Key Points
Question
Does the administration of dextroamphetamine combined with physiotherapy improve poststroke motor recovery?
Findings
In this pilot randomized clinical trial of 64 patients with ischemic stroke, no overall treatment-associated difference in the change in Fugl-Meyer motor scores from baseline to 3 months after treatment, no overall treatment-associated differences in any of the study’s secondary measures, and no differences in any subgroup based on stroke location or baseline severity were found. No adverse events were attributed to study treatments.
Meaning
This study found no evidence that dextroamphetamine combined with physiotherapy improves poststroke motor recovery.
This randomized clinical trial evaluates the effect of dextroamphetamine combined with physiotherapy vs placebo and physiotherapy for improving poststroke motor recovery among patients with ischemic stroke.
Abstract
Importance
Data from animal models show that the administration of dextroamphetamine combined with task-relevant training facilitates recovery after focal brain injury. Results of clinical trials in patients with stroke have been inconsistent.
Objectives
To collect data important for future studies evaluating the effect of dextroamphetamine combined with physiotherapy for improving poststroke motor recovery and to test the efficacy of the approach.
Design, Setting, Participants
This pilot, double-blind, block-randomized clinical trial included patients with cortical or subcortical ischemic stroke and moderate or severe motor deficits from 5 rehabilitation hospitals or units. Participants were screened and enrolled from March 2001 through March 2003. The primary outcome was assessed 3 months after stroke. Study analysis was completed December 31, 2015. A total of 1665 potential participants were screened and 64 were randomized. Participants had to begin treatment 10 to 30 days after ischemic stroke. Data analysis was based on intention to treat.
Interventions
Participants were allocated to a regimen of 10 mg of dextroamphetamine (n = 32) or placebo (n = 32) combined with a 1-hour physical therapy session beginning 1 hour after drug or placebo administration every 4 days for 6 sessions in addition to standard rehabilitation.
Main Outcomes and Measures
The primary outcome was the difference between groups in change in Fugl-Meyer motor scores from baseline to 3 months after stroke (intention to treat with dextroamphetamine). Secondary exploratory measures included the National Institutes of Health Stroke Scale, Canadian Neurological Scale, Action Research Arm Test, modified Rankin Scale score, Functional Independence Measure, Ambulation Speed and Distance, Mini-Mental State Examination, Beck Depression Inventory, and Stroke Impact Scale.
Results
Among the 64 patients randomized to dextroamphetamine vs placebo (55% men; median age, 66 years; age range, 27-91 years), no overall treatment-associated difference in the mean (SEM) change in Fugl-Meyer motor scores from baseline to 3 months after stroke was noted (−18.65 [2.27] points with dextroamphetamine vs −20.83 [2.94] points with placebo; P = .58). No overall treatment-associated differences in any of the study’s secondary measures and no differences in subgroups based on stroke location or baseline severity were found. No adverse events were attributed to study treatments.
Conclusions and Relevance
Treatment with dextroamphetamine combined with physical therapy did not improve recovery of motor function compared with placebo combined with physical therapy as assessed 3 months after hemispheric ischemic stroke. The studied treatment regimen was safe.
Trial Registration
ClinicalTrials.gov identifier: NCT01905371
Introduction
Stroke is the third leading cause of disability worldwide.1 More than 60% of Medicare beneficiaries in the United States receive care in inpatient rehabilitation or skilled nursing facilities after stroke.2 One study of 4-year survivors after stroke3 found 42% had ongoing disabilities, 28% could not fully participate in activities, and 78% reported that they had not completely recovered. Even after treatment with mechanical thrombectomy, 49% to 88% of participants have some disability after 90 days.4 These data highlight the need for additional approaches intended to enhance poststroke recovery.
Laboratory experiments performed during several decades in a variety of animal models show that systemic administration of dextroamphetamine days or weeks after stroke or other forms of injury to the cerebral cortex, when combined with task-relevant training, can facilitate postinjury functional recovery.5,6 Factors affecting efficacy include the frequency, type, and intensity of therapy sessions. Therefore, the results of variably designed clinical trials evaluating the approach, not surprisingly, have been inconsistent.7 Some studies suggest benefit,8,9 whereas others had negative findings.10,11,12,13,14 The Amphetamine-Enhanced Stroke Recovery Trial was an exploratory, phase 2, double-blind, placebo-controlled, multicenter randomized clinical trial intended to refine the target patient population, gain information to permit an accurate sample size calculation, determine appropriate outcome measures, develop management procedures for a subsequent study, and collect preliminary efficacy data.
