Abstract
Objective
To explore the clinical efficacy and complications of treating sternal tumors by resection and titanium mesh thoracic reconstruction.
Methods
This retrospective analysis of eight patients with sternal tumors treated in the Department of Orthopedic Surgery at the First Affiliated Hospital of Zhengzhou University from January 2008 to June 2012 included five men and three women aged 37–66 years (mean, 50.4 years). The histological diagnoses were chondrosarcoma (two cases), osteosarcoma (one), malignant fibrous histiocytoma (two), eosinophilic granuloma (one) and sternal metastasis from breast cancer (two). The tumors were invading the manubrium sterni (three cases), manubrium sterni and body (three) and sternal body (two). All patients underwent needle or incisional biopsy prior to sternal tumor resection and titanium mesh thoracic reconstruction.
Results
All patients were followed for 9 months to 4 years. There were no intraoperative complications or operative or postoperative deaths. One patient developed a deep wound hematoma 1 week postoperatively; incisional drainage and debridement resulting in healing within 2 weeks. There was no loosening or exsertion of the titanium mesh and no patients developed respiratory complications or thoracic deformity. One patient with malignant fibrous histiocytoma died of lung metastases 9 months postoperatively, another with malignant fibrous histiocytoma died of liver metastases 14 months postoperatively; the remaining patients survived without tumor recurrence.
Conclusion
Titanium mesh chest reconstruction after sternal tumor resection has the advantages of simplifying the procedure, achieving a good shape and having few complications. Titanium mesh is an ideal material for reconstruction of the sternum.
Keywords: Reconstructed titanium mesh, Sternal tumor, Surgical treatment
Introduction
The morbidity associated with sternal tumors is relatively low; they account for approximately 20% of all breast tumors and 0.94% of all bone tumors1. Most are malignant, including fibrous tissue cell tumor, rhabdomyosarcoma, lymphoma, osteosarcoma and chondrosarcoma, chondrosarcoma being the commonest2. Benign sternal tumors comprise osteochondroma, desmoid tumors and fibrous structures.
“Extended resection” is the preferred method of treatment. According to published reports, surgical treatment is indicated for benign tumors, malignant tumors without distant metastasis, and metastatic tumors (a survival benefit may be achieved in patients with controlled primary lesions and no other distant metastases)3, 4, 5, 6. Extended resection also offers the benefit of preventing relapses, which is important because even benign tumors can recur locally7. Postoperative chest wall defects can cause a floating chest wall, impacting lung function and extending ventilator time. Accordingly, it is important to reconstruct the chest wall. Chest wall reconstruction is beneficial in restoring chest wall integrity and stability, protecting vital organs within the chest and preventing cardiopulmonary dysfunction.
Materials used to repair sternal defects include artificial and biological tissues such as bone allografts and autografts, muscle flaps, omentum, steel wire (OsteoMed Co., Addison, TX, USA), polyester fabric, silicone rubber sheets and Marlex nets (C.R. Bard, Murray Hill, NY. USA)8, 9, 10, 11. However, most of these tissues provide inadequate support; are prone to loosening, breaking, rejection and infection; and can cause pain. Moreover, some of these materials are difficult to shape, resulting in a poor appearance or other shortcomings. One of the most common complications is infection; a 5% postoperative infection rate has been reported with Marlex mesh (C.R. Bard, Murray Hill, NY. USA)12.
Clearly, choosing the right materials to replace the sternum is vital. The ideal reconstruction material would meet the following criteria: good hardness and stability to prevent floating of the chest wall; not subject to rejection; suitable for long‐term retention in the body; easily cut, shaped, fixed, and sterilized; and radiopaque13. Titanium mesh has been widely used and achieved good results in clinical practice as a repair material for skull defects14. However, there are few reports of its use for sternal tumor resection and reconstruction.
In this retrospective study, we assessed the need for and efficacy of resection and reconstruction in patients with sternal tumors and explored the advantages and disadvantages of using titanium mesh for reconstruction.
