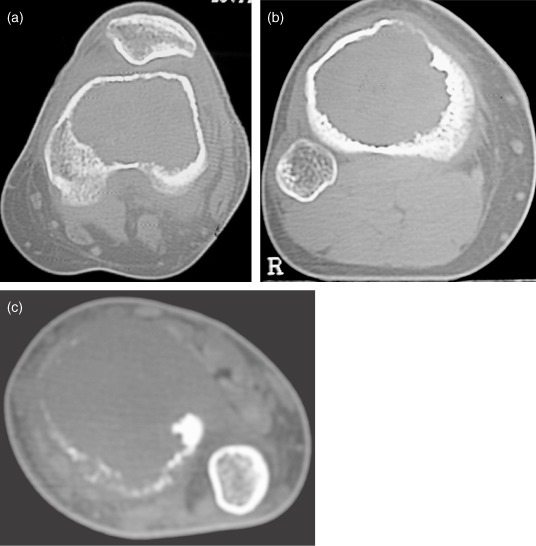
Based on the CT findings, a GCT patient of the distal femur can be classified as Class I (a) without any cortical break, Class II (b) with a cortical break confined to one surface and not exceeding one third of its circumference and Class III (c) with a cortical break more than one surface and more than one third of the bone's circumference.
Surgical Technique
The surgeries were performed by one of the authors (DP) at a single institution. For curettage; the lesions were approached through the site of cortical break irrespective of standard approaches to facilitate optimal clearance of the involved soft tissues. For example in a lesion of the lower femur or upper tibia, if the cortical break was in the posterior aspect, a posterior approach (by isolating the popliteal vessels and tibial nerve) was preferred. In patients with GCTs without cortical breaks (Class I), CT guided biopsies were taken from the thinnest area of cortex and the tumors surgically approached in such a way as to include the biopsy track. After exposure, the site of cortical break was identified by palpation and a circumferential area of 1 cm × 1 cm beyond the margin of the cortical break marked using cautery. The cortical break was widened with a small osteotome and the area with soft tissue extension (determined preoperatively by MRI) removed as a lid with scissors, taking care not to spill the tumor (Fig. 3a–d). After thorough curettage, the cavity was washed several times with hydrogen peroxide and normal saline, then cauterized with phenol and tightly filled with pre‐prepared femoral head allograft. In the cases described in this article, a posterior approach was used for 11 lower femoral and 6 upper tibial tumors. In the remaining cases of lower femoral and upper tibial tumors, the approaches were anteromedial or anterolateral, depending on the location of the cortical break as shown by the CT scan.
Figure 3.
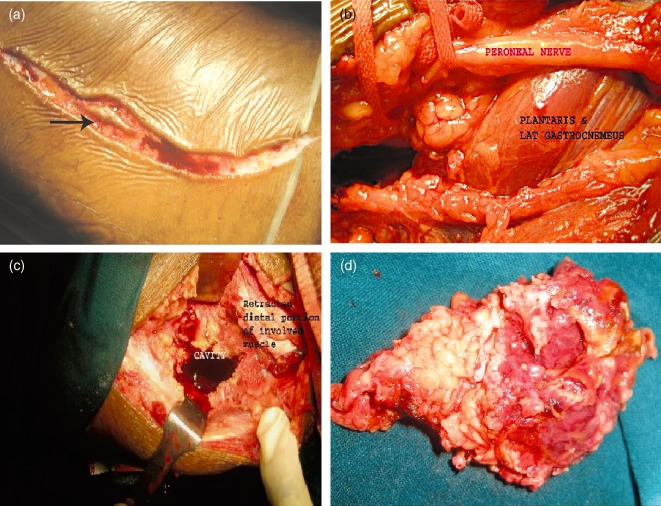
Operative photograph showing (a) the biopsy track (black arrow) being included in the incision and (b) posterior approach to the proximal tibia by isolating the popliteal vessels and peroneal nerve. (c) The proximal tibia was exposed after the cortical break had been widened with a small osteotome and the area where there was soft tissue extension was removed. (d) Operative photograph showing resected gross specimen.
In one case of GCT of the calcaneum, the cortical break was on its superior, nonarticular surface, where there was tumor extension into extraosseous tissue. This lesion was approached through a transverse incision on the lateral aspect, and the insertion of the Achilles tendon severed to achieve a wider area of removal of the tumor, along with the involved soft tissues, at the site of the cortical break. After curettage and bone grafting, the Achilles tendon was reinserted using pull out sutures.