Methods
Participants
A copy of the trial protocol is found in Supplement 1. Participants were screened and enrolled at 5 rehabilitation hospitals or units (Burke Rehabilitation Hospital, White Plains, New York; Helen Hayes Hospital, West Haverstraw, New York; Wake Forest University, Winston-Salem, North Carolina; Duke University, Durham, North Carolina; and Washington University in St Louis, St Louis, Missouri) from March 2001 through March 2003. The study protocol was approved by each participating center’s institutional review board, and all patients or their representatives provided written informed consent.
The Amphetamine-Enhanced Stroke Recovery Trial enrolled potential participants if they (1) had a documented (including neuroimaging) ischemic hemispheric stroke; (2) could start treatment from 10 to 30 days after stroke; (3) were independent before the index stroke (modified Rankin Scale score, 0 or 1 [range, 0-6, with 6 indicating death]); (4) had a moderate or severe stroke-associated motor impairment; (5) were capable of giving informed consent (or had a legal representative to do so); (6) would be available for the required follow-up evaluations; and (7) were physically able to receive the study drug or placebo. Potential participants were excluded if they had (1) uncontrolled hypertension, defined as systolic blood pressure of at least 160 mm Hg or diastolic blood pressure of at least 100 mm Hg at rest, determined by 3 readings during the 24 hours before randomization; (2) an index or a remote intracerebral or subarachnoid hemorrhage; (3) a history of active psychosis or bipolar disorder; (4) angina pectoris within the preceding 3 months; (5) a myocardial infarction within the preceding year; (6) inducible myocardial ischemia based on results of an exercise or pharmacologic stress test if performed within the prior year; (7) clinically significant congestive heart failure, defined as New York Heart Association classification III or IV; (8) atrial or ventricular arrhythmias, including atrial fibrillation, atrial flutter, ventricular tachycardia, ventricular fibrillation, and Wolff-Parkinson-White syndrome by history or electrocardiographic or Holter monitor findings; (9) a history of seizures or seizures associated with the index ischemic stroke; (10) an allergy to amphetamine; (11) current treatment with levodopa, another dopamine agonist, or a monoamine oxidase inhibitor; (12) glaucoma; (13) a need for treatment with a drug or a class thought to impair recovery based on laboratory and available clinical evidence (α1-adrenergic receptor antagonist, α2-adrenergic receptor agonist, benzodiazepine, dopamine receptor antagonist, phenobarbital, or phenytoin); (14) hyperthyroidism; (15) current pregnancy; (16) an expected rehabilitation stay of less than 3 weeks; (17) a mild stroke-associated motor impairment (adjusted Fugl-Meyer motor score ≥80 [range, 0-100, with higher scores indicating better motor performance]); (18) participation in another investigational protocol; or (19) any condition that in the view of the investigator would put the patient at risk through participation in the study.
Screening and Randomization
All potential participants were screened for study eligibility at the time of rehabilitation admission based on a standard admission evaluation, including a comprehensive medical, neurologic, and psychiatric history, review of medical records, results of physical and neurologic examinations, review of brain imaging reports, electrocardiographic findings, and pregnancy test results in premenopausal women. Eligible participants were invited to participate in the study. After consent, baseline testing was performed and participants were block randomized within each center based on stroke severity at the beginning of rehabilitation (Fugl-Meyer motor score, 0-35 for severe stroke and 36-79 for moderate stroke)15 and stroke subtype (subcortical vs cortical hemispheric ischemic stroke using the Oxfordshire criteria and neuroimaging data),16,17 resulting in 4 blocks with 1:1 randomization to dextroamphetamine or placebo within each block (severe cortical, severe subcortical, moderate cortical, and moderate subcortical stroke).
Study Drug and Placebo
Active and placebo capsules were prepared by the hospital pharmacy on-site at each institution. The pharmacies were given a randomization scheme generated by the study statistician (G.P.S.) at the coordinating center. Each patient’s study medications were prepared as a unit-dose kit according to this predetermined randomization schedule. The on-site pharmacies were not to reveal the randomization assignment to other personnel except in the extraordinary circumstance that the information would be required for a patient’s emergency treatment.
Two 5-mg dextroamphetamine tablets obtained commercially by each hospital pharmacy were split and placed in a single opaque blue gelatin capsule (Gallipot, Inc). The 5-mg tablets were cut with a pill splitter to allow them to fit into 1 size 0 capsule (any fragmented pills were discarded). To prevent movement within the capsules, lactose monohydrate powder (AMEND, Inc) was used as a filler. Identical placebo capsules were filled with 0.65 g of powdered lactose monohydrate (the amount of lactose does not represent a significant load and did not preclude those with lactose intolerance from participating in the study). The gelatin capsules dissolve in the stomach within 1 to 3 minutes and do not interfere with the absorption of the dextroamphetamine tablets.
