Key Points
Question
Does a gadolinium-enhanced magnetic resonance imaging pattern help in distinguishing a spinal dural arteriovenous fistula from other causes of myelopathy?
Findings
This medical record review included 51 patients with a spinal dural arteriovenous fistula and pretreatment magnetic resonance imaging with gadolinium. Of these, 44 (86%) had intraparenchymal contrast enhancement, and 19 of these patients (43%) displayed the missing-piece sign defined by at least 1 focal geographic nonenhancing area within a long segment of intense holocord gadolinium enhancement.
Meaning
Identifying this unique radiographic finding could potentially result in earlier time to angiography with improved outcomes for patients with spinal dural arteriovenous fistulas.
Abstract
Importance
Spinal dural arteriovenous fistula (sDAVF) is often misdiagnosed as an inflammatory or a neoplastic myelopathy, often because of intraparenchymal gadolinium enhancement on magnetic resonance imaging (MRI); proper early diagnosis is important because deficits are reversible and a delay in treatment is associated with permanent morbidity. Tortuous flow voids on MRI are not universally present; thus, recognition of a unique gadolinium enhancement pattern may also aid in the early recognition and treatment of sDAVF.
Objective
To describe a unique pattern of spinal cord gadolinium enhancement on MRI in sDAVF.
Design, Setting, and Participants
This retrospective evaluation included pretreatment MRIs from 80 patients referred to the Mayo Clinic, Rochester, Minnesota, from January 1, 1997, through December 31, 2017, with a confirmed diagnosis of sDAVF and a control group of 144 patients with alternative confirmed myelopathy diagnoses. All participants underwent a neurologic evaluation at the Mayo Clinic.
Main Outcomes and Measures
Evidence of at least 1 focal geographic nonenhancing area within a long segment of intense holocord gadolinium enhancement (termed the missing-piece sign) on MRI.
Results
Of 51 patients with an sDAVF and a pretreatment MRI with gadolinium enhancement, 44 (86%) had intraparenchymal contrast enhancement, and 19 of these patients (43%) displayed the characteristic missing-piece sign. Of these 19 patients, symptom onset occurred at a median age of 67 years (range, 27-80 years); 15 patients were men. Progressive myelopathy features affecting the lower extremities occurred during a median of 33 months (range, 1-84 months). Eleven patients (58%) received an alternative diagnosis before confirmation of sDAVF. Tortuous flow voids were present on T2-weighted MRI in 13 of 19 patients. More than 1 digital subtraction angiogram was required for 5 patients to confirm the diagnosis. The missing-piece sign was not seen in any patients from the control group.
Conclusions and Relevance
This unique gadolinium enhancement pattern in sDAVF was not found in a large control group of patients with other myelopathy. Identifying the missing-piece sign on MRI could potentially result in earlier time to angiography with improved outcomes for patients with an sDAVF.
This medical record review described a unique pattern of spinal cord gadolinium enhancement on magnetic resonance imaging in patients with spinal dural arteriovenous fistulas.
Introduction
Spinal dural arteriovenous fistulas (sDAVFs) are a rare cause of myelopathy with heterogeneous clinical and radiographic features1,2,3; they are often mistaken for an inflammatory or a neoplastic myelopathy.2,4 Recognizing an sDAVF early is important because clinical deficits are reversible and a delay in diagnosis is associated with severe permanent morbidity.2 Although abnormal vascular flow voids are a critical radiographic feature that distinguishes sDAVF from other myelopathies,3 they are not universally present. Thus, we should be cognizant of other clinical and radiographic features in sDAVF that may facilitate its timely diagnosis. Although spinal cord gadolinium enhancement typically raises concern for transverse myelitis4 or a neoplasm,5 it also occurs in a number of noninflammatory myelopathies,4,6 including sDAVF.7 In sDAVF, the gadolinium enhancement pattern on magnetic resonance imaging (MRI) is typically described as nonspecific and hazy.8 Recently we have noticed an unusual MRI pattern in sDAVF in which abrupt pieces or segments are seemingly missing amidst an intense area of contrast enhancement, a finding we term the missing-piece sign. We sought to assess the frequency of this finding in a pretreatment group of patients with sDAVF and in a large control group of patients with myelopathies of alternative specific causes.
