Abstract
Objective
To determine whether the prevalence of severe spinal osteoarthritis (OA) increases with the number of metabolic syndrome (MetS) risk factors.
Methods
Data from a single surgeon's high volume, spine surgery practice were reviewed. Severe OA was defined as degenerative spondylolisthesis or cervical or lumbar stenosis causing neurologically based symptoms and early OA as lumbar and cervical spondylosis causing axial pain only. Logistic regression modeling was used to determine the odds (adjusted for age and sex) of having severe spine OA with more numerous MetS risk factors.
Results
Severe spinal OA was identified in 839/1502 patients (55.9%) and early OA in the remaining 663 individuals (44.1%). The overall prevalence of MetS was 30/1502 (2.0%): 26/839 (3.1%) in the severe OA group and 4/663 (0.6%) in the early OA group (P = 0.001). Presence of all four MetS risk factors was associated with almost quadruple the odds of having severe OA as compared with absence of risk factors (OR 3.9 [1.4–11.6], P < 0.01).
Conclusion
The components of MetS are more prevalent in subjects with severe spinal OA than in those with spondylosis causing axial pain. Future study of the association between MetS and the incidence of OA is required.
Keywords: Metabolic syndrome, Obesity, Osteoarthritis, Spine
Introduction
Metabolic syndrome (MetS), a concurrence of disturbed glucose and insulin metabolism, overweight and abdominal fat distribution, mild dyslipidemia, and hypertension, is reportedly a risk factor for a number of chronic illnesses such as cardiovascular disease (CVD), including myocardial infarction and stroke1, and dementia2. Moreover, the individual risk factors that make up MetS (central obesity, diabetes, high blood pressure and dyslipidemia) have been shown to be independently associated with degenerative joint disease3, 4, 5, 6, 7, 8, 9. It has been postulated that MetS‐associated atheromatous vascular disease, small vessel occlusion, and venous stasis may predispose patients to subchondral bone ischemia, leading to poor nutrient and gas exchange in articular cartilage4, 10. Further ischemia leads to osteocyte apoptosis and increased stiffness of subchondral bone, thereby leading to the articular cartilage layer being vulnerable to damage from impact loads11, 12. Combined, these factors may predispose patients with MetS to developing osteoarthritis (OA).
Obesity is now regarded as a mild, chronic inflammatory state in which white adipose tissue (WAT) releases proinflammatory cytokines such as tumor necrosis factor‐α and interleukin‐6 into the systemic circulation13, 14, 15, 16, 17. Insulin resistance potentiates systemic inflammation through increased lipolysis of abdominal fat and high systemic concentrations of free fatty acids13. Systemic inflammation is further promoted by release from WAT of the peptide hormone leptin5, 8, 18, which is strongly believed be the metabolic link between obesity and joint degeneration19. This marked inflammatory state has been linked to chondrocyte apoptosis and cartilage matrix degeneration20, 21 and may represent an additional mechanism for the increased risk of OA in patients with MetS.
Although most investigations of associations between Mets and OA have focused on the appendicular skeleton, spinal degeneration (e.g. synovial facet joints) is in many ways similar, if not identical, to extremity joint OA, and thus is likely to be associated with, and influenced by, similar factors22. A number of investigators have examined the relationship between MetS risk factors and spine OA23, 24, 25, 26. However, the capacity of MetS to predict the incidence or prevalence of this disease has not been studied. In the present study, we asked whether the prevalence of spinal OA increases with the number of MetS risk factors. We hypothesized that the prevalence of severe spinal OA causing neurogenic symptoms would be greatest in individuals with all four MetS risk factors and that individuals with spondylosis causing axial pain would have fewer MetS risk factors.
Materials and Methods
Data from all patients who presented between 2002 and 2007 to a high volume, single surgeon, tertiary referral spine surgery practice were retrospectively reviewed. All patients were over the age of 18 years and had presented for consultation for degenerative spinal conditions. All participants gave informed consent to participate in this study, which was approved by the local Research Ethics Board.