Treatment Regimen
Based on the results of a small trial suggesting benefit,9 enrolled participants were randomized to double-blind treatment with a regimen of 10 mg of dextroamphetamine or placebo combined with a 1-hour session of active physical therapy directed at a primary motor impairment beginning 1 hour after drug or placebo administration and delivered every 4 days for a total of 6 sessions. A target motor impairment for physical therapy intervention was designated (usually gait, but in some cases arm function). An outline indicating a range and level of physical therapy interventions was provided to the therapists, and the level and target of therapy were recorded. Throughout the rehabilitation hospitalization (and including the 3-week study treatment phase), all participants also received standard, comprehensive rehabilitation services.
Protocol-Specified Criteria for Withdrawal
The protocol specified that participants would be withdrawn from the study if they experienced any of the following adverse events: (1) moderate or severely elevated blood pressure (systolic blood pressure of ≥180 mm Hg or diastolic blood pressure of ≥110 mm Hg); (2) angina pectoris; (3) myocardial infarction; (4) stroke or transient ischemic attack; (5) New York Heart Association classification III or IV congestive heart failure or a cardiac arrhythmia (as listed in exclusion criteria 8); (6) psychosis; (7) hallucinations; (8) agitation requiring treatment; (9) need for treatment with a drug thought to impair recovery (listed in exclusion criteria 13); or (10) any other condition that the investigator believed might reasonably be associated with dextroamphetamine treatment and present a risk to the patient.
Outcome Assessments
The primary, prespecified outcome was the change in Fugl-Meyer motor score18 (exclusive of sensation and reflexes) assessed 3 months after stroke. Secondary measures included the National Institutes of Health Stroke Scale,19 Canadian Neurological Scale,20 Action Research Arm Test,21 modified Rankin Scale score,22 Functional Independence Measure,23 Ambulation Speed and Distance (6-minute walk test),24,25 Mini-Mental State Examination,26 Beck Depression Inventroy,27 and Stroke Impact Scale.28 Assessments were also performed at the end of treatment.
Statistical Analysis
Although the study was not primarily intended to determine the efficacy of the intervention, we hypothesized that the addition of treatment with dextroamphetamine to targeted physical therapy would result in at least a 12.6-point difference in improvement in the Fugl-Meyer motor score at 3 months after stroke, which was anticipated to be a clinically important effect. To detect a 12.6-point difference in Fugl-Meyer motor scores between the groups with a 2-sided α = .05 and a power of 80%, a sample size of 25 participants per group for each regimen was calculated as adequate. Based on preliminary data, we anticipated that approximately 24% of participants meeting exclusion criteria would be withdrawn because of complications normally occurring during poststroke rehabilitation hospitalizations (eg, angina, myocardial infarction, new cardiac arrhythmia, stroke, seizure, psychosis, agitation, or transfer to an acute care hospital). Therefore, a total of 65 participants were to be enrolled to allow for a 30% dropout rate.
The analysis of the primary end point was based on the intention to treat with dextroamphetamine for participants who received at least 1 dose of study drug. A 2-sample paired t test was first used to compare the intervention and placebo groups with respect to change in Fugl-Meyer motor scores. This analysis was repeated for each of the secondary outcomes (the Wilcoxon rank sum test was used for group comparisons for ordinal data). The frequencies of serious adverse events were compared with χ2 analyses. Because of the exploratory nature of the analyses, P values were not adjusted for multiple comparisons. P < .05 indicated significance.