Methods
We retrospectively evaluated 80 patients referred to the Mayo Clinic, Rochester, Minnesota, from January 1, 1997, through December 31, 2017, with a confirmed diagnosis of sDAVF. All pretreatment MRIs were assessed for the presence of gadolinium enhancement and the missing-piece sign, which was defined as at least 1 focal geographic nonenhancing area within a long (>2 vertebral segments) segment of intense holocord (whole cross-sectional area of spinal cord) gadolinium enhancement; findings were verified by a neurologist (N.L.Z.) and a neuroradiologist (W.B.). Neuroimaging was performed with 1.5-T and 3-T MRI machines. Images from diagnostic centers outside the Mayo Clinic were reviewed and compared with Mayo Clinic MRIs. Typical sequences for evaluation of myelopathy include sagittal T1- and T2-weighted, short-tau inversion recovery and axial T2-weighted sequences. Gadolinium was administered at the discretion of the ordering and performing services. The institutional review board of the Mayo Clinic approved this study, and patients provided written consent for the use of their records for medical research.
The control group of 144 patients with MRIs and a confirmed alternative myelopathy diagnosis that included a gadolinium-enhancing spinal cord lesion were also assessed for this particular radiographic feature. Diagnoses included spinal cord sarcoidosis in 21 patients, spondylotic compressive myelopathy treated surgically with stability or resolution in 20, intramedullary spinal cord lymphoma in 18, multiple sclerosis in 17, neuromyelitis optica with aquaporin 4–IgG seropositivity in 17, ependymoma in 17, myelitis with myelin oligodendrocyte glycoprotein–IgG seropositivity in 13, spinal cord metastases in 12, and astrocytoma in 9.
Results
Of our group of 80 patients with a confirmed sDAVF, 51 had a pretreatment MRI available to review in which gadolinium was administered. Forty-four of 51 patients (86%) had intraparenchymal contrast enhancement, and 19 of these patients (43%) displayed the characteristic missing-piece sign (Figure 1). Of these 19 patients, symptom onset occurred at a median age of 67 years (range, 27-80 years). Fifteen patients were men and 4 were women. Progressive myelopathy features affecting the lower extremities occurred during a median of 33 months (range, 1-84 months), with symptoms including sensory loss (n = 18), weakness (n = 17), urinary tract problems (n = 13), back and/or leg pain (n = 11), and exertional worsening of deficits (n = 8). Eleven patients (58%) received an alternative diagnosis before confirmation of sDAVF, including probable spinal cord tumor in 5 (1 with a spinal cord biopsy), lumbar spondylosis with radiculopathy in 2, peripheral neuropathy in 2, seronegative neuromyelitis optica in 1, and motor neuron disease in 1.
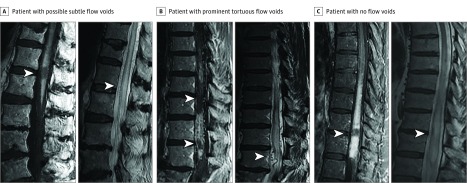
Figure 1. Sagittal Magnetic Resonance Imaging (MRI) of the Missing-Piece Sign in Patients With Spinal Dural Arteriovenous Fistulas (sDAVFs).
A T1-weighted gadolinium enhancement pattern with abrupt “pieces” of contrast enhancement missing (left panels of A-C; arrowheads) is shown in 3 patients with an sDAVF. Accompanying sagittal T2-weighted imaging (right panels of A-C) shows prominent tortuous flow voids in 1 patient (B, right panel), possible subtle flow voids in 1 patient (A, right panel), and no flow voids (C, right panel) in a patient with recognition of sDAVF only at the time of durotomy (arrowheads).
