Key Points
Question
How do caregiver-reported measures compare with performance-based measures in describing neuropsychological outcomes of children resuscitated after cardiac arrest who were initially comatose after return of circulation?
Findings
This secondary analysis of 2 clinical trials found that, of 160 survivors of pediatric cardiac arrest without significant developmental delay before cardiac arrest, 114 had favorable caregiver-rated outcomes 1 year later. However, significant performance-based neuropsychological deficits were evident across measures.
Meaning
These data provide clinicians with a greater understanding of neuropsychological outcomes in pediatric cardiac arrest survivors and of strong correlations in young children and moderate correlations in older children between caregiver-reported outcomes used in clinical trials and performance-based cognitive outcomes.
Abstract
Importance
Little is known about neuropsychological outcomes of children who survived pediatric cardiac arrest (CA).
Objective
To describe the neuropsychological outcomes of CA survivors enrolled in the Therapeutic Hypothermia After Pediatric Cardiac Arrest In-Hospital (THAPCA-IH) and Out-of-Hospital (THAPCA-OH) trials and compare the results with the primary outcome measure for these trials.
Design, Setting, and Participants
Secondary analysis of 222 CA survivors aged 1 to 18 years who received chest compressions for 2 minutes or more, remained comatose and required mechanical ventilation after return of circulation, and were enrolled in targeted temperature-management trials from 41 pediatric intensive care units. Data were collected from September 3, 2009, to February 3, 2016, and analyzed from March 10, 2017, to April 20, 2018.
Main Outcomes and Measures
The Vineland Adaptive Behavior Scales, Second Edition (VABS-II), a standardized measure of neurobehavioral functioning based on caregiver report (age-corrected mean [SD] scores = 100 [15]), was used to evaluate pre-CA functioning within 24 hours after enrollment; VABS-II<70 indicated significant developmental delays; VABS-II and neuropsychological testing were completed 1 year after CA. Neuropsychological testing included the Mullen Scales of Early Learning (Mullen) for children younger than 6 years and the Wechsler Abbreviated Scale of Intelligence (WASI) and neuropsychological measures of attention, memory, processing speed, and executive functioning for older children.
Results
Of 160 participants who completed neuropsychological testing, 96 (60.0%) were male; the median (interquartile range [IQR]) age was 2.5 years (1.3-6.1 years). Ninety-six (60.0%) were white, 41 (25.6%) were black, and 23 (14.4%) were of other/unknown race; 34 (21.2%) were Hispanic or Latino; 119 (74.4%) were non-Hispanic or Latino; and 7 (4.4%) were of unknown ethnicity. One hundred fourteen participants (71.2%) were classified as having favorable outcomes (VABS-II ≥70). Impairments (>2 SD below the mean for age) across neuropsychological measures ranged from 7% to 61%. Correlations between global cognitive and VABS-II scores were strong for younger children (Mullen, r = 0.69-0.87) but moderate for older children (r = 0.21-0.54 for the WASI). Of 111 children with favorable outcomes on VABS-II, 25.2% had global cognitive impairment and 30 of 35 older children (85.7%) had selective neuropsychological deficits.
Conclusions and Relevance
In this prospectively evaluated cohort of pediatric CA survivors who were initially comatose, although 71.2% were classified as having favorable outcomes, significant neuropsychological deficits were identified in pediatric CA survivors who were classified as having favorable outcomes. The findings provide clinicians with a greater understanding of the spectrum of neuropsychological outcomes of pediatric CA survivors and the complex relationship between standardized caregiver-reported functional outcome measures incorporated in clinical trials and performance-based neuropsychological assessments.
This secondary analysis of results from 2 randomized clinical trials compares caregiver-reported functional outcome measures with performance-based neuropsychological assessments at 1 year among pediatric survivors of cardiac arrest who were initially comatose after return of circulation.
Introduction
Little is known about the neuropsychological outcomes of children who survive pediatric cardiac arrest (CA). Two multicenter randomized clinical trials, the Therapeutic Hypothermia After Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) trial and the Therapeutic Hypothermia After Pediatric Cardiac Arrest In-Hospital (THAPCA-IH) trial, which compared the outcomes of 2 targeted temperature-management strategies (hypothermia [33°C] and normothermia [36.8°C]) for children who remained comatose after CA resuscitation, provide the largest prospective data set of long-term neurobehavioral and neuropsychological outcomes of survivors of pediatric CA. Children aged 48 hours to 18 years were eligible for trial enrollment if they required at least 2 minutes of cardiopulmonary resuscitation and were comatose and required mechanical ventilation after return of circulation. In both trials, hypothermia did not confer a significant benefit on 1-year survival with a favorable neurobehavioral outcome, defined as a score of 70 or greater (no more than 2 SD below the age-corrected mean [100]) on the Vineland Adaptive Behavior Scales, Second Edition (VABS-II), a standardized measure based on caregiver report of daily functioning in multiple domains.1,2
The VABS-II was selected as the primary outcome measure because it is appropriate for the entire age range of participants in the Therapeutic Hypothermia After Pediatric Cardiac Arrest In-Hospital and Out of Hospital (THAPCA-IH/OH) trials and for the spectrum of outcomes of survivors of CA (from vegetative state to full recovery). Using a measure based on caregiver report allowed for standardized assessment of pre-CA neurobehavioral functioning, which was essential to identify children with significant developmental delays prior to CA and to enable direct assessment of change from pre-CA function. Neuropsychological testing was incorporated in the trial protocols to provide complementary, objective, performance-based outcome data for 1-year survivors.
Secondary analyses of outcome data from both trials found substantial neurobehavioral morbidity in survivors 1 year after out-of-hospital CA3,4 and better overall outcomes after in-hospital CA.2 Among out-of-hospital CA survivors, 33% displayed severe to profound impairments, although one-half had 1-year VABS-II scores in the favorable outcomes range.3 One year after in-hospital CA, approximately three-fourths of survivors attained VABS-II scores within this range.5
The present study reports performance-based neuropsychological outcomes 1 year after CA among survivors classified as having favorable or unfavorable outcomes based on their VABS-II composite scores. For adult out-of-hospital CA survivors, memory is most impaired, and deficits in executive functioning, fine motor skills, and visuospatial skills are often present.6,7,8,9,10,11 Because these specific domains of neuropsychological functioning are not as well developed or readily testable in young children, we describe neuropsychological outcomes separately for younger and older children. The goal of the present study is to provide novel information about the spectrum of neuropsychological outcomes of children after CA by age group and more precisely delineate neuropsychological functioning of children in both age groups classified as having favorable outcomes.