Results
Study Population
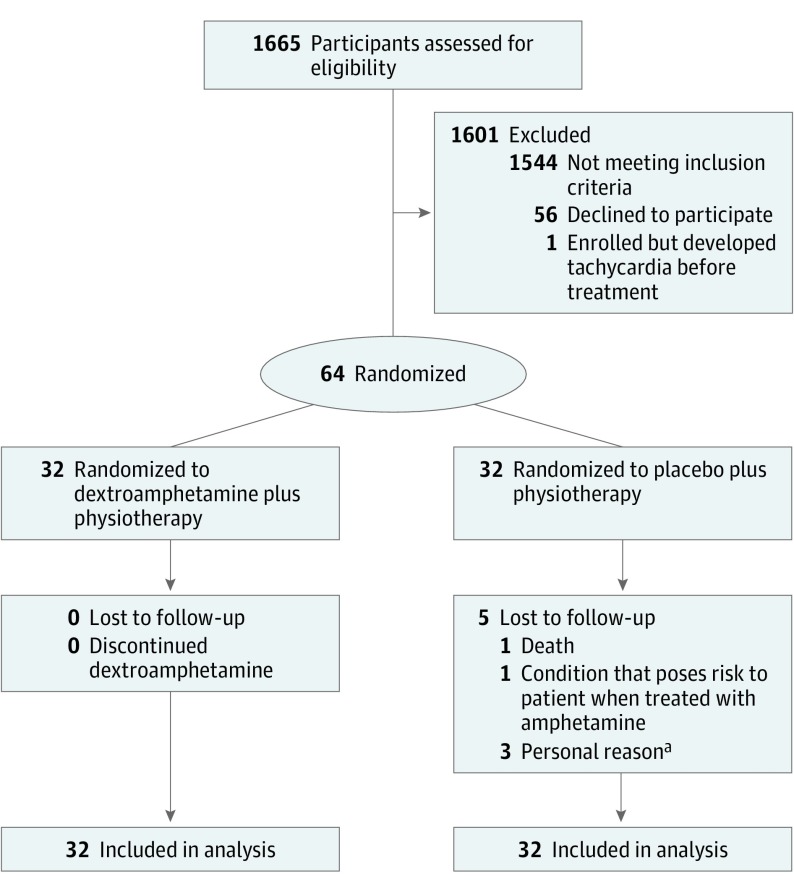
A total of 1665 potential participants were screened, and 64 were randomized (1544 did not meet inclusion criteria, 56 declined to participate, and 1 was enrolled but excluded because of development of tachycardia before any study procedures) (Figure 1). Patients were most frequently excluded because they had a mild stroke-related impairment (n = 536), an index brain hemorrhage (n = 249), or an expected rehabilitation stay of less than 3 weeks or were deemed by the investigator to be at high risk for complications of participation (n = 153). A total of 64 participants (35 men [55%] and 29 women [45%]; median age, 66 years; age range, 27-91 years) were randomized (32 per treatment group, dextroamphetamine vs placebo). The groups were balanced according to the block randomization plan among severe cortical, severe subcortical, moderate cortical, and moderate subcortical strokes (eFigure in Supplement 2). Table 1 gives the baseline characteristics of the participants by treatment group (eTable 1 in Supplement 2 further gives baseline characteristics of the participants by randomization block and treatment group). Demographics, vascular risk factors, medications, and the results of baseline assessments were similar for dextroamphetamine- and placebo-treated participants.
Figure 1. Study Flowchart.
aIncludes withdrew owing to depression and family’s desire to have patient closer to home (n = 1); early withdrawal owing to lack of transportation (n = 1); and discharge home with family (n = 1).
Table 1. Characteristics of Participants at Baseline.
| Characteristic | Treatment Group | P Value | |
|---|---|---|---|
| Dextroamphetamine (n = 32) | Placebo (n = 32) | ||
| Age, median (IQR) | 67.5 (57.0-77.5) | 65.5 (56.0-75.0) | .69 |
| Sex, No. (%) | |||
| Male | 19 (59) | 16 (50) | .45 |
| Female | 13 (41) | 16 (50) | |
| Race/ethnicity, No. (%) | |||
| White | 26 (81) | 23 (72) | .11 |
| Black | 2 (6) | 8 (25) | |
| Latino or Hispanic | 2 (6) | 0 | |
| Other | 2 (6) | 1 (3) | |
| History, No. (%) | |||
| Stroke | 4 (13) | 4 (13) | .96 |
| Hypertension | 23 (72) | 23 (72) | .84 |
| Diabetes | 7 (22) | 12 (38) | .15 |
| Smoking | 8 (25) | 9 (28) | .78 |
| Medication type, No. (%) | |||
| Antihypertensive | 13 (41) | 14 (44) | .97 |
| Antiplatelet | 28 (88) | 25 (78) | .32 |
| Benzodiazepine | 1 (3) | 1 (3) | >.99 |
| Statin | 16 (50) | 14 (44) | .62 |
| Antidepressant, No. (%) | |||
| SSRI | 2 (6) | 3 (9) | .55 |
| TCA | 1 (3) | 0 | |
| Assessments | |||
| Fugl-Meyer motor score, mean (SEM)a | 23.2 (3.6) | 24.5 (3.6) | .75 |
| Modified Rankin Scale score, median (IQR)b | 4 (4-4) | 4 (4-4) | .09 |
| National Institutes of Health Stroke Scale score, mean (SEM)c | 13.1 (1.1) | 13.2 (1.1) | .86 |
| Canadian Neurological Scale score, mean (SEM)d | 5.75 (0.4) | 5.84 (0.3) | .91 |
| Functional Independence Measure score, mean (SEM)e | 60 (4) | 61 (3) | .82 |
| Ambulation Distance and Speed, mean (SEM) | |||
| Distance, m | 52.4 (18.3) | 54.9 (5.9) | .93 |
| Speed, m/min | 16.5 (8.2) | 11.9 (3.4) | .79 |
| Action Research Arm Test, mean (SEM)f | 62 (4) | 63 (3) | .38 |
| MMSE score, mean (SEM)g | 18 (2) | 22 (1) | .27 |
| Beck Depression Index score, mean (SEM)h | 12 (1) | 12 (2) | .52 |
| Stroke Impact Scale score, mean (SEM)i | 36 (2) | 36 (2) | .78 |
Abbreviations: IQR, interquartile range; MMSE, Mini-Mental State Examination; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Scores range from 0 to 100, with higher scores indicating better motor performance.