The patients were clinically assessed preoperatively and postoperatively. They were followed up at 6 week interval until 6 months, then at 3 month intervals until 1 year, and annually thereafter. All patients were assessed for any intraoperative, postoperative, and final follow up complications. Functional results were evaluated using Musculoskeletal Tumor Society (MSTS) scores. Radiographic evaluation was performed using standard anteroposterior and lateral radiographs. Local recurrence was suspected when a progressive area of radiolucency appeared within, or adjacent to, a previously treated area (Fig. 4).
Figure 4.
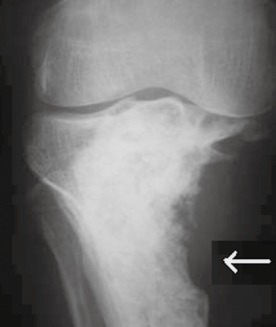
Radiographic evaluation was performed using standard anteroposterior radiograph. Radiograph showing recurrence of GCT of the proximal tibia with area of radiolucency within and adjacent to the previously treated area.
To assess how the results of this procedure compared to standard/anatomic curettage and bone grafting performed in similarly aged patients (mean, 33 years) at a similar length of follow up (mean, 72 months), the authors carried out a retrospective review of a separate contemporaneous cohort of 20 patients (group B) who had been treated by curettage and bone grafting based on Campanacci grading, stage of the tumor and location (without applying the CT based selection criteria) and compared their outcomes to those of the present study. The surgical approaches used in this group of patients were standard. For statistical analysis, we used Epi info 3 and SPSS 10 for Windows.
Results
Of 51 patients with primary GCT of bone, 31 (group A) were evaluated by CT and further grouped into two classes (class I: 1 case, no recurrence; class II: 30 cases, 4 recurrences). Twenty seven of the 31 patients who had undergone curettage and bone grafting had no local recurrence at long term follow up (mean, 72 months). No recurrences occurred before 24 months.
Patients with recurrence were further evaluated by MRI and repeat biopsy. One patient with recurrence in the lower femur was treated by wide resection and a custom‐made prosthesis. Another patient with recurrence in the upper tibia did not come to us for further treatment. One in the lower radius was treated by en bloc resection and reconstruction with non‐vascularized proximal fibula. The fourth recurrence was in theupper tibia and treated by wide resection and arthrodesis by long intramedullary interlocking nail and bone grafting.
In group B, of 20 patients treated by standard curettage and bone grafting without prior CT classification, 6 (30%) had local recurrences. Five patients had recurrences within 24 months.
The long term clinical success rate (absence of recurrence) of 87.1% (27 of 31) for group A patients was significantly better (z = 2.112, P < 0.05) than that of group B at similar follow up (72 months) in patients with a similar mean age (33 years). There was no significant difference between the two groups in mean MSTS scores (group A, 26.9 points; group B, 26.77 points; P = 0.4). No pathologic fractures, wound healing problems, or nerve injuries occurred in either study group (Table 1).
Table 1.
Summary of results of the CT classified and non CT classified groups
| Results | CT classified | Non‐CT classified |
|---|---|---|
| Recurrence (total) | 4 (12.9%) | 6 (30%) |
| Recurrence at 24 months | None | 5 (25%) |
| MSTS score (final) | 26.9 (1.37 SD) | 26.77 (2.2 SD) |
| Other complications | None | None |
Discussion
We thought that it made intuitive sense to surgically approach GCT tumors through the site of cortical break to attain adequate clearance of possible soft tissue extensions and thus more reliably achieve local control of this annoying and recidivistic tumor. The aim of approaching the lesion at the site of cortical break was to achieve wide excision of any involved soft tissue. We asked whether this strategy reduced recurrence in comparison to a group of patients with GCT who had been treated by standard surgical approaches. Only patients with primary GCT, belonging to class I and class II on CT based classification were selected for curettage. All class III and recurrent GCTs were treated by wide resection.
Our findings suggest that group A and group B had comparable wound problems and functional outcomes. Although we cannot draw a solid conclusion regarding wound complications because of the insufficient power of the statistical analysis, our experience during the past 14 years of approaching the tumor through the site of cortical break, irrespective of standard surgical approach, support the safety of this approach in terms of wound complications. Our study indicates that CT classification and approaching the tumor through the site of cortical break does not create clinically different functional outcomes.