Among patients with the missing-piece sign, prominent perimedullary surface veins seen as tortuous flow voids were not universally present (Figure 1C, right panel) but were demonstrated on T2-weighted imaging in 13 of 19 patients (Figure 1B, right panel) and on T1-weighted imaging with gadolinium administration in 8 of 19 patients. Thirteen of 15 patients had identification of an sDAVF on spinal magnetic resonance angiography. A digital subtraction angiogram confirmed sDAVF on the initial angiogram in 14 of 19 patients, on the second angiogram in 3 of 19, and on the third angiogram in 1 of 19. The remaining patient had 2 angiograms with normal findings, and sDAVF was identified at the time of durotomy for a planned spinal cord biopsy. Gadolinium enhancement in those patients with sDAVF without the missing-piece sign was generally nonspecific and hazy with some central canal prominence. All patients with identification of an sDAVF were treated with endovascular or surgical occlusion of the fistula; the subsequent course with expected clinical and radiographic response ruled out alternative causes of myelopathy. Six of 8 patients with posttreatment MRIs available to review from our institution (median, 10 months; range, 3-29 months) showed similar or less residual T2-hyperintense signal and preserved spinal cord volume at the site of missing gadolinium enhancement compared with the adjacent spinal cord; correlation was indeterminate in the other 2 patients. The missing-piece sign was not seen in any patients from the control group, but other typical gadolinium enhancement patterns were seen that were helpful clues to an alternative myelopathy diagnosis (Figure 2).
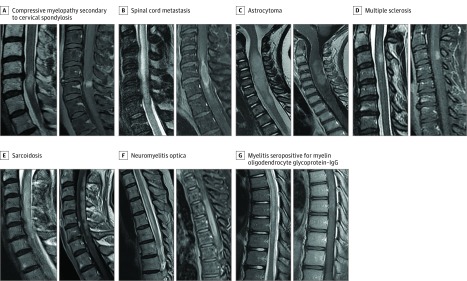
Figure 2. Gadolinium Enhancement Patterns in a Control Group With Alternative Causes of Myelopathy.
Control group of patients with alternative contrast-enhancing spinal cord lesions, with T2-weighted imaging on the left and accompanying T1-weighted imaging with gadolinium administration on the right. A, Compressive myelopathy secondary to cervical spondylosis, with a pancakelike band of enhancement just below the maximal area of stenosis. B, Spinal cord metastasis from squamous cell lung cancer with rim and flame enhancement. C, Astrocytoma demonstrating T2-weighted hyperintense mass with nonspecific accompanying enhancement. D, Multiple sclerosis with well-defined homogenous ovoid enhancing lesion in the dorsal spinal cord. E, Sarcoidosis displaying long dorsal subpial enhancement. F, Neuromyelitis optica with aquaporin 4–IgG seropositivity with a long contrast-enhancing spinal cord lesion and a component of central sparing, forming a ringlike enhancement. G, Myelitis with myelin oligodendrocyte glycoprotein–IgG seropositivity with a long T2-hyperintense lesion and faint patchy gadolinium enhancement.
Discussion
Spinal cord gadolinium enhancement was common (86%) in our pretreatment group of patients with sDAVF; furthermore, many patients demonstrated a unique pattern of enhancement termed the missing-piece sign that was not seen in a large control group with alternative myelopathies, suggesting specificity for sDAVF. As in other causes of myelopathy, spinal cord contrast enhancement occurs owing to breakdown of the blood-cord barrier, allowing leakage of gadolinium into the parenchyma, which in sDAVF is secondary to progressive venous hypertension. We suspect the peculiar imaging pattern of the missing-piece sign may be owing to inconsistency of the intrinsic venous system of the spinal cord,3 with the abrupt segments without enhancement likely having better venous egress routes than the adjacent cord with contrast enhancement.