Scores range from 0 to 6, with 6 indicating death.
Scores range from 0 to 20, with higher scores indicating greater severity.
Scores range from 0 to 8, with higher scores indicating less severity.
Scores range from 7 to 126, with higher scores indicating more dependence.
Scores range from 0 to 57, with higher scores indicating better performance.
Scores range from 0 to 30, with higher scores indicating better cognition.
Scores range from 0 to 27, with higher scores indicating more depression.
Scores range from 0 to 100, with higher scores indicating better recovery.
Outcomes
Table 2 gives the overall baseline, end of treatment, and 30-day posttreatment findings and baseline to 30-day difference for the primary outcome (Fugl-Meyer motor score) and by randomization strata (intention to treat). The overall and within-randomization strata differences between dextroamphetamine- and placebo-treated participants were not significant, although participants with moderate cortical strokes tended to have greater improvements with placebo (mean [SEM] difference, −34.75 [7.60] vs −14.17 [1.96]; P = .054).
Table 2. Change in Fugl-Meyer Motor Scoresa.
| Stroke Type by Treatment | Mean (SEM) Fugl-Meyer Motor Score | |||
|---|---|---|---|---|
| Baseline | End of Treatment | 3 mo After Treatment | Difference, Baseline to 3 mo | |
| Severe cortical | ||||
| Dextroamphetamine | 14.75 (3.52) | 27.13 (5.61) | 34.38 (6.75) | 19.63 (3.64) |
| Placebo | 13.78 (4.54) | 26.50 (10.99) | 19.75 (5.78) | 13.50 (5.07) |
| P value | .63 | .44 | .20 | .35 |
| Severe subcortical | ||||
| Dextroamphetamine | 13.75 (2.42) | 24.09 (4.67) | 28.09 (4.09) | 14.55 (2.28) |
| Placebo | 20.25 (4.34) | 34.27 (5.75) | 40.92 (5.95) | 21.18 (4.55) |
| P value | .23 | .16 | .15 | .39 |
| Moderate cortical | ||||
| Dextroamphetamine | 42.50 (11.13) | 50.00 (11.56) | 56.67 (12.27) | 14.17 (1.96) |
| Placebo | 36.75 (9.63) | 59.75 (7.69) | 71.50 (4.52) | 34.75 (7.60) |
| P value | .75 | >.99 | .39 | .054 |
| Moderate subcortical | ||||
| Dextroamphetamine | 34.17 (10.47) | 53.50 (12.56) | 63.50 (11.82) | 29.3 (8.88) |
| Placebo | 40.83 (10.65) | 45.20 (13.72) | 52.20 (12.71) | 14.80 (3.92) |
| P value | .63 | .65 | .58 | .36 |
| All | ||||
| Dextroamphetamine | 23.22 (3.63) | 35.58 (4.41) | 42.10 (4.61) | 18.65 (2.27) |
| Placebo | 24.48 (3.58) | 38.50 (4.77) | 44.68 (4.89) | 20.83 (2.94) |
| P value | .75 | .63 | .70 | .58 |
Fugl-Meyer motor score 0-35 indicates severe stroke and 36-79, moderate stroke.