For each patient, relevant clinical data of age, sex and body mass index (BMI) were reviewed. BMI was defined as body weight in kilograms (kg) divided by the square of height in meters. Medical comorbidities were collected by patient self‐report on a standardized pre‐consultation questionnaire, as well as through medication review. The final study cohort of 1502 patients comprised all individuals for whom complete clinical and comorbidity data were available out of a total of 1836 that were screened for eligibility (82%). The mean age of these patients was 55.3 years (SD = 15.5), with a mean BMI of 27.4 kg/m2 (SD = 5.2). Fifty percent of the patients were male.
Because the significance of insulin resistance is debated, there is no consensus on the definition of MetS. The World Health Organization defines MetS as insulin resistance (type 2 diabetes, impaired fasting glucose, or impaired glucose tolerance) plus any two of the following27: (i) blood pressure of at least 140/90 mm Hg; (ii) plasma triglyceride concentration of at least 150 mg/dL; (iii) high‐density lipoprotein (HDL) not exceeding 35 mg/dL (male) or 40 mg/dL (female); (iv) BMI of at least 30 kg/m2 and/or waist‐hip ratio ≥0.9 (male) or ≥0.85 (female); and (v) urinary albumin of at least 20 mg/min, albumin‐creatinine ratio of at least 30 mg/g. The National Cholesterol Education Program's Adult Treatment Panel III report (ATP III) defines MetS as three or more of the following risk factors28: (i) waist circumference: ≥102 cm (male) or ≥88 cm (female); (ii) plasma triglyceride concentration of at least 150 mg/dL; (iii) HDL cholesterol <40 mg/dL (male) or <50 mg/dL (female); (iv) blood pressure of at least 130/85 mm Hg; and (v) fasting blood glucose concentration of at least 100 mg/dL.
Waist circumference and laboratory values of cholesterol, fasting glucose and blood pressure were not routinely collected as part of our dataset. Consequently, MetS was defined in our patients as BMI ≥30 kg/m2 and patient self‐report of diabetes, hypertension and hypercholesterolemia.
Patients with spine OA clearly associated with neurological signs and symptoms of cervical/lumbar stenosis were classified as having severe OA. Symptoms of cervical stenosis included myelopathy and or radiculopathy (due to foraminal stenosis). Symptoms of lumbar stenosis included neurogenic claudication in the lower extremities with or without neurological deficits. Patients with degenerative spondylolisthesis were also considered to have severe spinal OA. Those with spondylosis or lumbar/cervical degenerative disc disease with axial pain only (i.e. no extremity or cord‐based stenotic symptoms) were classified as having mild OA. Patients with cervical or lumbar radiculopathy due to acute disc herniation, isthmic spondylolisthesis, primary coronal or sagittal deformity, or inflammatory, infectious, traumatic or tumor‐related spinal disorders were excluded. Patients with multifactorial chronic pain disorders were also excluded.
Statistical Analysis
Continuous data such as age and BMI were compared between groups using Student's t‐test following normality testing. Continuous variables are expressed as means and standard deviations. Categorical data such as sex and prevalence of MetS are expressed as frequencies and were compared using Fisher's exact test.
Logistic regression modeling was used to examine the relationship between the number of MetS risk factors and the prevalence of severe spinal OA. Patients were categorized according to how many MetS risk factors they had (i.e. none, one, two, three or four). The group with no risk factors was designated as the reference group. All odds ratios (OR) were adjusted for age and sex.
All statistical analyses were performed using the SPSS version 13.0 (Chicago, IL, USA) software package. Odds ratios for regression modeling and their 95% confidence intervals (CI) are reported. All reported P values are 2‐tailed with an α of 0.05.