Methods
Study Setting and Population
The THAPCA trials were conducted in 37 North American pediatric intensive care units (eAppendix in the Supplement). Children from 4 UK sites were also enrolled, but those sites did not offer neuropsychological testing. Data were collected from September 3, 2009, until February 3, 2016. Trials were approved by the institutional review boards at all sites and at data coordinating and outcome centers. Written informed consent was obtained from the child’s parent or legal guardian before enrollment. Data were analyzed between March 10, 2017, and April 20, 2018.
Children older than 48 hours and younger than 18 years who were resuscitated after out-of-hospital CA or in-hospital CA with chest compressions for 2 minutes or longer and were unresponsive and required mechanical ventilation after return of circulation met inclusion criteria. Major exclusion criteria included trauma, inability to randomize within 6 hours of return of circulation, a Glasgow Coma Scale motor score of 5 or 6 (ie, purposeful lateralizing response to painful stimulus), a clinical decision to withhold aggressive treatment, or non–English-speaking or Spanish-speaking parent or guardian. Inclusion and exclusion criteria are detailed in the primary outcome studies.1,2 Eligibility for inclusion in the primary outcome analyses included absence of significant development delays before CA (VABS-II score ≥70). Of 624 children randomized, there were 222 one-year survivors without significant developmental delays before CA (135 from the THAPCA-IH trial and 87 from the THAPCA-OH trial).
The present report analyzes the neuropsychological outcomes of 160 survivors whose caregivers completed 12-month VABS-II interviews and who subsequently underwent neuropsychological evaluations (eFigure in the Supplement). One hundred forty-eight children younger than 6 years at the 12-month follow-up were invited for an on-site evaluation; 27 families declined. Of 69 survivors who were at least 6 years of age at the 12-month follow-up, 50 with functional means of communication (based on the VABS-II) were deemed eligible for neuropsychological testing; 8 families declined. For 3 children (2 younger than 6 years, and 1 older) who underwent testing, reliable scores were not obtained. Demographic variables were similar between eligible patients with VABS-II and on-site testing data and those whose families declined either or both evaluations (eTables 1 and 2 in the Supplement).
Outcome Measures
Neurobehavioral Functioning
The VABS-II measures caregiver-reported functional skills and provides age-corrected standard scores (mean [SD], 100 [15]) in 4 domains (communication, daily living, socialization, and motor skills) and an overall adaptive behavior composite.12 Higher scores denote better functioning. Favorable outcomes were defined as scores no more than 2 SD below the age-corrected standard score mean (≥70) among 1-year survivors. The rationale for the selection of this outcome measure and cutoff has been reported elsewhere.13 Each domain includes subdomains with developmentally sequenced items, starting with skills typically observed in infancy. The VABS-II includes a parent or caregiver rating form and a survey interview that yield comparable scores.12 The survey interview can be administered by telephone from a single central location.14 Examples of typical developmental skills within various ages and score ranges have been previously described.5
The Mullen Scales of Early Learning (Mullen),15 a measure of cognitive functioning designed for young children, was administered to children younger than 6 years. The Mullen Scales of Early Learning has 4 scales: visual reception, fine motor, receptive language, and expressive language. Normative data are available through the age of 5 years and 8 months. Children were tested with the Mullen up through 5 years, 8 months, 30 days. Children who were at least 5 years and 9 months but younger than 6 years were tested after their sixth birthday. For children 6 years and older, a battery of tests (Table 1) measured a range of domains including intellectual functioning, processing speed, attention, learning and memory, executive functioning, and visuomotor functioning.
Table 1. Neuropsychological Test Battery for Children 6 Years or Older.
| Test, Domain, and Constructs Measures | Test Variables |
|---|---|
| WASI for intelligence | |
| Overall cognitive ability | Full-scale IQ (includes 2 subtests below) |
| Verbal reasoning | Vocabulary score |
| Visual reasoning | Matrix reasoning score |
| WISC-IV coding or WAIS-III digit symbol for processing speeda | |
| Graphomotor speed | No. of symbols copied in time limit |
| WISC-IV digit span or WAIS-III digit span for attentiona | |
| Attention span or verbal working memory | Total No. of digits recited correctly |
| CVLT-C or CVLT-II for learning and memorya | |
| Verbal learning | No. of words recalled in trials 1-5 |
| Verbal memory | No. of words retrieved for short-delay free recall, short-delay cued recall, long-delay free recall, long-delay cued recall, and recognition memory |
| ROCF recall and recognition for learning and memory | |
| Visual memory | Short-term and long-term recall, recognition memory |
| ROCF copy for executive functioning | |
| Visual organization | Total accuracy score |
| COWA for executive functioning | |
| Novel verbal generation | Total No. of words generated |
| Grooved Pegboard Test for visual-motor functioning | |
| Fine motor dexterity for visual-motor functioning | Time to place all the pegs in the board |
| VMI-5 | |
| Graphomotor accuracy | Total accuracy score |
Abbreviations: COWA, Controlled Oral Word Association; CVLT-C, California Verbal Learning Test–Children; CVLT-II, California Verbal Learning Test, Second Edition; ROCF, Rey Osterrieth Complex Figure Test; VMI-5, Beery Buktenica Developmental Test of Visual-Motor Integration, Fifth Edition; WAIS-III, Wechsler Adult Intelligence Scale, Third Edition; WASI, Wechsler Abbreviated Scale of Intelligence; WISC-IV, Wechsler Intelligence Scale for Children, Fourth Edition.
The WAIS-III and CVLT-II were used for participants 17 years or older at follow-up.
All measures have published norms that covered the participants’ age range. All age-corrected standardized scores (t scores, z scores, and scaled scores) were transformed to standard scores (mean [SD], 100 [15]), with higher scores representing better performance. The lowest scores across measures ranged from 55 to 40. To minimize the effects of outliers on measures that lacked floors, floors were set at 40 (4 SD < mean). Scores greater than 115 were classified as above average, 85 to 115 as average, 70 to 84 as below average, 50 to 69 as impaired, and less than 50 as severely impaired.
Other Measures
Family functioning before CA was measured using the General Functioning Scale of the Family Assessment Device,16 a 12-item self-reported measure scored on a scale of 0 to 4, with lower scores indicating better functioning. Scores of 2 or greater indicate abnormal functioning.