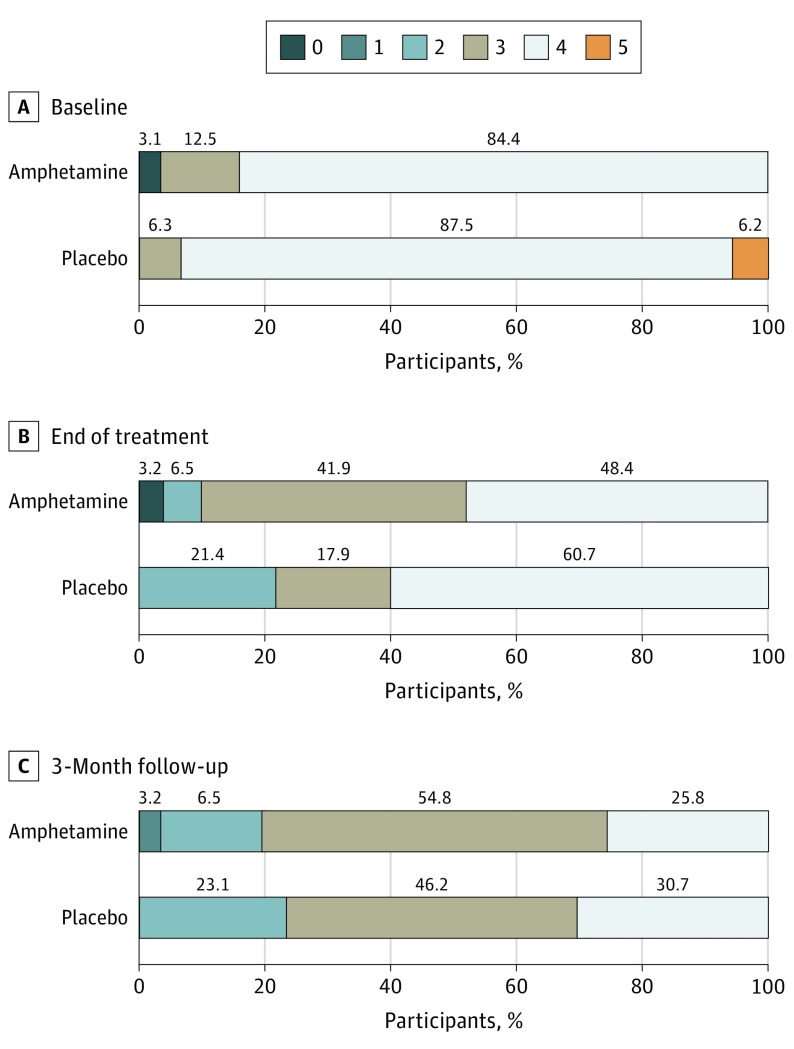
Figure 2 gives the baseline, end of treatment, and 30-day posttreatment results for the modified Rankin Scale score. The difference in improvement between the groups from baseline to 3 months was not significant (mean [SEM] difference, 0.71 [0.14] vs 0.92 [0.13] points; P = .29). eTables 2 to 9 in Supplement 2 give the baseline, end of treatment, 3-month poststroke, and baseline to 3-month differences by strata and overall for the National Institutes of Health Stroke Scale (overall mean [SEM] difference for dextroamphetamine vs placebo groups, −4.84 [0.75] vs −4.96 [0.77] points; P = .97), Canadian Neurological Score (mean [SEM] difference, 1.95 [0.27] vs 2.04 [0.33] points; P = .66), Functional Independence Measure (mean [SEM] difference, 38.29 [3.31] vs 34.46 [2.84] points; P = .46), Ambulation Distance (mean [SEM] difference, 109.64 [21.36] vs 67.71 [19.55] m; P = .16), Ambulation Speed (mean [SEM] difference, 9.41 [7.69] vs 9.49 [2.85] m/min; P = .21), Research Action Arm Test (mean [SEM] difference, 7.24 [2.72] vs 12.83 [3.69] points; P = .08), Mini-Mental State Examination (mean [SEM] difference, 4.90 [2.23] vs 17.58 [3.49] points; P = .12), and Beck Depression Inventory (mean [SEM] difference, −3.03 [1.16] vs −1.43 [1.66] points; P = .15). eTable 10 in Supplement 2 gives the baseline, 3-month poststroke, and baseline to 3-month differences by strata and overall for the Stroke Impact Scale (mean [SEM] difference, 18.04 [7.69] vs 9.49 [2.85] points; P = .21). We found no overall differences between the groups for any measure. Those with severe subcortical strokes had somewhat greater improvements with placebo on the Action Research Arm test (mean [SEM] difference, 17.33 [5.42] vs 2.27 [1.89] points; P = .01, uncorrected for multiple comparisons) (eTable 7 in Supplement 2).
Figure 2. Distribution of Modified Rankin Scale Scores at Baseline, End of Treatment, and 3 Months After Stroke.
Scores range from 0 (no deficit) to 6 (death). None of the participants died during the study period. Numbers above the bars indicate the percentage of participants with each score at each point for those randomized to dextroamphetamine (n = 32) or placebo (n = 32). The difference between the groups between baseline and 3 months is not significant (P = .29).