Materials and Methods
Patient and Tumor Characteristics
The study included eight patients with sternal tumors diagnosed in the First Affiliated Hospital of Zhengzhou University between January 2008 and June 2012. Five patients were male and three female, and their mean age was 50.4 years (range, 37–66 years). Two patients had chondrosarcoma, one osteosarcoma, two malignant fibrous histiocytoma, one eosinophilic granuloma and two sternal metastases from breast cancer. All of the patients presented with sternal masses with a hard texture. Sternal tenderness was present in three cases. Six patients reported chest wall pain and two pain radiating into the back. On examination, three tumors had invaded the manubrium sterni; the size of these tumors ranged from 12 cm × 10 cm to 5 cm × 4 cm. Three tumors had invaded both the manubrium and corpus of the sternum; these ranged in size from 9 cm × 8 cm to 4 cm × 3 cm. In two cases, only the corpus sterni had been invaded; these tumors measured 11 cm × 9 cm and 6 cm × 4 cm. The diagnoses of all eight patients were confirmed preoperatively by needle or incisional biopsy. X‐ray imaging showed osteolytic bone destruction and CT and MRI revealed the presence of a sternal mass or osteolytic lesion in all cases. Bone scans were performed in all cases and confirmed isolated lesions in seven cases; one patient with breast cancer had bone metastases in the fifth thoracic vertebrae.
Preoperative Evaluation
All patients underwent comprehensive preoperative assessment. Imaging examinations included plain radiography, CT, MRI, and bone scans, as described above. The following variables were assessed: tumor size, sternal and soft tissue invasion, tumor blood supply, general patient condition including cardiac and pulmonary function, serum album concentration, presence and location of metastases; and the patients evaluated for anesthesia and tolerance of surgery. Seven cases were Cardiac Level 1 and one Cardiac Level 2 (the New York Heart Association (NYHA)). In all patients, maximum ventilation percentagein one minute was greater than 80%, and forced expiratory volume in one second/forced vital capacity greater than 80%.
Surgical Procedure
Anesthesia and Positioning
Surgery was performed under general anesthesia; patients were placed in a lateral position with the upper limbs abducted 90° and restrained.
Surgical Approach
A T‐shaped or median sternotomy incision was made through the skin and subcutaneous tissues. Resections generally extended 2 cm beyond the tumor margins for benign tumors (eosinophilic granuloma) and at least 3 cm beyond the tumor for malignant lesions (Fig. 1A).
Figure 1.
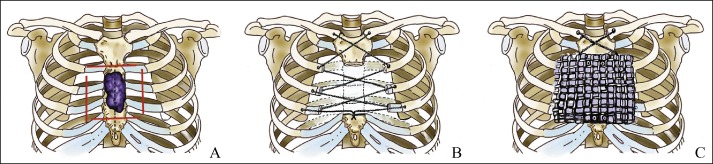
Diagrammatic representation of sternal tumor resection and insertion of titanium mesh. (A) Diagram showing incision and tumor resection; the skin is initially cut along the dotted line, the solid line shows the boundary of the resected tissue. Resections generally extend 2 cm beyond the tumor margins for benign tumors and at least 3 cm beyond the tumor for malignant lesions. (B) The clavicular heads and ribs on both sides are tied together with titanium cable. (C) Titanium mesh is put in place and adjusted to align with the edges of the remaining sternum.
Resection of Lesion and Titanium Mesh Installation
In three cases, resection of lesion included resection of the manubrium sterni, both sides of the clavicular head and the sternal ends of the first and second costal cartilages. In another three cases, the manubrium and body of the sternum were resected and the involved ribs widely excised (Fig. 1B). In the remaining two cases, the body of the sternum was resected and the involved ribs widely excised.
To reconstruct the chest wall and stabilize it, titanium mesh was put in place and adjusted to align with the edges of the remaining sternum (Fig. 1C), then fixed with stainless steel wire or titanium screws. Next, the reconstruction was covered with the pectoral muscle and subcutaneous tissues. Vacuum suction drainage devices were placed in the wound.
Postoperative Management
Postoperatively, the surgical wounds were fully drained and the patients treated with conventional antibiotics. Patients' vital signs were closely observed and they were monitored for paradoxical motion of the chest wall. Particular attention was paid to the respiratory tract to detect and prevent pulmonary complications. Patency of the drainage tube was maintained until the drainage was less than 50 mL/day, at which time the tubes were removed. The sutures were removed after 2 weeks. Patients with osteosarcoma and chondrosarcoma received T7 chemotherapy (Cyclophosphamide, doxorubicin, vincristine, actinomycin D), those with malignant fibrous histiocytoma received combination dimethyl triazeno imidazole carboxamide and doxorubicin chemotherapy, and those with bone metastases from breast cancer received docetaxel, doxorubicin and cyclophosphamide. Additionally, all patients except the one with eosinophilic granuloma received zoledronic acid (4 mg per dose, four times per week) for 12–18 months.