Recognition of particular gadolinium enhancement patterns of the spinal cord that may suggest a specific diagnosis is important,4 especially in the context of sDAVF, where a misdiagnosis as an inflammatory or neoplastic myelopathy is common, as shown in this study and others,2 and can result in unnecessary harm, with corticosteroid therapy known to worsen sDAVF2 and spinal cord biopsy with its inherent risk. As displayed in Figure 2, helpful gadolinium enhancement patterns have been described in compressive myelopathy (a pancakelike transverse band [width of enhancement greater than the height, enhancement just below maximal stenosis]),6 multiple sclerosis (homogeneous or ring),9 aquaporin 4 IgG–seropositive neuromyelitis optica (ringlike or patchy),10 sarcoidosis (dorsal subpial and 3-pronged axial enhancement [trident sign]),11,12 and spinal cord metastases (rim [more intense thin rim of enhancement] and flame [flame shaped at the superior and inferior aspect of lesion]).5 Identification of particular imaging findings can help physicians pursue the correct subsequent diagnostic or therapeutic steps in a timely manner (eg, obtaining computed tomography of the chest for sarcoidosis vs urgent surgical decompression for compressive myelopathy). Although some myelopathy origins often have particularly helpful contrast enhancement patterns, others are nonspecific and can be highly variable (eg, lymphoma, astrocytoma, myelin oligodendrocyte glycoprotein IgG–seropositive myelitis). In the case of sDAVF, however, recognition of the contrast-enhancing pattern termed the missing-piece sign could result in an earlier time to angiography with improved patient outcomes. Our study is limited by its retrospective design, relatively small numbers, and the variability in imaging protocols and timing.
Conclusions
We have described a unique gadolinium enhancement pattern in sDAVF that we have not seen in a large control group or in other patients with myelopathy at our institution. Identifying the missing-piece sign on MRI may serve as an important piece of the puzzle in solving the diagnosis of sDAVF.
References
- 1.Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129(pt 12):3150-3164. doi: 10.1093/brain/awl220 [DOI] [PubMed] [Google Scholar]
- 2.Brinjikji W, Nasr DM, Morris JM, Rabinstein AA, Lanzino G. Clinical outcomes of patients with delayed diagnosis of spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2016;37(2):380-386. doi: 10.3174/ajnr.A4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JM. Imaging of dural arteriovenous fistula. Radiol Clin North Am. 2012;50(4):823-839. doi: 10.1016/j.rcl.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 4.Zalewski NL, Flanagan EP, Keegan BM. Evaluation of idiopathic transverse myelitis revealing specific myelopathy diagnoses. Neurology. 2018;90(2):e96-e102. doi: 10.1212/WNL.0000000000004796 [DOI] [PubMed] [Google Scholar]
- 5.Rykken JB, Diehn FE, Hunt CH, et al. . Rim and flame signs: postgadolinium MRI findings specific for non-CNS intramedullary spinal cord metastases. AJNR Am J Neuroradiol. 2013;34(4):908-915. doi: 10.3174/ajnr.A3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flanagan EP, Krecke KN, Marsh RW, Giannini C, Keegan BM, Weinshenker BG. Specific pattern of gadolinium enhancement in spondylotic myelopathy. Ann Neurol. 2014;76(1):54-65. doi: 10.1002/ana.24184 [DOI] [PubMed] [Google Scholar]
- 7.Terwey B, Becker H, Thron AK, Vahldiek G. Gadolinium-DTPA enhanced MR imaging of spinal dural arteriovenous fistulas. J Comput Assist Tomogr. 1989;13(1):30-37. doi: 10.1097/00004728-198901000-00006 [DOI] [PubMed] [Google Scholar]
- 8.Luetmer PH, Lane JI, Gilbertson JR, Bernstein MA, Huston J III, Atkinson JL. Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol. 2005;26(4):711-718. [PMC free article] [PubMed] [Google Scholar]
- 9.Klawiter EC, Benzinger T, Roy A, Naismith RT, Parks BJ, Cross AH. Spinal cord ring enhancement in multiple sclerosis. Arch Neurol. 2010;67(11):1395-1398. doi: 10.1001/archneurol.2010.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalewski NL, Morris PP, Weinshenker BG, et al. . Ring-enhancing spinal cord lesions in neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2017;88(3):218-225. doi: 10.1136/jnnp-2016-314738 [DOI] [PubMed] [Google Scholar]
- 11.Flanagan EP, Kaufmann TJ, Krecke KN, et al. . Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann Neurol. 2016;79(3):437-447. doi: 10.1002/ana.24582 [DOI] [PubMed] [Google Scholar]
- 12.Zalewski NL, Krecke KN, Weinshenker BG, et al. . Central canal enhancement and the trident sign in spinal cord sarcoidosis. Neurology. 2016;87(7):743-744. doi: 10.1212/WNL.0000000000002992 [DOI] [PubMed] [Google Scholar]