Results
The overall prevalence of severe spinal OA in the study cohort was 839/1502 (55.9%). Patients with severe spinal OA were significantly older, included a greater percentage of females, and had higher BMIs than those with early spinal OA (P < 0.05, Table 1). The prevalence of severe spinal OA varied according to the number of MetS risk factors as follows: 353/748 (47.2%) in those with no MetS risk factors; 236/392 (60.2%) in those with one MetS risk factor; 148/228 (64.9%) in those with two MetS risk factors; 76/104 (73.1%) in those with three MetS risk factors; and 26/30 (86.7%) in those with all four MetS risk factors. The overall prevalence of MetS was 30/1502 (2.0%), comprising 26/839 (3.1%) in the severe OA group and 4/663 (0.6%) in the early OA group (P = 0.001).
Table 1.
Unadjusted analysis comparing relevant clinical data between patients with and without severe spinal OA
| Index | Early OA (663 cases) | Severe OA (839 cases) | P |
|---|---|---|---|
| Age (mean ± SD, yrs) | 49.8 ± 15.0 | 58.8 ± 14.8 | <0.05 |
| Male (%) | 53 | 48 | 0.03 |
| BMI (mean ± SD, kg/m2) | 26.7 (5.1) | 27.7 (5.2) | <0.05 |
| Prevalence of DM (cases [%]) | 59 (9) | 99 (12) | 0.03 |
| Prevalence of HTN (cases [%]) | 173 (26) | 335 (40) | <0.05 |
| Prevalence of HCL (cases [%]) | 93 (14) | 196 (23) | <0.05 |
| Prevalence of MetS (cases [%]) | 4 (0.6) | 26 (3.1) | <0.05 |
BMI, body mass index; DM, diabetes mellitus; HCL, hypercholesterolemia; HTN, hypertension; MetS, metabolic syndrome; OA, osteoarthritis; SD, standard deviation.
Logistic regression modeling showed that the odds of having severe spinal OA increased with increasing number of MetS risk factors, relative to patients with no risk factors (Fig. 1). Patients with MetS had almost quadruple the odds of having severe spinal OA when compared to those with no MetS risk factors, adjusted for age and sex (OR 3.9 [1.4–11.6], P < 0.01] (Fig. 1).
Figure 1.
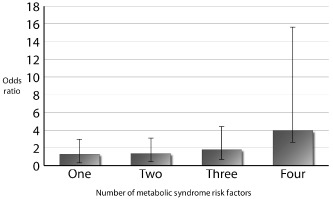
Odds ratios for the prevalence of severe spinal osteoarthritis by number of metabolic syndrome risk factors compared to controls.
Discussion
In the 1920's, Kylin described a clustering of metabolic risk factors including hypertension, hyperglycemia, and gout29. Twenty years later, it was noted that central obesity was commonly associated with the chronic diseases of diabetes and CVD27; these observations have contributed to our present understanding of MetS. All of the individual risk factors of obesity, hypertension, impaired fasting glucose and hypercholesterolemia have now been shown to have independent relationships with cartilage degeneration3, 4, 5, 6, 7, 8, 9, a hallmark of OA. The present study showed that the presence of a greater number of MetS risk factors is associated with increasing odds of severe spinal OA. Furthermore, the presence of all four risk factors significantly increases the odds of severe spinal OA over that of patients without any risk factors.
Our findings indicate that the impact of the MetS is greater than simply a cumulative effect of the four individual risk factors and confirms the World Health Organization state ment that these metabolic factors become more “powerful” in combination30, 31. Klein et al. examined the relationship between the number of MetS risk factors and the incidence of CVD32. Similar to our findings, they reported that increasing odds of disease were associated with a higher number of risk factors, four risk factors having double the odds of disease of three risk factors32. However, to the best of our knowledge, the present study is the first to evaluate the potential impact of MetS in patients with spine OA.