Follow‐Up
Follow‐up was performed at 3, 6 and 12 months after surgery, then annually.
Results
Intraoperative Findings
The tumors were surrounded by edematous soft tissue. The eosinophilic granuloma was encapsulated, whereas the remaining lesions were encapsulated by intact false capsules. The lesions were irregular and oval in shape and surrounded by infiltration. After the titanium mesh had been installed, 300 mL of normal saline was poured into the wound and the site closely inspected for bubbles; a lack of bubbles confirmed undamaged pleura.
Evaluation of Patients and Fixation Devices
All eight patients underwent surgery safely; there were no intraoperative complications or intraoperative or postoperative deaths. During follow‐up, there was no loosening of the titanium mesh, exposure of the mesh, breathing difficulties, chest pain or discomfort. All patients had normal cardiopulmonary function. Review of chest X‐ray films showed no deformity of the thorax and no loosening or fracture of the fixation devices (Figs 2 and 3).
Figure 2.
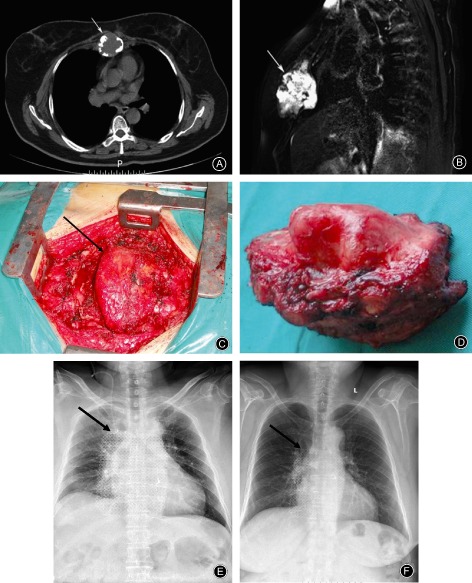
Female, 62 years old, chondrosarcoma of the sternal manubrium. (A) CT image showing tumor‐induced bone destruction (arrow); the tumor is eroding the sternal bone and surrounding soft‐tissue. (B) Preoperative MRI T2 fat suppression image showing invasion of surrounding tissue by the tumor (arrow), demonstrated as long T1 and T2 signals. (C) Intraoperative photograph showing the tumor tissue. (D) The tumor has been completely resected. (E) X‐ray film taken one week postoperatively (titanium mesh shown by the arrow). (F) X‐ray film taken after one year (titanium mesh shown by the arrow). There has been no displacement or fracture of the titanium mesh.
Figure 3.

Male, 37 years old, malignant fibrous histiocytoma of sternum. (A) Preoperative CT image showing tumor‐induced bone destruction, the tumor has eroded the sternal bone and surrounding soft‐tissue (arrow). (B) Preoperative MRI T2 fat suppression image showing invasion of surrounding tissue by the tumor extending to the right pectoralis major, demonstrated as long T1 and T2 signals (arrow). (C) One week after the operation, X‐ray film showing no displacement of the titanium mesh (shown by the arrow). (D) X‐ray film after a year, the position of the titanium mesh (arrow) is correct, no movement or fracture has occurred.
Complications
One deep hematoma occurred 1 week postoperatively, evidenced by development of dyspnea and a pink liquid exudate from the wound. The patient underwent emergency surgery, during which no active bleeding was identified. Loosening of the intercostal vessels may have caused the deep hematoma. The hematoma was removed and the drain repositioned; the wound healed within 2 weeks. In the remaining cases, the incisions healed without any respiratory problems subcutaneous emphysema, pneumothorax, infection or other complications.
Follow‐Up
The eight cases were followed up for from 9 months to 4 years postoperatively. One patient with malignant fibrous histiocytoma died of lung metastases 9 months postoperatively and another patient with malignant fibrous histiocytoma died of liver metastases after 14 months. The remaining patients had no evidence of tumor recurrence during follow‐up.