The Pediatric Cerebral Performance Category measures neurologic functioning. The Pediatric Overall Performance Category measures overall health (including neurologic functioning).17,18 These clinician-rated scales (scored from 1 to 6, with lower scores reflecting better function) provide no detailed measurements but are often used to report pediatric CA outcomes and facilitated comparisons with other studies.19
Data Collection Procedures
Within 24 hours of enrollment, a primary caregiver completed the VABS-II form to determine pre-CA functioning. Site research coordinators reviewed VABS-II responses, collected demographic and CA-related variables, and rated pre-CA functioning (with the Pediatric Cerebral Performance Category and the Pediatric Overall Performance Category). Race and ethnicity data were collected according to standard National Institutes of Health guidelines.
One year after CA, a trained research assistant at Kennedy Krieger Institute in Baltimore, Maryland, conducted a telephone interview to assess neurobehavioral function (including the VABS-II). Subsequently, children participated in on-site neuropsychological testing. Doctoral-level psychologists or neuropsychologists at each site participated in training (1-hour webinar) before conducting evaluations and either completed the testing directly or supervised psychometricians who completed the testing. Spanish-speaking children were tested by Spanish-speaking examiners; for Spanish-speaking children 6 years or older due to concerns with validity of language-based measures, only nonverbal measures (eg, visual reasoning/organization/memory, fine motor tasks) were administered. All neuropsychological data were deidentified and reviewed centrally by one of us (B.S.S.); discrepancies were adjudicated through discussion with site psychologists.
Statistical Analysis
Categorical characteristics were compared between survivors with favorable VABS-II 12-month outcomes and survivors with unfavorable VABS-II 12-month outcomes by using the Fisher exact test, and continuous characteristics were compared by using Wilcoxon rank sum tests or t tests. Spearman correlations quantified relationships between VABS-II and Mullen or Wechsler Abbreviated Scale of Intelligence (WASI) scores; magnitudes of correlation were compared by using the Fisher z transformation.20 Neuropsychological testing scores were summarized by using mean values for survivors with favorable VABS-II outcomes and by using median values for survivors overall owing to skewed distributions. For comparing score distributions to normative populations (mean and median = 100), the nonparametric Wilcoxon signed rank test was used, with corresponding upper 95% confidence limits calculated for the Hodges-Lehmann location parameter. Distributions of scores were compared between groups using t tests. Statistical significance was set at P < .05. Correlation comparisons were performed in R, version 3.3.3., using the package cocor.21 All other analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Cohort Characteristics
Of 160 survivors without significant developmental delays before CA who completed neuropsychological evaluations, 96 (60.0%) were male, and the median (interquartile range [IQR]) age was 2.5 years (1.3-6.1 years). Ninety-six (60.0%) were white, 41 (25.6%) were black, and 23 (14.4%) were of other/unknown race; 34 (21.2%) were Hispanic or Latino; 119 (74.4%) were non-Hispanic or Latino; and 7 (4.4%) were of unknown ethnicity. One hundred fourteen (71.2%) were classified as having favorable outcomes (VABS-II ≥70). Table 2 compares survivors with favorable outcomes with survivors with unfavorable outcomes. The favorable outcome group had less pre-CA impairment based on the Pediatric Overall Performance Category (ie, better overall health). Age, race/ethnicity (data not shown), and assigned temperature treatment group did not differ. Although survival was greater in the THAPCA-IH trial than in the THAPCA-OH trial,1,2 proportions of favorable vs unfavorable outcomes were similar. The favorable group had shorter post-CA hospital lengths of stay and less neurologic impairment (based on the Pediatric Cerebral Performance Category) at hospital discharge.
Table 2. Sample Characteristics by Outcome Classification.
| Characteristic | Children, No. (%) | P Value | ||
|---|---|---|---|---|
| Overall (N = 160) | Month 12 VABS-IIa | |||
| Favorable (n = 114) | Unfavorable (n = 46) | |||
| Age at assessment, median (IQR), y | ||||
| Overall | 2.5 (1.3-6.1) | 2.8 (1.3-8.6) | 2.0 (1.3-4.3) | .24b |
| By assessment group, y | ||||
| <6 (Mullen) | 1.6 (1.2-3.1) | 1.6 (1.2-2.8) | 1.8 (1.2-3.4) | .52b |
| ≥6 (WASI and neuropsychological battery) | 14.3 (10.3-16.4) |
13.9 (9.7-16.4) |
15.5 (12.7-17.8) |
.22b |
| Male | 96 (60) | 67 (59) | 29 (63) | .72c |
| Caregiver’s highest education received | ||||
| Some high school or less | 27 (17) | 21 (18) | 6 (13) | .10c |
| High school graduate or GED | 44 (28) | 24 (21) | 20 (43) | |
| Vocational school or some college | 39 (24) | 31 (27) | 8 (17) | |
| College degree | 31 (19) | 23 (20) | 8 (17) | |
| Graduate or doctoral degree | 19 (12) | 15 (13) | 4 (9) | |
| FAD score, mean (SD) | 1.5 (0.45) | 1.5 (0.41) | 1.4 (0.55) | .60d |
| Pre-CA PCPC | ||||
| Normal = 1 | 119 (74) | 89 (78) | 30 (65) | .10b |
| Mild disability = 2 | 26 (16) | 16 (14) | 10 (22) | |
| Moderate disability = 3 | 14 (9) | 8 (7) | 6 (13) | |
| Severe disability = 4 | 1 (1) | 1 (1) | 0 (0) | |
| Pre-CA POPC | ||||
| Good = 1 | 94 (59) | 73 (64) | 21 (46) | .03b |
| Mild disability = 2 | 45 (28) | 29 (25) | 16 (35) | |
| Moderate disability = 3 | 18 (11) | 10 (9) | 8 (17) | |
| Severe disability = 4 | 3 (2) | 2 (2) | 1 (2) | |
| Pre-CA VABS-II adaptive behavior composite score, mean (SD) | ||||
| Overall | 97.4 (16.03) | 98.9 (15.57) | 93.7 (16.74) | .08d |
| By assessment group, y | ||||
| <6 (Mullen) | 95.2 (14.21) | 96.4 (13.64) | 92.8 (15.16) | .21d |
| ≥6 (WASI and neuropsychological battery) | 103.9 (19.18) | 104.5 (18.19) | 100.2 (25.94) | .71d |