The pathogenesis of degenerative disc disease is believed to be mediated through atherosclerosis and insufficient blood supply33. A systematic review of this topic summarized findings from post‐mortem and clinical studies and concluded that stenoses of the middle sacral and fourth lumbar arteries are highly correlated with lumbar disc degeneration. However, clinical studies suggest a more multifactorial causation, several studies demonstrating associations between smoking34, 35, high blood pressure24, high cholesterol24, 25 and low back pain (LBP).
Obesity has been well established as a risk factor for OA of weight bearing joints such as hips and knees, mechanical overload being the causative link36, 37, 38. However, studies have also identified obesity as a predictor of OA in non‐weight bearing joints such as those of the hand, supporting the influence of a systemic metabolic effect whereby WAT secretes inflammatory mediators into the systemic circulation and these directly impact cartilage degeneration37, 39, 40. Assessment of the role of inflammatory mediators in inter‐vertebral disc degeneration has consistently shown that cytokines such as tumor necrosis factor‐α, interleukin‐6, and nitric oxide are present at higher concentrations in degenerative discs and likely play a role in disease progression41.
Published reports concerning the relationship between obesity and spine OA are inconsistent23, 26, 42, 43. Despite the frequent use of BMI as a measure of habitus, some of the methodologically strongest longitudinal studies have found that BMI is not a predictor of progression of disc degeneration23, 42. However, because visceral and truncal fat is believed to be the most metabolically active fat, other measures of habitus such as waist‐hip ratio or waist circumference may best reflect the biochemical association between habitus and OA. Studies have shown that waist circumference, independent of BMI, best predicts the risks of hypertension, dyslipidemia and myocardial infarction44, 45.
The relationship between metabolic factors and knee OA has been examined. Using a cross sectional study design, Hart et al. reported that hypertension, hypercholesterolemia and high blood glucose concentrations were associated with increased prevalence of knee OA in female subjects, independent of BMI46. In contrast, Martin et al. found that this relationship was no longer significant after adjusting for age and BMI47. These findings lend support to the postulate that OA has a systemic and metabolic causative component, rather than being dependent on BMI alone.
The overall prevalence of MetS in our study subjects was 2%. In contrast, using the ATP III definition, the estimated prevalence of MetS is 22% in the general population of the USA48, 25% in the general Canadian population49 and 10% in the general French population50. Several factors may explain the difference in the prevalence of MetS between our study and the general population. First, because we did not measure serum lipid or blood glucose concentrations, a number of patients with true MetS may have been allocated to the non‐MetS group. Second, it has been estimated that the prevalence in the general Canadian population of undiagnosed diabetes is as high as 5%51, of undiagnosed dyslipidemia 9%52, and of hypertension 12%53. Because we relied on patient self‐reporting of comorbidities, it is again possible that we failed to correctly identify a number of patients who did indeed have MetS. Third, because we relied on BMI as a measure of truncal obesity rather than waist circumference, a number of patients with true MetS may have been allocated to the non‐Mets group. Finally, we did not adjust for the effect of ethnicity in our regression model, although the prevalence of MetS is known to vary by ethnicity54. In particular, South Asian and East Asian individuals are believed to develop metabolic abnormalities at a lower BMI and waist circumference than other ethnic groups55, 56; thus, some of these patients may be incorrectly classified as not having MetS. However, all of these possibilities would have led to underdiagnosis, thus resulting in under‐estimation of the association between MetS and the odds of severe spine OA, suggesting that the true magnitude of association may be even greater than we identified. In addition, our study cohort was pre‐selected by presentation to a tertiary referral center with symptoms severe enough to warrant surgical assessment; some of them had been pre‐screened for the presence of pathology potentially amenable to surgical management.