Discussion
Necessity for and Therapeutic Effect of Sternal Tumor Resection and Reconstruction
Because the sternum is important for maintaining the integrity of the thoracic bones, sternal resection significantly affects respiratory and circulatory function. To preserve the integrity of the chest, maintain negative intrathoracic pressure and protect vital organs, it is important to reconstruct the thoracic cage15. In combination with postoperative radiotherapy and chemotherapy, this reduces recurrence and metastasis and improves survival rates of patients with malignant tumors5. Previous studies have recommended en bloc resection of the tumor with at least 3 cm margins for malignant tumors and at least 2 cm margins for benign tumors5, 16. To allow for defect closure and wound repair, muscle and skin resection should be appropriately conservative. The principles involved in chest wall reconstruction are: (i) reconstruction of the chest wall to restore its sturdiness and stability; and (ii) covering the chest wall reconstruction with soft tissues and skin to restore the rigidity of the chest wall. To appropriately plan the extent of each patient's resection, they all underwent preoperative imaging and pathological examination. During the operation, care was taken to meticulously dissect tissue that was adherent to the tumor and to protect blood vessels and nerves.
Advantages and Disadvantages of Titanium Mesh Reconstruction of Sternal Defects
Reconstruction should achieve stability of the chest structure and closure of the pleural cavity, prevent abnormal breathing and restore the normal morphology of the thorax without affecting postoperative assessment and treatment. Currently, no commercial sternal reconstruction materials are available. Because there is a lack of consensus regarding the material that should be selected for sternal reconstruction, many different materials are presently used. They can primarily be divided into autologous tissues, allograft tissues and artificial materials. Autologous sternal reconstruction materials include ribs17, ilium18, fibula19, fascia20 and muscle flaps21, 22. The limited availability of sufficiently rigid autologous tissues increases the difficulty of repairing sternal defects. Obtaining autologous tissues also increases the operative difficulty and surgery time, thereby increasing patient risk and adverse effects23. However, allograft tissues are susceptible to rejection and infection and are also difficult to obtain24. In this group of patients, we wanted to use iliac allografts in two patients; however, they were not willing to wait for a source of bone. Synthetic materials, such as nylon, polyester networks (Prolene) (PermacolTM implant,Covidien, USA) and fiberglass (Normopost, Normon, Biolonen, Saronno, Italy), are rarely used because of their low tensile strength, ease of deformity with long‐term implantation, and susceptibility to rejection. At present, some surgeons outside of China prefer to use Marlex net (C.R. Bard, Murray Hill, NY. USA) because of its maneuverability and long‐term tolerability; additionally, it does not readily cause rejection and is radiopaque25, 26, 27. However, because of its elasticity, it can only play a role in covering and isolating surgical sites; it is unable to restore chest wall stability or protect the thoracic organs. Accordingly, it can only be used to cover small sternal defects. Medical plexiglass can be shaped into the form of a rib; sterilized, shaped intraoperatively, placed in the bone defect and attached to the corresponding ribs with steel wire28. To prevent floating of the chest wall, its deep tissues should be firmly tied to the ribs. However, because medical plexiglass provides poor compression, it is not suitable for reconstructing large defects.
Surface‐perforated titanium mesh is used to repair skull defects. In our study, we found that titanium mesh is convenient for rebuilding chest wall defects. Titanium closely matches the hardness and elastic properties of bone. It is thin and easy to shape, thus facilitating achieving a curvature similar to that of normal anatomic structures and being able to combine closely with the residual sternum and ribs. Moreover, titanium offers low density, high strength, good biocompatibility, low rejection, impact resistance, radiopacity, is non‐carcinogenic and non‐allergenic, and does not deform after sterilization14, 29, 30.
In our study, the results of sternal defect repair with titanium mesh were satisfactory. To prevent the artificial materials from being in direct contact with the skin, it is important to fasten both sides of the pectoralis major muscle to the midline during surgery, covering the titanium net. Fibrous granulation tissue then forms and readily penetrates titanium's mesh‐like structure, creating coverage by muscle and connective tissues, thereby sealing the chest wall, preserving its integrity, and reducing the chances of infection. Titanium mesh does have the limitations of being expensive and attenuating radiotherapy31. The most common postoperative complication is fluid surrounding the implant, which may lead to secondary infections and subsequent operation failure. In this study, one deep hematoma occurred 1 week after surgery, accompanied by dyspnea. The key to prevention of hematomas is good intraoperative hemostasis, postoperative drainage and bandaging.