The rate of recurrence was significantly higher in group B (30%), a finding which is consistent with that of available published reports. Group A had a significantly lower long term recurrence rate (12.9%), which is lower than that of most reported studies (without using liquid nitrogen) to date4, 5, 6, 8, 9, 10. CT classification and approaching the tumor through the site of cortical break leads to more precise patient selection and soft tissue clearance, which seems to lead to less tumor recurrence by 2 years postoperatively. Whether to perform intralesional or en‐bloc resection of Campanacci grade III or aggressive GCTs of bone remains controversial15, 16. Grade III Campanacci lesions have traditionally been treated by wide resection because of their potential for local recurrence16. However, based on our CT classification system, some of the grade III lesions which have previously been treated by wide resection could achieve a recurrence rate comparable with grade I and II lesions if treated by intralesional curettage. We believe that CT classification and soft tissue clearance are better predictors of local recurrence than is Campanacci grading.
To date, the postoperative recurrence rate reported for GCTs has been surprisingly high (Table 2). In So far, only two large series of GCTs with overall recurrence rates of less than 15% after curettage have been published. In both series liquid nitrogen was used as an adjuvant7, 13. Because there is no clear evidence as to which adjuvants are effective; their use remains controversial17, 18. We did not use liquid nitrogen as an adjuvant because of its potential complications, which include pathologic fractures, wound healing problems, and nerve injuries19. Though phenol has also been reported to be associated with the above‐mentioned complications in more recent studies, none of the patients in our study groups had such complications20. Use of phenol and hydrogen peroxide as adjuvants does not seem to have affected the long term recurrence rate in our patients. In retrospect, further investigation is needed to establish or refute the usefulness of adjuvants in the treatment of GCTs.
Table 2.
Summary of review of published reports concerning local recurrence after curettage of giant cell tumor
| Author | Year | No. of patients | Additional treatment | Recurrence (%) |
|---|---|---|---|---|
| Turcotte18 | 2006 | 120 | PMMA, LN | 12 |
| Prosser et al.11 | 2005 | 137 | None | 19 |
| Malawer et al.7 | 1999 | 102 | Burr, LN | 8 |
| Oda et al.10 | 1998 | 47 | Burr | 50 |
| Masui et al.8 | 1998 | 47 | None | 47 |
| O'Donnell et al.9 | 1994 | 60 | PMMA | 25 |
| Campanacci et al.4 | 1987 | 106 | None | 34 |
| Capanna et al.5 | 1990 | 280 | None | 45 |
| Larsson et al.6 | 1975 | 75 | None | 42 |
Note: LN, liquid nitrogen; PMMA, polymethylmethacrylate.
The biological basis for using polymethylmethacrylate (PMMA) has not been clarified. As, in most cases, there is less than 3 mm of cancellous bone between PMMA implants and the subchondral bone layer, regions of subchondral bone are also exposed to thermal necrotic conditions. Because its thermal effect on the adjacent joint cartilage may eventually lead to degenerative changes, we prefer bone graft over PMMA as a filler material after curettage12, 21, 22.
The findings of this study should be viewed after considering the following limitations. First, we did not include a group without CT classification in which the approaches to the lesions were through cortical breaks. We therefore cannot conclude from this study whether the CT based selection strategy alone offers advantages or disadvantages in comparison to no selection. How to take CT guided core biopsies from lesions without cortical breaks and approach the tumors surgically has not yet been clarified. GCTs usually become symptomatic when the cortex has been broken (98.5% in our series). Only one patient presented to us before the cortex had broken. In that patient, we took a biopsy from the thinnest area of the cortex and included the biopsy track in the excised soft tissue. Furthermore, despite the lack of definitive proof regarding the advantages of adjuvant therapy, phenol and hydrogen peroxide were used in all patients after curettage; therefore their effects in our patients cannot be statistically validated.
We achieved long‐term success in this challenging patient population. We believe our CT based selection strategy is a valid preoperative tool for evaluating GCTs. Further, the lesions are better approached for curettage through the sites of cortical break, irrespective of standard approaches, so that adequate soft tissue clearance can be achieved. In addition, we believe that recurrent GCTs and extraosseous lesions that have broken through the cortex at more than one surface or extended into more than one third of its circumference are better treated by wide resection.
Acknowledgements
We acknowledge Dr Asha RV and Mr Kiran P for critical comments they made while we were preparing this article.