Degenerative spine OA is a multifactorial process, with contributions from both systemic and local factors, and it is possible that the relative contributions of these factors vary based on severity of disease. Thus, our cohort may not be representative of the larger population of patients with back or neck pain and may therefore have a significantly different prevalence of MetS than the general population. This possibility is supported by Gandhi et al.'s report of a prevalence of MetS of 9.2% in their study of patients having elective knee replacements57. This prevalence is substantially lower than would be expected in the general population, suggesting that the phenomenon of underrepresentation of subjects with MetS is not isolated to patients with severe spinal OA. In addition, our patients' mean age is more than 10 years less than that of the patients with knee OA reported by Gandhi et al. The prevalence of MetS is known to increase with age, one study citing a prevalence that increased from 6.7% among those aged 20–29 years up to 42.0% for those aged 60 and older48. Thus, the relatively young age of our study cohort may partly explain the lower prevalence of MetS.
We wish to emphasize that our study does not establish a “cause and effect relationship” between metabolic risk factors and spine OA, but rather demonstrates a cross‐sectional association. Further assessment of population‐based prevalence (e.g. comparison of the incidence of MetS in individuals with and without spinal symptoms/degeneration) and longitudinal studies would help clarify whether these combined risk factors are predictors of disease. Given the enormous societal burden associated with symptomatic spinal degeneration, we strongly believe that this probable association warrants further investigation. This would be particularly prudent given the potentially modifiable nature of some of the risk factors associated with MetS.
In conclusion, the components of MetS are more prevalent in patients with severe spinal OA causing neurological symptoms than in those with spondylosis causing axial pain. Further longitudinal studies will help to clarify whether these metabolic factors play a causative role in the incidence of spinal OA. Lifestyle modification and aggressive primary care management of these risk factors may have an influence on the prevalence of spinal degeneration.
Disclosure: None of the authors have any potential conflicts of interest to disclose that could be perceived to influence the present work.
References
- 1. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab, 2004, 89: 2595–2600. [DOI] [PubMed] [Google Scholar]
- 2. Yaffe K, Kanaya A, Lindquist K, et al The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA, 2004, 292: 2237–2242. [DOI] [PubMed] [Google Scholar]
- 3. Aspden RM, Scheven BA, Hutchison JD. Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet, 2001, 357: 1118–1120. [DOI] [PubMed] [Google Scholar]
- 4. Conaghan PG, Vanharanta H, Dieppe PA. Is progressive osteoarthritis an atheromatous vascular disease? Ann Rheum Dis, 2005, 64: 1539–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumond H, Presle N, Terlain B, et al Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum, 2003, 48: 3118–3129. [DOI] [PubMed] [Google Scholar]
- 6. Rojas‐Rodríguez J, Escobar‐Linares LE, Garcia‐Carrasco M, Escárcega RO, Fuentes‐Alexandro S, Zamora‐Ustaran A. The relationship between the metabolic syndrome and energy‐utilization deficit in the pathogenesis of obesity‐induced osteoarthritis. Med Hypotheses, 2007, 69: 860–868. [DOI] [PubMed] [Google Scholar]
- 7. Singh G, Miller JD, Lee FH, Pettitt D, Russell MW. Prevalence of cardiovascular disease risk factors among US adults with self‐reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care, 2002, 8 (15 Suppl.): S383–S391. [PubMed] [Google Scholar]
- 8. Terlain B, Presle N, Pottie P, Mainard D, Netter P. Leptin: a link between obesity and osteoarthritis? Bull Acad Natl Med, 2006, 190: 1421–1435. [DOI] [PubMed] [Google Scholar]
- 9. Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem, 2007, 14: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 10. Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford), 2007, 46: 1763–1768. [DOI] [PubMed] [Google Scholar]