In summary, we successfully used titanium mesh for chest wall reconstruction after sternal tumor resection in this group of eight patients. We achieved good shaping of the chest wall, all patients were able to breathe freely after surgery, and no loosening of the titanium mesh occurred postoperatively, confirming that titanium mesh is a good material for reconstructing the thorax. Because this was a small study with a short duration of follow‐up, further study and a longer observation period are required for ascertaining long‐term efficacy.
Disclosure: No funds were received in support of this work.
References
- 1. Koo KS. Thoraco‐Cardiac Operation Surgery. Shanghai: Shanghai Science and Technology Press, 2003: 586–591. [Google Scholar]
- 2. McAfee MK, Pairolero PC, Bergstralh EJ, et al Chondrosarcoma of the chest wall: factors affecting survival. Ann Thorac Surg, 1985, 40: 535–541. [DOI] [PubMed] [Google Scholar]
- 3. Cheng YE. Surgical treatment of primary tumor of the sternum. Zhonghua Wai Ke Za Zhi, 1983, 21: 621–622. (In Chinese). [PubMed] [Google Scholar]
- 4. Shamberger RC, Laquaglia MP, Krailo MD, et al Ewing sarcoma of the rib: results of an intergroup study with analysis of outcome by timing of resection. J Thorac Cardiovasc Surg, 2000, 119: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 5. Chapelier AR, Missana MC, Couturaud B, et al Sternal resection and reconstruction for primary malignant tumors. Ann Thorac Surg, 2004, 77: 1001–1006. Discussion 1006–1007. [DOI] [PubMed] [Google Scholar]
- 6. Eguchi K, Ishi S, Sugiura H, Noga K. Angiosarcoma of the chest wall in a patient with fibrous dysplasia. Eur J Cardiothorac Surg, 2002, 22: 654–655. [DOI] [PubMed] [Google Scholar]
- 7. Laredo J, Morris DJ, Thurer RL. Metastatic melanoma to the manubrium sternum. Eur J Cardiothorac Surg, 1998, 14: 629–630. [DOI] [PubMed] [Google Scholar]
- 8. Landes G, Harris PG, Sampalis JS, et al Outcomes in the management of sternal dehiscence by plastic surgery: a ten‐year review in one university center. Ann Plast Surg, 2007, 59: 659–666. [DOI] [PubMed] [Google Scholar]
- 9. Strecker T, Rösch J, Horch RE, Weyand M, Kneser U. Sternal wound infections following cardiac surgery: risk factor analysis and interdisciplinary treatment. Heart Surg Forum, 2007, 10: E366–E371. [DOI] [PubMed] [Google Scholar]
- 10. Schimmer C, Keith P, Neukam K, Beissert M, Leyh R. Large thoracic wall hematoma following sternal reconstruction with transversal plate fixation after deep sternal wound infection. Thorac Cardiovasc Surg, 2007, 55: 402–405. [DOI] [PubMed] [Google Scholar]
- 11. Veronesi G, Scanagatta P, Goldhirsch A, et al Results of chest wall resection for recurrent or locally advanced breast malignancies. Breast, 2007, 16: 297–302. [DOI] [PubMed] [Google Scholar]
- 12. King RM, Pairolero PC, Trastek VF, Piehler JM, Payne WS, Bernatz PE. Primary chest wall tumors: factors affecting survival. Ann Thorac Surg, 1986, 41: 597–601. [DOI] [PubMed] [Google Scholar]
- 13. LeRoux BT. Maintenance of chest wall stability. Thorax, 1964, 19: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Rosa V, Ionna F, Mozzillo N, Parascandolo S, Ziviello M. 3D spiral computerized tomography in the reconstructive treatment of malignant maxillofacial tumors. Radiol Med, 2000, 100: 424–428. [PubMed] [Google Scholar]