| Primary etiology of CA arrest | ||||
| Cardiac | 66 (41) | 52 (46) | 14 (30) | .16c |
| Respiratory | 69 (43) | 44 (39) | 25 (54) | |
| Other or unknown | 25 (16) | 18 (16) | 7 (15) | |
| Total No. of doses of epinephrine administered by EMS and at hospital, median (IQR) | 3.0 (1.0-4.0) | 2.0 (1.0-4.0) | 3.0 (2.0-7.0) | .09b |
| Estimated duration of chest compressions, min | ||||
| Unable to determine | 2 (1) | 1 (1) | 1 (2) | .07c |
| ≤15 | 68 (43) | 55 (48) | 13 (28) | |
| >15 to ≤30 | 40 (25) | 26 (23) | 14 (30) | |
| >30 | 50 (31) | 32 (28) | 18 (39) | |
| Treatment assigned | ||||
| Hypothermia | 92 (58) | 64 (56) | 28 (61) | .60c |
| Normothermia | 68 (43) | 50 (44) | 18 (39) | |
| Post-CA length of stay, median (IQR), d | 29.0 (16.0-60.0) |
25.0 (14.0-50.0) |
43.0 (27.0-78.0) |
<.001b |
| PCPC at hospital discharge | ||||
| Normal = 1 | 58 (36) | 53 (46) | 5 (11) | <.001c |
| Mild disability = 2 | 38 (24) | 30 (26) | 8 (17) | |
| Moderate disability = 3 | 29 (18) | 17 (15) | 12 (26) | |
| Severe disability = 4 | 24 (15) | 13 (11) | 11 (24) | |
| Coma or vegetative state = 5 | 10 (6) | 1 (1) | 9 (20) | |
| Missing | 1 (1) | 0 (0) | 1 (2) | |
| THAPCA trial study | ||||
| In-hospital | 99 (62) | 75 (66) | 24 (52) | .15c |
| Out-of-hospital | 61 (38) | 39 (34) | 22 (48) | |
Abbreviations: CA, cardiac arrest; EMS, emergency medical services; FAD, Family Assessment Device; GED, general equivalency diploma; IQR, interquartile range; Mullen, Mullen Scales of Early Learning; PCPC, Pediatric Cerebral Performance Category; POPC, Pediatric Overall Performance Category; THAPCA, Therapeutic Hypothermia After Pediatric Cardiac Arrest; VABS-II, Vineland Adaptive Behavior Scales, Second Edition; WASI, Wechsler Abbreviated Scale of Intelligence.
Favorable outcomes classified as VABS-II Composite score of 70 or higher; unfavorable outcomes classified as VABS-II Composite score of lower than 70.
Based on the Wilcoxon rank sum test.
Based on the Fisher exact test.
Based on the 2-sided t test with unpooled variance.
Neuropsychological Outcomes
Table 3 summarizes the neuropsychological outcomes. In addition to calculating median values, to illustrate the broad ranges of performance, we examined distributions of cases within 3 categorical groups (with scores of <70, 70-99, and ≥100). For children younger than 6 years (n = 119), the median (IQR) Mullen Early Learning Composite score was 67 (49-83). Composite and scale scores were significantly lower (P < .001) than the normative reference group with median scores ranging from 67 to 72 compared to the normative population scores of 100. Approximately half had scores in the impaired range (at least 2 SD below the mean for age). Yet 14 of the 117 children (12.0%) had Mullen Early Learning Composite scores at or the above age-corrected mean values.
Table 3. Neuropsychological Outcomes.
| Test Name or Test Name by Cognitive Domain | Overall Score | Score, No./Total No. (%) | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | Upper 95% CLa | P Valueb | <70 | 70-99 | ≥100 | |
| Children <6 y (n = 119) | ||||||
| Mullen Early Learning Composite | 67 (49-83) | 72 | <.001 | 65/117 (56) | 38/117 (32) | 14/117 (12) |
| Visual reception | 71 (55-91) | 80 | <.001 | 55/118 (47) | 41/118 (35) | 22/118 (19) |
| Fine motor | 72 (55-93) | 78 | <.001 | 59/118 (50) | 41/118 (35) | 18/118 (15) |
| Receptive language | 70 (55-90) | 77 | <.001 | 58/118 (49) | 46/118 (39) | 14/118 (12) |
| Expressive language | 67 (55-90) | 76 | <.001 | 61/118 (52) | 44/118 (37) | 13/118 (11) |
| Children ≥6 y (n = 41) | ||||||
| Intelligence | ||||||
| WASI full-scale IQ | 90 (79-103) | 96 | <.001 | 6/40 (15) | 23/40 (58) | 11/40 (28) |
| Vocabulary | 87 (73-102) | 93 | <.001 | 9/40 (23) | 16/40 (40) | 15/40 (38) |
| Matrix reasoning | 93 (85-105) | 99 | .01 | 5/41 (12) | 20/41 (49) | 16/41 (39) |
| Processing speed | ||||||
| WISC-IV coding/WAIS-III digit symbol | 85 (70-95) | 88 | <.001 | 9/41 (22) | 24/41 (59) | 8/41 (20) |
| Attention | ||||||
| WISC-IV coding/WAIS-III digit span total | 90 (80-100) | 92 | <.001 | 3/41 (7) | 25/41 (61) | 13/41 (32) |
| Learning and memory | ||||||
| CVLT-C (or II) list A total trials | 96 (75-111) | 101 | .06 | 9/40 (23) | 14/40 (35) | 17/40 (43) |
| CVLT-C (or II) short-delay free recall | 93 (74-100) | 93 | <.001 | 8/40 (20) | 13/40 (33) | 19/40 (48) |
| CVLT-C (or II) short-delay cued recall | 89 (70-108) | 96 | .002 | 8/40 (20) | 16/40 (40) | 16/40 (40) |
| CVLT-C (or II) long-delay free recall | 96 (78-108) | 96 | .002 | 7/40 (18) | 13/40 (33) | 20/40 (50) |
| CVLT-C (or II) long-delay cued recall | 93 (70-108) | 100 | .01 | 5/40 (13) | 18/40 (45) | 17/40 (43) |
| CVLT-C (or II) recognition | 100 (85-108) | 104 | .14 | 5/40 (13) | 10/40 (25) | 25/40 (63) |
| ROCF Immediate recall | 70 (55-84) | 79 | <.001 | 20/41 (49) | 16/41 (39) | 5/41 (12) |
| ROCF Delayed recall | 69 (55-84) | 77 | <.001 | 21/41 (51) | 17/41 (41) | 3/41 (7) |
| ROCF recognition | 91 (68-106) | 94 | .003 | 12/40 (30) | 17/40 (43) | 11/40 (28) |
| Executive functioning | ||||||
| ROCF Accuracy for copy | 62 (40-81) | 69 | <.001 | 25/41 (61) | 15/41 (37) | 1/41 (2) |
| COWA | 75 (57-91) | 82 | <.001 | 16/39 (41) | 19/39 (49) | 4/39 (10) |
| Visuomotor functioning | ||||||
| Grooved Pegboard Test, dominant hand | 85 (58-100) | 91 | <.001 | 12/38 (32) | 16/38 (42) | 10/38 (26) |
| Grooved Pegboard Test, nondominant hand | 77 (40-100) | 86 | <.001 | 16/37 (43) | 11/37 (30) | 10/37 (27) |
| VMI-5 | 77 (64-87) | 83 | <.001 | 16/41 (39) | 20/41 (49) | 5/41 (12) |
Abbreviations: CL, confidence limit; COWA, Controlled Oral Word Association; CVLT-C, California Verbal Learning Test–Children; CVLT-II, California Verbal Learning Test, Second Edition; IQR, interquartile range; ROCF, Rey Osterrieth Complex Figure Test; VMI-5, Beery-Buktenica Developmental Test of Visual-Motor Integration, Fifth Edition; WAIS-III, Wechsler Adult Intelligence Scale, Third Edition; WASI, Wechsler Abbreviated Scale of Intelligence; WISC-IV, Wechsler Intelligence Scale for Children, Fourth Edition.