Safety
Five participants (8%) did not complete the protocol through the 3-month poststroke follow-up evaluation (Figure 1). None were withdrawn owing to a protocol-specified cause and all were allocated to the placebo group. One patient withdrew consent, 1 moved away from the study site, and 1 transferred to a rehabilitation facility closer to home. One participant with a remote history of a seizure disorder had a possible uncomplicated partial seizure. A fifth participant had bilateral lower-extremity deep vein thromboses, was transferred to an acute care hospital, and was then found to have colon cancer with hepatic metastases. That patient was subsequently found to have a second stroke on magnetic resonance imaging without clinical manifestations and died of sepsis in the acute care hospital.
Adverse events that did not prompt withdrawal from the study included 1 participant who had a second stroke 2 months after completing treatment and after completing the last study assessment. An additional participant had a deep vein thrombosis that was treated with an inferior vena cava filter. No treatment-associated serious adverse events occurred.
Discussion
The primary result of this pilot clinical trial was that intention to treat with dextroamphetamine combined with physical therapy did not improve recovery of motor function compared with placebo combined with physical therapy in similar participants as assessed 3 months after hemispheric ischemic stroke based on the Fugl-Meyer motor score. Although only highly selected patients in whom amphetamine treatment was considered safe and who were thought to most likely benefit from the intervention were included, no difference in any of the trial’s secondary measures occurred.
A meta-analysis that included data from 9 trials (total of 114 amphetamine-treated participants and 112 controls)29 found no overall effect of treatment with dextroamphetamine on motor recovery (standard mean difference, −0.08; 95% CI, −0.34 to 0.19); in 4 trials, the meta-analysis found no effect on activities of daily living (total of 58 amphetamine-treated participants and 55 controls; standard mean difference, 3.85; 95% CI, −5.75 to 13.49) after stroke. More deaths occurred at the end of follow-up among participants randomized to amphetamine (8.5% vs 2.2%; odds ratio, 2.8; 95% CI, 0.9-8.6), which may have been owing to baseline imbalances between the groups, but the difference was not significant. The trials, however, were small and had important differences in patient populations, drug treatment regimens, physiotherapy protocols, and outcome measures.30 Although intended as a pilot, the present study represents, to our knowledge, the second largest randomized trial of dextroamphetamine conducted to date. Despite the choice of a dosing and treatment regimen modeled after a prior trial that suggested benefit in a smaller, single-site study that also included careful linking of drug treatment with targeted physiotherapy,9 the present study also failed to find a treatment effect.
Preclinical laboratory studies of the effect of amphetamine administration on recovery after brain injury were limited to experiments in which the lesion was restricted to the cerebral cortex.31 Therefore, no preclinical data evaluated the potential effects of treatment with dextroamphetamine on recovery after injury to subcortical structures. Because the present study was intended to be exploratory, participants with strokes affecting the cerebral cortex or subcortical hemispheric structures resulting in motor deficits were included. Although not powered for subgroup analyses, our participants were randomized according to stroke subtype (cortical vs subcortical) and functional severity. Those participants with moderately severe cortical strokes tended to have more benefit with placebo, with no differences in other subgroups. As in our study, another trial14 found no effect of treatment in subgroups of participants with a cortically based stroke.
We anticipated that 30% of enrolled participants would be withdrawn owing to expected complications during rehabilitation. During the trial, only 5 participants (8%) did not complete the 3-month assessment, providing sufficient power to evaluate the primary efficacy end point. None of the adverse events that occurred during the trial were considered to have been associated with study treatments. This finding is consistent with those of other studies that found no clinically important adverse events related to treatment with dextroamphetamine during poststroke rehabilitation.14,32,33 As in our study, these clinical trials carefully excluded participants who might be at higher risk of amphetamine-related complications.
Limitations
This study has several important limitations. Although powered to detect what was anticipated to be a clinically important effect, this pilot trial included participants for whom there was no preclinical evidence of treatment benefit. An alternative dosing regimen was not assessed. Although study-related physiotherapy sessions followed a standard protocol, other physiotherapy was permitted, which could obscure a treatment effect. Whether subgroups of participants (eg, those with a cortical stroke causing a moderate motor deficit) might benefit cannot be assessed. Whether a different amphetamine dose or administration schedule (eg, more or less frequent treatment or beginning sooner after the stroke) or a modified patient population (eg, more severely affected) might show a benefit would need to be evaluated in other studies. Since our study was conducted, acute thrombectomy has been proven effective,34 and physiotherapeutic approaches may have evolved,35 both of which might influence the effect of drugs such as dextroamphetamine on the trajectory of functional recovery after stroke.
Conclusions
Despite supportive preclinical data, we found no evidence that treatment with dextroamphetamine combined with physiotherapy leads to an important improvement in poststroke recovery. Future studies could assess other strategies for modulating central neurotransmitters and other subgroups of participants.