- 15. Tunçözgür B, Elbeyli L, Güngör A, Işik F, Akay H. Chest wall reconstruction with autologas rib grafts in dogs and report of a clinic case. Eur J Cardiothorac Surg, 1999, 16: 292–295. [DOI] [PubMed] [Google Scholar]
- 16. Mansour KA, Anderson TM, Hester TR. Sternal resection and reconstruction. Ann Thorac Surg, 1993, 55: 838–842. Discussion 843. [DOI] [PubMed] [Google Scholar]
- 17. Bisgard JD, Swenson SA Jr. Tumors of the sternum; report of a case with special operative technic. Arch Surg, 1948, 56: 570–578. [DOI] [PubMed] [Google Scholar]
- 18. Brodin H, Linden K. Resection of the whole of the sternum and the cartilaginous parts of costae I–IV: a case report. Acta Chir Scand, 1959, 118: 13–15. [PubMed] [Google Scholar]
- 19. Ogawa J, Kawada S, Koide S, et al Reconstruction of sternal defects with autogenous bone graft and myocutaneous flap of latissimus dorsi muscle (author's translation). Kyobu Geka, 1980, 33: 696–699. (In Japanese). [PubMed] [Google Scholar]
- 20. Watson WL, James AG. Fascia lata grafts for chest wall defects. J Thorac Surg, 1947, 16: 399–406. [PubMed] [Google Scholar]
- 21. Khalil el‐SA, EI‐Zohairy MA, Bukhari M. Reconstruction of large full thickness chest wall defects following resection of malignant tumors. J Egypt Natl Canc Inst, 2010, 22: 19–27. [PubMed] [Google Scholar]
- 22. Chapelier A. Resection and reconstruction for primary sternal tumors. Thorac Surg Clin, 2010, 20: 529–534. [DOI] [PubMed] [Google Scholar]
- 23. McCormack PM. Use of prosthetic materials in chest‐wall reconstruction. Assets and liabilities. Surg Clin North Am, 1989, 69: 965–976. [DOI] [PubMed] [Google Scholar]
- 24. Marulli G, Hamad AM, Cogliati E, Breda C, Zuin A, Rea F. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J Thorac Cardiovasc Surg, 2010, 139: e69–e70. [DOI] [PubMed] [Google Scholar]
- 25. Haraguchi S, Yamashita Y, Yamashita K, Hioki M, Matsumoto K, Shimizu K. Sternal resection for metastasis from thyroid carcinoma and reconstruction with the sandwiched Marlex and stainless steel mesh. Jpn J Thorac Cardiovasc Surg, 2004, 52: 209–212. [DOI] [PubMed] [Google Scholar]
- 26. Suehara Y, Yazawa Y, Hitachi K, Terakado A. Clear cell sarcoma arising from the chest wall: a case report. J Orthop Sci, 2004, 9: 171–174. [DOI] [PubMed] [Google Scholar]
- 27. Athanassiadi K, Kalavrouziotis G, Rondogianni D, Loutsidis A, Hatzimichalis A, Bellenis I. Primary chest wall tumors: early and long‐term results of surgical treatment. Eur J Cardiothorac Surg, 2001, 19: 589–593. [DOI] [PubMed] [Google Scholar]
- 28. Haraguchi S, Hioki M, Hisayoshi T, et al Resection of sternal tumors and reconstruction of the thorax: a review of 15 patients. Surg Today, 2006, 36: 225–229. [DOI] [PubMed] [Google Scholar]
- 29. Wu X, Chen M, Yu F. Application of titanium plate and Teflon patch in chest wall reconstruction after sternal tumor resection. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2011, 25: 1224–1226. (In Chinese). [PubMed] [Google Scholar]
- 30. Bullens PH, Schreuder BH, Malefijt MC, Veth RP, Buma P, The VN. stability of impacted morsellized bone grafts in a metal cage under dynamic loaded conditions: an in vitro reconstruction of a segmental diaphyseal bone defect. Arch Orthop Trauma Surg, 2009, 129: 575–581. [DOI] [PubMed] [Google Scholar]
- 31. Zhang JY, Zeng ZC, Dose SJ. Perturbation of metal objects in patients and a study for the physical causation. Zhongguo Yi Xue Wu Li Xue Za Zhi, 2005, 22: 505–510. (In Chinese). [Google Scholar]