Upper 95% CL for Hodges-Lehmann Location Parameter for the Overall Score.
Overall scores compared with normative reference distribution using a Wilcoxon signed rank test.
For children 6 years and older (n = 41), the median WASI full-scale IQ was 90 (IQR, 79-103). Scores on this measure and most neuropsychological tests were significantly lower (at least P < .05) than the normative reference groups for each measure, with median scores for those significantly different from the population median ranging from 62 to 96. Children deemed to lack the functional means of communication based on preceding VABS-II assessments did not participate in neuropsychological testing, thereby excluding 19 survivors with severe impairment. Among those tested, the percentage of impairment ranged from 7% to 61%, with impairment most frequent on tasks of executive functioning and visual memory. The percentage of survivors with scores at or greater than 100 ranged from 2% (the Rey Osterrieth Complex Figure Test [ROCF] for accuracy) to 63% (the California Verbal Learning Test–Children for recognition).
Relationships Among Outcome Measures
Because different global cognitive measures were appropriate for younger (Mullen) and older (WASI) children, we compared relationships between VABS-II scores and these cognitive test scores separately. The VABS-II overall and domain scores were strongly correlated with Early Learning Composites and each Mullen scale; correlations ranged from 0.69 to 0.87 (P < .001; eTable 3A in the Supplement). The correlation was strongest between VABS-II and Mullen composite scores. Correlations between VABS-II overall and domain scores and WASI composite and subtest scores ranged from 0.21 to 0.54 (eTable 3B in the Supplement) and were highest between VABS-II communication and WASI vocabulary. The VABS-II socialization scores were not significantly correlated with the WASI. The relationship between the VABS-II and Mullen composite scores was significantly stronger than the relationship between the VABS-II composite and the full-scale IQ (0.87 vs 0.45, P < .001).
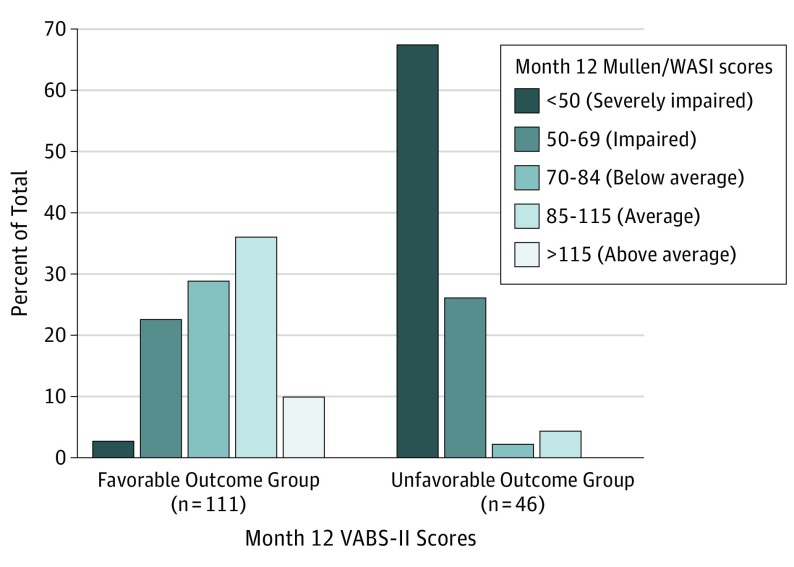
The Figure compares distributions of cognitive composite scores (Mullen Early Learning Composite or 2-subtest WASI IQ composite) for the favorable and unfavorable outcome groups. Fifty-one of 111 (45.9%) in the favorable group had average cognitive functioning; 2 of 46 (4.3%) of the children in the unfavorable group had scores in this range. Yet, within the favorable outcome group, 28 of 111 (25.2%) had scores less than 70. Of 46 children in the unfavorable group, 31 of 46 (67.4%) had scores less than 50 (all younger than 6 years because the lowest possible WASI score is 55).
Figure. Distributions of Cognitive Composite Scores (Early Learning Composite From Mullen or 2-Subtest WASI IQ Composite) for Favorable and Unfavorable Outcome Groups.
Mullen indicates Mullen Scales of Early Learning; VABS-II, Vineland Adaptive Behavior Scales, Second Edition; and WASI indicates Wechsler Abbreviated Scale of Intelligence.
Neuropsychological Outcomes in Favorable Outcome Groups
To more precisely delineate neuropsychological profiles in the subset of survivors who were classified as having favorable outcomes, we compared subgroups with VABS-II scores between 70 to less than 85 (favorable I: >1-2 SDs below the population mean [n = 34]) with those with scores of 85 or greater (favorable II: ≥1 SD below the population mean [n = 80]) (Table 4).