Disclosure: The authors declare no conflict of interest. No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1. Muramatsu K, Ihara K, Taguchi T. Treatment of giant cell tumor of long bones: clinical outcome and reconstructive strategy for lower and upper limbs. Orthopedics, 2009, 32: 491. [DOI] [PubMed] [Google Scholar]
- 2. Ruka W, Rutkowski P, Morysinski T, et al The megavoltage radiation therapy in treatment of patients with advanced or difficult giant cell tumors of bone. Int J Radiat Oncol Biol Phys, 2010, 78: 494–498. [DOI] [PubMed] [Google Scholar]
- 3. Blackley HR, Wunder JS, Davis AM, et al Treatment of giant‐cell tumors of long bones with curettage and bone‐grafting. J Bone Joint Surg Am, 1999, 81: 811–820. [DOI] [PubMed] [Google Scholar]
- 4. Campanacci M, Baldini N, Boriani S, et al Giant‐cell tumor of bone. J Bone Joint Surg Am, 1987, 69: 106–114. [PubMed] [Google Scholar]
- 5. Capanna R, Fabbri N, Bettelli G. Curettage of giant cell tumor of bone. The effect of surgical technique and adjuvants on local recurrence rate. Chir Organi Mov, 1990, 75 (Suppl. 1): S206. [PubMed] [Google Scholar]
- 6. Larsson SE, Lorentzon R, Boquist L. Giant‐cell tumor of bone. A demographic, clinical, and histopathological study of all cases recorded in the Swedish Cancer Registry for the years 1958 through 1968. J Bone Joint Surg Am, 1975, 57: 167–173. [PubMed] [Google Scholar]
- 7. Malawer MM, Bickels J, Meller I, et al Cryosurgery in the treatment of giant cell tumor. A long‐term followup study. Clin Orthop Relat Res, 1999, 359: 176–188. [DOI] [PubMed] [Google Scholar]
- 8. Masui F, Ushigome S, Fujii K. Giant cell tumor of bone: a clinicopathologic study of prognostic factors. Pathol Int, 1998, 48: 723–729. [DOI] [PubMed] [Google Scholar]
- 9. O'Donnell RJ, Springfield DS, Motwani HK, et al Recurrence of giant‐cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am, 1994, 76: 1827–1833. [DOI] [PubMed] [Google Scholar]
- 10. Oda Y, Miura H, Tsuneyoshi M, et al Giant cell tumor of bone: oncological and functional results of long‐term follow‐up. Jpn J Clin Oncol, 1998, 28: 323–328. [DOI] [PubMed] [Google Scholar]
- 11. Prosser GH, Baloch KG, Tillman RM, et al Does curettage without adjuvant therapy provide low recurrence rates in giant‐cell tumors of bone? Clin Orthop Relat Res, 2005, 435: 211–218. [DOI] [PubMed] [Google Scholar]
- 12. Radev BR, Kase JA, Askew MJ, et al Potential for thermal damage to articular cartilage by PMMA reconstruction of a bone cavity following tumor excision: a finite element study. J Biomech, 2009, 42: 1120–1126. [DOI] [PubMed] [Google Scholar]
- 13. Turcotte RE, Wunder JS, Isler MH, et al Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res, 2002, 397: 248–258. [DOI] [PubMed] [Google Scholar]
- 14. Abdelrahman M, Bassiony AA, Shalaby H, et al Cryosurgery and impaction subchondral bone graft for the treatment of giant cell tumor around the knee. HSS J, 2009, 5: 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faisham WI, Zulmi W, Halim AS, et al Aggressive giant cell tumor of bone. Singapore Med J, 2006, 47: 679–683. [PubMed] [Google Scholar]
- 16. Lackman RD, Hosalkar HS, Ogilvie CM, et al Intralesional curettage for grades II and III giant cell tumors of bone. Clin Orthop Relat Res, 2005, 438: 123–127. [DOI] [PubMed] [Google Scholar]
- 17. Becker WT, Dohle J, Bernd L, et al Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am, 2008, 90: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 18. Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am, 2006, 37: 35–51. [DOI] [PubMed] [Google Scholar]
- 19. Robert K, Heck J. Benign (occasionally aggressive) tumors of bone In: Canale ST, ed. Campbell's Operative Orthopaedics, 10th edn. Pennsylvania: Mosby, 2003; 813–816. [Google Scholar]
- 20. Robert K, Heck J. Benign/aggressive tumors of bone In: Canale ST, ed. Campbell's Operative Orthopaedics, 11th edn. Pennsylvania: Mosbay, 2007; 883–886. [Google Scholar]
- 21. Fraquet N, Faizon G, Rosset P, et al Long bones giant cells tumors: treatment by curettage and cavity filling cementation. Orthop Traumatol Surg Res, 2009, 95: 402–406. [DOI] [PubMed] [Google Scholar]
- 22. Morii T, Yabe H, Morioka H, et al Curettage and allograft reconstruction for giant cell tumors. J Orthop Surg (Hong Kong), 2008, 16: 75–79. [DOI] [PubMed] [Google Scholar]