- 11. Dequeker J, Mohan S, Finkelman RD, Aerssens J, Baylink DJ. Generalized osteoarthritis associated with increased insulin‐like growth factor types I and II and transforming growth factor beta in cortical bone from the iliac crest. Possible mechanism of increased bone density and protection against osteoporosis. Arthritis Rheum, 1993, 36: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 12. Powell A, Teichtahl AJ, Wluka AE, Cicuttini FM. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br J Sports Med, 2005, 39: 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation, 2005, 111: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 14. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet, 2005, 365: 1415–1428. [DOI] [PubMed] [Google Scholar]
- 15. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin‐6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab, 2001, 280: E745–E751. [DOI] [PubMed] [Google Scholar]
- 16. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C‐reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA, 2001, 286: 327–334. [DOI] [PubMed] [Google Scholar]
- 17. Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin‐6 in relation to adiposity, insulin action, and insulin secretion. Obes Res, 2001, 9: 414–417. [DOI] [PubMed] [Google Scholar]
- 18. La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med (Berl), 2004, 82: 4–11. [DOI] [PubMed] [Google Scholar]
- 19. Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum, 2009, 60: 2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gualillo O. Further evidence for leptin involvement in cartilage homeostases. Osteoarthritis Cartilage, 2007, 15: 857–860. [DOI] [PubMed] [Google Scholar]
- 21. Simopoulou T, Malizos KN, Iliopoulos D, et al Differential expression of leptin and leptin's receptor isoform (Ob‐Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage, 2007, 15: 872–883. [DOI] [PubMed] [Google Scholar]
- 22. Milgram JW. Osteoarthritic changes at the severely degenerative disc in humans. Spine (Phila Pa 1976), 1982, 7: 498–505. [DOI] [PubMed] [Google Scholar]
- 23. Hassett G, Hart DJ, Manek NJ, Doyle DV, Spector TD. Risk factors for progression of lumbar spine disc degeneration: the Chingford Study. Arthritis Rheum, 2003, 48: 3112–3117. [DOI] [PubMed] [Google Scholar]
- 24. Leino‐Arjas P, Kaila‐Kangas L, Solovieva S, Riihimaki H, Kirjonen J, Reunanen A. Serum lipids and low back pain: an association? A follow‐up study of a working population sample. Spine (Phila Pa 1976), 2006, 31: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 25. Leino‐Arjas P, Solovieva S, Kirjonen J, Reunanen A, Riihimaki H. Cardiovascular risk factors and low‐back pain in a long‐term follow‐up of industrial employees. Scand J Work Environ Health, 2006, 32: 12–19. [DOI] [PubMed] [Google Scholar]
- 26. Symmons DP, van Hemert AM, Vandenbroucke JP, Valkenburg HA. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women. II. Radiographic findings. Ann Rheum Dis, 1991, 50: 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world‐wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med, 2006, 23: 469–480. [DOI] [PubMed] [Google Scholar]
- 28. Grundy SM, Brewer HB Jr, Cleeman JI, et al Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation, 2004, 109: 433–438. [DOI] [PubMed] [Google Scholar]
- 29. Kylin E. Studien uber das Hypertonie‐Hyperglyka “mie‐Hyperurika” miesyndrom. Zentralbl Inn Med, 1923, 44: 105–127 (in German). [Google Scholar]
- 30. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med, 1998, 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 31. Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol, 2006, 47: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 32. Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care, 2002, 25: 1790–1794. [DOI] [PubMed] [Google Scholar]
- 33. Kurunlahti M, Tervonen O, Vanharanta H, Ilkko E, Suramo I. Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine (Phila Pa 1976), 1999, 24: 2080–2084. [DOI] [PubMed] [Google Scholar]
- 34. Frymoyer JW. Back pain and sciatica. N Engl J Med, 1988, 318: 291–300. [DOI] [PubMed] [Google Scholar]