Table 4. Comparison of Neuropsychological Test Scores for Children With Outcomes Classified as Favorable I vs Favorable IIa.
| Test Name or Test Name by Cognitive Domain | Classification Based on Month 12 VABS-II Adaptive Behavior Composite Score | Difference (95% CI) | P Value, Favorable I vs IIb | Favorable II, Upper 95% CLc | P Value, Favorable II vs Normative Reference Distributiond | |
|---|---|---|---|---|---|---|
| Favorable I | Favorable II | |||||
| Children <6 y (Favorable I, n = 24; Favorable II, n = 55) | ||||||
| Mullen Scales of Early Learning composite and scaled scores, mean (range) | ||||||
| Early learning composite | 65 (49 to 95) | 88 (53 to 131) | 23.6 (15.8 to 31.5) | <.001 | 95 | <.001 |
| Visual reception | 72 (55 to 111) | 93 (55 to 138) | 21.1 (11.7 to 30.5) | <.001 | 99 | .02 |
| Fine motor | 70 (55 to 99) | 93 (55 to 145) | 23.0 (14.0 to 31.9) | <.001 | 99 | .01 |
| Receptive language | 70 (55 to 100) | 89 (55 to 129) | 18.8 (11.4 to 26.3) | <.001 | 94 | <.001 |
| Expressive language | 70 (55 to 97) | 87 (55 to 129) | 16.9 (9.2 to 24.6) | <.001 | 93 | <.001 |
| Children ≥6 y (Favorable I, n = 10; Favorable II, n = 25) | ||||||
| Neuropsychological testing scores, mean (range) | ||||||
| Intelligence | ||||||
| WASI full scale IQ | 87 (59 to 110) | 95 (68 to 123) | 8.3 (−5.1 to 21.7) | .20 | 101 | .10 |
| Vocabulary | 86 (55 to 118) | 92 (55 to 120) | 5.7 (−9.7 to 21.2) | .44 | 100 | .04 |
| Matrix reasoning | 87 (55 to 117) | 97 (73 to 120) | 10.3 (−3.9 to 24.5) | .14 | 103 | .34 |
| Processing speed | ||||||
| WISC-IV coding/WAIS-III digit symbol | 79 (55 to 110) | 88 (55 to 120) | 9.0 (−5.4 to 23.4) | .20 | 95 | .001 |
| Attention | ||||||
| WISC-IV/WAIS-III digit span total | 82 (55 to 110) | 93 (75 to 125) | 11.2 (−4.5 to 26.9) | .15 | 100 | .04 |
| Learning and memory | ||||||
| CVLT-C (or II) list A total trials | 85 (66 to 117) | 98 (55 to 123) | 13.1 (−2.8 to 29.0) | .10 | 108 | .98 |
| CVLT-C (or II) short-delay free recall | 81 (48 to 115) | 95 (40 to 115) | 13.4 (−3.3 to 30.2) | .11 | 104 | .27 |
| CVLT-C (or II) short-delay cued recall | 81 (55 to 108) | 95 (55 to 130) | 14.2 (−2.9 to 31.3) | .10 | 107 | .20 |
| CVLT-C (or II) long-delay free recall | 81 (40 to 115) | 95 (40 to 130) | 14.1 (−3.8 to 32.0) | .12 | 107 | .33 |
| CVLT-C (or II) long-delay cued recall | 84 (55 to 115) | 96 (55 to 123) | 12.4 (−3.8 to 28.7) | .12 | 104 | .44 |
| CVLT-C (or II) recognition | 81 (40 to 108) | 101 (78 to 115) | 20.4 (2.9 to 38.0) | .03 | 108 | .85 |
| ROCF Immediate recall | 65 (55 to 91) | 81 (55 to 126) | 15.9 (4.7 to 27.0) | .007 | 89 | <.001 |
| ROCF Delayed recall | 63 (55 to 84) | 79 (55 to 127) | 15.5 (5.3 to 25.6) | .004 | 86 | <.001 |
| ROCF recognition | 77 (55 to 109) | 92 (55 to 130) | 14.8 (−0.7 to 30.3) | .06 | 102 | .11 |
| Executive functioning | ||||||
| ROCF Accuracy for copy | 55 (40 to 99) | 70 (40 to 105) | 15.5 (−1.2 to 32.2) | .07 | 81 | <.001 |
| COWA | 72 (40 to 95) | 80 (42 to 118) | 8.8 (−6.7 to 24.3) | .25 | 90 | <.001 |
| Processing speed | ||||||
| Grooved Pegboard Test (dominant hand) | 71 (40 to 106) | 87 (40 to 118) | 16.2 (−8.4 to 40.8) | .17 | 99 | .03 |
| Grooved Pegboard Test (nondominant hand) | 70 (40 to 97) | 82 (40 to 115) | 12.0 (−11.7 to 35.7) | .29 | 98 | .03 |
| VMI-5 | 70 (48 to 92) | 83 (62 to 121) | 13.4 (1.8 to 25.0) | .03 | 89 | <.001 |
Abbreviations: CL, confidence limit; COWA, Controlled Oral Word Association; CVLT-C, California Verbal Learning Test–Children; CVLT-II, California Verbal Learning Test, Second Edition; ROCF, Rey Osterrieth Complex Figure Test; VABS-II, Vineland Adaptive Behavior Scales, Second Edition; VMI-5, Beery-Buktenica Developmental Test of Visual-Motor Integration, Fifth Edition; WAIS-III, Wechsler Adult Intelligence Scale, Third Edition; WASI, Wechsler Abbreviated Scale of Intelligence; WISC-IV, Wechsler Intelligence Scale for Children, Fourth Edition.
Favorable outcomes classified as VABS-II Composite score of 70 to less than 85 for the favorable I outcome group and of 85 or higher for the favorable II outcome group.
Based on the t test.
Upper 95% CL for Hodges-Lehmann location parameter.
Based on the Wilcoxon signed rank test.
For children younger than 6 years (Table 4) in the favorable I group, the mean Mullen scores ranged from 65 to 72 (SD, 12-18) (approximately 2 SDs below average). In contrast, for children in the favorable II group, the mean Mullen scores ranged from 87 to 93 (SD, 18-23) (<1 SD below average); however, all scores were significantly lower than in the normative reference group. Within both groups, the mean scores were similar across the 4 scales (favorable I, 70-72 [SD, 14-18]; favorable II, 87-93 [SD, 18-23]).
For children 6 years or older in the favorable group 30 of 35 older children (85.7%) had impairment (score of <70) on at least 1 test variable. There were few differences between the 2 favorable subgroups based on neuropsychological measures; visual memory recall and verbal memory recognition were significantly higher for the favorable II group (Table 4). Within both subgroups, the lowest scores were on measures of executive functioning (ROCF Accuracy for copy, favorable I = 55, favorable II = 70) and visual memory (ROCF Immediate recall, favorable I = 65, favorable II = 81; ROCF Delayed recall, favorable I = 63, favorable II = 79).
Discussion
To our knowledge, this is the first prospective study of the long-term neuropsychological outcomes of children who survived pediatric CA who remained comatose after return of circulation and survived for at least 1 year. We used data collected rigorously as part of 2 clinical trials to define distinct, well-characterized study populations. The THAPCA trials required standardized methods to evaluate pre-CA neurobehavioral function and long-term outcomes. In both trials, the rates of favorable neurobehavioral outcomes were similar in both temperature-management arms.1,2 Neuropsychological testing provided complementary outcomes; in both trials, composite cognitive scores did not differ between treatment arms.1,2,3 This study provides novel information about the spectrum of neuropsychological outcomes of children after CA and about neuropsychological functioning of children classified as having favorable outcomes in the THAPCA trials.
We found high rates of impairment across neuropsychological measures. Our results are consistent with other pediatric studies that also found impairments in a range of neuropsychological domains after CA; unlike the THAPCA trials, the other studies did not exclude children who regained consciousness quickly after return of circulation.22,23,24,25 In our cohort of children at very high risk for neurologic morbidity, although deficits in many areas of neuropsychological functioning were common, a substantial minority of children obtained neuropsychological test scores that were at or above the age-corrected mean values.
The results highlight global cognitive impairments in younger children and domain-specific impairments in older children. Although we identified age-related differences in outcomes, these trends in part reflect challenges inherent in measuring and comparing cognitive functioning in heterogeneous pediatric populations and must be interpreted with caution. For example, although the frequency of impairment based on global cognitive composite scores differed by age (65 of 117 [55.6%] had global cognitive impairment based on the Mullen Scales of Early Learning and 6 of 40 [15.0%] had global cognitive impairment based on the WASI 2-subtest full-scale IQ), the much smaller sample size in the older group and the exclusion of survivors with the most severe impairment from testing in this group likely explain this difference.
We sought to evaluate whether specific domains of neuropsychological functioning were selectively affected. However, in young children, the specific domains of neuropsychological functioning that may be most sensitive to hypoxic-ischemic brain injury are not as well developed (or readily testable in the first year after CA). In older children, we identified selective impairments. We found the lowest median scores and the highest rates of impairment based on measures of executive functioning, fine motor skill, visuomotor skills, and visual memory, whereas IQ was generally spared. Similarly, for adults after out-of-hospital CA, memory is the domain most significantly affected, although executive functioning, fine motor, and visuospatial skills are also adversely affected.6,7,8,9,10,11 We found the greatest impairment based on measures of visual memory, with relatively less impairment based on verbal memory measures; this pattern (greater visual relative to verbal memory impairment) has previously been reported for survivors of pediatric CA.22 In this population, individualized neuropsychological assessment can guide appropriate cognitive rehabilitation and educational services, especially for older children.
We found substantial age-related differences in the relationship between cognitive performance and caregiver-rated function. Similar to previous studies,26,27 we found moderate correlations among caregiver-reported neurobehavioral outcomes and cognitive performance in older children; in contrast, we found strong correlations between these measures in the younger group. In both age groups, performance-based neuropsychological testing provided complementary insights about deficits in children with less obvious functional impairment. In fact, younger children with VABS-II scores no lower than 1 SD below the mean (favorable II group) had significantly lower mean scores relative to the normative reference group on all 4 Mullen scales. In the corresponding older group, deficits were more selective and reflected domains most vulnerable to hypoxic-ischemic injury.
We anticipated a broad range of neurobehavioral outcomes in the THAPCA-OH and THAPCA-IH trial survivors and that the VABS-II would provide a robust measure to encompass and distinguish functional performance. An invaluable feature of caregiver-reported measures, such as the VABS-II, is the ability to assess pre-CA function retrospectively at the time of enrollment so that preexisting deficits can be distinguished from deficits attributable to hypoxic-ischemic brain injury. Although the VABS-II met these goals, in future clinical trials in similar populations, neuropsychological evaluations may provide sensitive complementary measures of intervention efficacy.
Limitations
Our results should be considered in the context of several limitations. Although 71.2% of survivors were classified as having favorable outcomes, a minority of patients from either trial survived to 1 year post-CA (96 of 287 [33.4%] in the THAPCA-OH trial and 155 of 327 [47.4%] in the THAPCA-IH trial), and concern for poor neurologic outcome with redirection of goals of care and brain death declaration were frequent causes of death in both trials.1,2 Because older children with severe hypoxic-ischemic brain injury were excluded from participation, our results underestimate impairment in this age group. Finally, the trial protocols did not enable us to collect data about variables that might predict or influence neuropsychological outcome, such as neuroimaging abnormalities, seizure burden, coma duration, medications after hospital discharge, and rehabilitation services.
Nonetheless, these data provide clinicians with a better understanding of the range of neuropsychological outcomes in this population and highlight the complementary insights that neuropsychological assessments can provide about children who have incurred hypoxic-ischemic brain injury, even when overall functional recovery appears favorable. Furthermore, in younger survivors of pediatric CA, the full extent of deficits may only become apparent with maturation, and neuropsychological functioning may need to be reassessed subsequently to ensure that cognitive deficits are identified and managed within the context of increasing developmental expectations.
Conclusions
We identified significant neuropsychological deficits in this cohort of 160 children who experienced in-hospital or out-of-hospital CA, although 114 (71.2%) were classified as having favorable outcomes. In the subset of children with favorable outcomes, 28 of 111 (25.2%) have global cognitive impairment, with selective neuropsychological deficits evident in older children. Our data provide clinicians with a greater understanding of the spectrum of neuropsychological outcomes of survivors of pediatric CA and of the complex relationship between standardized functional outcome measures used in clinical trials and performance-based cognitive outcomes.