- 35. Goldberg MS, Scott SC, Mayo NE. A review of the association between cigarette smoking and the development of nonspecific back pain and related outcomes. Spine (Phila Pa 1976), 2000, 25: 995–1014. [DOI] [PubMed] [Google Scholar]
- 36. Bourne R, Mukhi S, Zhu N, Keresteci M, Marin M. Role of obesity on the risk for total hip or knee arthroplasty. Clin Orthop Relat Res, 2007, 465: 185–188. [DOI] [PubMed] [Google Scholar]
- 37. Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow‐up. BMC Musculoskelet Disord, 2008, 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sowers MF, Yosef M, Jamadar D, Jacobson J, Karvonen‐Gutierrez C, Jaffe M. BMI vs. body composition and radiographically defined osteoarthritis of the knee in women: a 4‐year follow‐up study. Osteoarthritis Cartilage, 2008, 16: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol, 1993, 20: 331–335. [PubMed] [Google Scholar]
- 40. Waldron HA. Association between osteoarthritis of the hand and knee in a population of skeletons from London. Ann Rheum Dis, 1997, 56: 116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol (Noisy‐le‐grand), 2007, 53: 4–18. [PubMed] [Google Scholar]
- 42. O'Neill TW, McCloskey EV, Kanis JA, et al The distribution, determinants, and clinical correlates of vertebral osteophytosis: a population based survey. J Rheumatol, 1999, 26: 842–848. [PubMed] [Google Scholar]
- 43. van Saase JL, Vandenbroucke JP, van Romunde LK, Valkenburg HA. Osteoarthritis and obesity in the general population. A relationship calling for an explanation. J Rheumatol, 1988, 15: 1152–1158. [PubMed] [Google Scholar]
- 44. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity‐related health risk. Am J Clin Nutr, 2004, 79: 379–384. [DOI] [PubMed] [Google Scholar]
- 45. Yusuf S, Hawken S, Ounpuu S, et al Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet, 2005, 366: 1640–1649. [DOI] [PubMed] [Google Scholar]
- 46. Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol, 1995, 22: 1118–1123. [PubMed] [Google Scholar]
- 47. Martin K, Lethbridge‐Cejku M, Muller DC, et al Metabolic correlates of obesity and radiographic features of knee osteoarthritis: data from the Baltimore Longitudinal Study of Aging. J Rheumatol, 1997, 24: 702–707. [PubMed] [Google Scholar]
- 48. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA, 2002, 287: 356–359. [DOI] [PubMed] [Google Scholar]
- 49. Anand SS, Yi Q, Gerstein H, et al Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation, 2003, 108: 420–425. [DOI] [PubMed] [Google Scholar]
- 50. Guize L, Thomas F, Pannier B, Bean K, Danchin N, Benetos A. Metabolic syndrome: prevalence, risk factors and mortality in a French population of 62 000 subjects. Bull Acad Natl Med, 2006, 190: 685–697; discussion 697–700. [PubMed] [Google Scholar]
- 51. Leiter LA, Barr A, Belanger A, et al Diabetes Screening in Canada (DIASCAN) Study: prevalence of undiagnosed diabetes and glucose intolerance in family physician offices. Diabetes Care, 2001, 24: 1038–1043. [DOI] [PubMed] [Google Scholar]
- 52. Petrella RJ, Merikle E, Jones J. Prevalence and treatment of dyslipidemia in Canadian primary care: a retrospective cohort analysis. Clin Ther, 2007, 29: 742–750. [DOI] [PubMed] [Google Scholar]
- 53. Petrella RJ, Merikle E. A retrospective analysis of the prevalence and treatment of hypertension and dyslipidemia in Southwestern Ontario, Canada. Clin Ther, 2008, 30: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 54. Meigs JB, Wilson PW, Nathan DM, D'Agostino RB Sr, Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes, 2003, 52: 2160–2167. [DOI] [PubMed] [Google Scholar]
- 55. Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr, 2007, 2: 267–275. [DOI] [PubMed] [Google Scholar]
- 56. Razak F, Anand SS, Shannon H, et al Defining obesity cut points in a multiethnic population. Circulation, 2007, 115: 2111–2118. [DOI] [PubMed] [Google Scholar]
- 57. Gandhi R, Razak F, Tso P, Davey JR, Mahomed NN. Asian ethnicity and the prevalence of metabolic syndrome in the osteoarthritic total knee arthroplasty population. J Arthroplasty, 2010, 25: 416–419. [DOI] [PubMed] [Google Scholar]