eAppendix. Personnel, Sites, Funding and Acknowlegements
eTable 1. Demographic Variables Between Eligible Cases With VABS-II Interview and Onsite Testing Data and Those Whose Families Declined Either or Both Evaluations -<6 Years
eTable 2. Demographic Variables Between Eligible Cases With VABS-II Interview and Onsite Testing Data and Those Whose Families Declined Either or Both Evaluations ->6 Years
eTable 3. Spearman Correlations Among Vineland Adaptive Behavior Scales – Second Edition (VABS-II) and Overall Cognitive Outcome Measures
eFigure. Description of Eligible Survivors Who Were Included and Excluded
References
- 1.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators . Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372(20):1898-1908. doi: 10.1056/NEJMoa1411480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators . Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017;376(4):318-329. doi: 10.1056/NEJMoa1610493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slomine BS, Silverstein FS, Christensen JR, et al. ; THAPCA Trial Group . Neurobehavioral outcomes in children after out-of-hospital cardiac arrest. Pediatrics. 2016;137(4):e20153412. Published online March 3, 2016. doi: 10.1542/peds.2015-3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverstein FS, Slomine BS, Christensen J, et al. ; Therapeutic Hypothermia to Improve Survival After Cardiac Arrest Trial Group . Functional outcome trajectories after out-of-hospital pediatric cardiac arrest. Crit Care Med. 2016;44(12):e1165-e1174. doi: 10.1097/CCM.0000000000002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slomine BS, Silverstein FS, Christensen JR, et al. ; Therapeutic Hypothermia after Paediatric Cardiac Arrest (THAPCA) Trial Investigators . Neurobehavioural outcomes in children after in-hospital cardiac arrest. Resuscitation. 2018;124:80-89. doi: 10.1016/j.resuscitation.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander MP, Lafleche G, Schnyer D, Lim C, Verfaellie M. Cognitive and functional outcome after out of hospital cardiac arrest. J Int Neuropsychol Soc. 2011;17(2):364-368. doi: 10.1017/S1355617710001633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CR, Botha JA, Tiruvoipati R. Cognitive function, quality of life and mental health in survivors of out-of-hospital cardiac arrest: a review. Anaesth Intensive Care. 2015;43(5):568-576. [DOI] [PubMed] [Google Scholar]
- 8.Lim C, Verfaellie M, Schnyer D, Lafleche G, Alexander MP. Recovery, long-term cognitive outcome and quality of life following out-of-hospital cardiac arrest. J Rehabil Med. 2014;46(7):691-697. doi: 10.2340/16501977-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80(3):297-305. doi: 10.1016/j.resuscitation.2008.10.034 [DOI] [PubMed] [Google Scholar]
- 10.Ørbo M, Aslaksen PM, Larsby K, et al. Determinants of cognitive outcome in survivors of out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1462-1468. doi: 10.1016/j.resuscitation.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Torgersen J, Strand K, Bjelland TW, et al. Cognitive dysfunction and health-related quality of life after a cardiac arrest and therapeutic hypothermia. Acta Anaesthesiol Scand. 2010;54(6):721-728. doi: 10.1111/j.1399-6576.2010.02219.x [DOI] [PubMed] [Google Scholar]
- 12.Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales: Survey Forms Manual. 2nd ed Minneapolis, MN: NCS Pearson; 2005. [Google Scholar]
- 13.Holubkov R, Clark AE, Moler FW, et al. Efficacy outcome selection in the Therapeutic Hypothermia After Pediatric Cardiac Arrest trials. Pediatr Crit Care Med. 2015;16(1):1-10. doi: 10.1097/PCC.0000000000000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limperopoulos C, Majnemer A, Steinbach CL, Shevell MI. Equivalence reliability of the Vineland Adaptive Behavior Scale between in-person and telephone administration. Phys Occup Ther Pediatr. 2006;26(1-2):115-127. doi: 10.1080/J006v26n01_08 [DOI] [PubMed] [Google Scholar]
- 15.Mullen EM. Mullen Scales of Early Learning. Circle Pine, MN: American Guidance Service; 1995. [Google Scholar]
- 16.Epstein NB, Baldwin LM, Bishop DS. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9(2):171-180. doi: 10.1111/j.1752-0606.1983.tb01497.x [DOI] [Google Scholar]
- 17.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68-74. doi: 10.1016/S0022-3476(05)82544-2 [DOI] [PubMed] [Google Scholar]
- 18.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of Pediatric Overall Performance Category and Pediatric Cerebral Performance Category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28(7):2616-2620. doi: 10.1097/00003246-200007000-00072 [DOI] [PubMed] [Google Scholar]
- 19.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the Pediatric Utstein Style: a statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Resuscitation. 1995;30(2):95-115. doi: 10.1016/0300-9572(95)00884-V [DOI] [PubMed] [Google Scholar]
- 20.Fisher RA. Statistical Methods for Research Workers. Edinburgh, Scotland: Oliver and Boyd; 1925. [Google Scholar]
- 21.Diedenhofen B, Musch J. cocor: a comprehensive solution for the statistical comparison of correlations [published correction in PLoS One. 2015;10(6):e0131499]. PLoS One. 2015;10(3):e0121945. doi: 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015;41(6):1057-1066. doi: 10.1007/s00134-015-3789-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloom AA, Wright JA, Morris RD, Campbell RM, Krawiecki NS. Additive impact of in-hospital cardiac arrest on the functioning of children with heart disease. Pediatrics. 1997;99(3):390-398. doi: 10.1542/peds.99.3.390 [DOI] [PubMed] [Google Scholar]
- 24.Morris RD, Krawiecki NS, Wright JA, Walter LW. Neuropsychological, academic, and adaptive functioning in children who survive in-hospital cardiac arrest and resuscitation. J Learn Disabil. 1993;26(1):46-51. doi: 10.1177/002221949302600105 [DOI] [PubMed] [Google Scholar]
- 25.Maryniak A, Bielawska A, Walczak F, et al. Long-term cognitive outcome in teenage survivors of arrhythmic cardiac arrest. Resuscitation. 2008;77(1):46-50. doi: 10.1016/j.resuscitation.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 26.Silver CH. Ecological validity of neuropsychological assessment in childhood traumatic brain injury. J Head Trauma Rehabil. 2000;15(4):973-988. doi: 10.1097/00001199-200008000-00002 [DOI] [PubMed] [Google Scholar]
- 27.Perry A, Flanagan HE, Dunn Geier J, Freeman NL. Brief report: the Vineland Adaptive Behavior Scales in young children with autism spectrum disorders at different cognitive levels. J Autism Dev Disord. 2009;39(7):1066-1078. doi: 10.1007/s10803-009-0704-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Personnel, Sites, Funding and Acknowlegements
eTable 1. Demographic Variables Between Eligible Cases With VABS-II Interview and Onsite Testing Data and Those Whose Families Declined Either or Both Evaluations -<6 Years
eTable 2. Demographic Variables Between Eligible Cases With VABS-II Interview and Onsite Testing Data and Those Whose Families Declined Either or Both Evaluations ->6 Years
eTable 3. Spearman Correlations Among Vineland Adaptive Behavior Scales – Second Edition (VABS-II) and Overall Cognitive Outcome Measures
eFigure. Description of Eligible Survivors Who Were Included and Excluded