Trial Protocol
eFigure. Stratification of Participants by Stroke Severity and Type
eTable 1. Baseline Demographics and Participant Characteristics
eTable 2. Mean National Institutes of Health Stroke Scale Score (SEM)
eTable 3. Mean Canadian Neurological Scale Score (SEM)
eTable 4. Mean Functional Independence Measure Score (SEM)
eTable 5. Mean Ambulation Distance (6-Minute Walk Test) (SEM)
eTable 6. Mean Ambulation Speed (6-Minute Walk Test) (SEM)
eTable 7. Mean Research Arm Test Score (SEM)
eTable 8. Mean Mini-Mental State Examination Score (SEM)
eTable 9. Mean Beck Depression Index Score (SEM)
eTable 10. Mean Stroke Impact Scale Score (SEM)
References
- 1.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439-448. doi: 10.1161/CIRCRESAHA.116.308413 [DOI] [PubMed] [Google Scholar]
- 2.Buntin MB, Colla CH, Deb P, Sood N, Escarce JJ. Medicare spending and outcomes after postacute care for stroke and hip fracture. Med Care. 2010;48(9):776-784. doi: 10.1097/MLR.0b013e3181e359df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gadidi V, Katz-Leurer M, Carmeli E, Bornstein NM. Long-term outcome poststroke: predictors of activity limitation and participation restriction. Arch Phys Med Rehabil. 2011;92(11):1802-1808. doi: 10.1016/j.apmr.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 5.Phillips JP, Devier DJ, Feeney DM. Rehabilitation pharmacology: bridging laboratory work to clinical application. J Head Trauma Rehabil. 2003;18(4):342-356. doi: 10.1097/00001199-200307000-00005 [DOI] [PubMed] [Google Scholar]
- 6.Goldstein LB. Effects of amphetamines and small related molecules on recovery after stroke in animals and man. Neuropharmacology. 2000;39(5):852-859. [DOI] [PubMed] [Google Scholar]
- 7.Adkins DL, Schallert T, Goldstein LB. Poststroke treatment: lost in translation. Stroke. 2009;40(1):8-9. doi: 10.1161/STROKEAHA.108.534248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crisostomo EA, Duncan PW, Propst M, Dawson DV, Davis JN. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol. 1988;23(1):94-97. doi: 10.1002/ana.410230117 [DOI] [PubMed] [Google Scholar]
- 9.Walker-Batson D, Smith P, Curtis S, Unwin H, Greenlee R. Amphetamine paired with physical therapy accelerates motor recovery after stroke: further evidence. Stroke. 1995;26(12):2254-2259. doi: 10.1161/01.STR.26.12.2254 [DOI] [PubMed] [Google Scholar]
- 10.Reding MJ, Solomon B, Borucki SJ. Effect of dextroamphetamine on motor recovery after stroke. Neurology. 1995;45(suppl 4):A222. [Google Scholar]
- 11.Sonde L, Nordström M, Nilsson C-G, Lökk J, Viitanen M. A double-blind placebo-controlled study of the effects of amphetamine and physiotherapy after stroke. Cerebrovasc Dis. 2001;12(3):253-257. doi: 10.1159/000047712 [DOI] [PubMed] [Google Scholar]
- 12.Martinsson L, Eksborg S, Wahlgren NG. Intensive early physiotherapy combined with dexamphetamine treatment in severe stroke: a randomized, controlled pilot study. Cerebrovasc Dis. 2003;16(4):338-345. doi: 10.1159/000072555 [DOI] [PubMed] [Google Scholar]
- 13.Treig T, Werner C, Sachse M, Hesse S. No benefit from d-amphetamine when added to physiotherapy after stroke: a randomized, placebo-controlled study. Clin Rehabil. 2003;17(6):590-599. doi: 10.1191/0269215503cr653oa [DOI] [PubMed] [Google Scholar]
- 14.Gladstone DJ, Danells CJ, Armesto A, et al. ; Subacute Therapy with Amphetamine and Rehabilitation for Stroke Study Investigators . Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2006;37(1):179-185. doi: 10.1161/01.STR.0000195169.42447.78 [DOI] [PubMed] [Google Scholar]
- 15.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke: outcome assessment and sample size requirements. Stroke. 1992;23(8):1084-1089. doi: 10.1161/01.STR.23.8.1084 [DOI] [PubMed] [Google Scholar]
- 16.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521-1526. doi: 10.1016/0140-6736(91)93206-O [DOI] [PubMed] [Google Scholar]
- 17.Lindley RI, Warlow CP, Wardlaw JM, Dennis MS, Slattery J, Sandercock PAG. Interobserver reliability of a clinical classification of acute cerebral infarction. Stroke. 1993;24(12):1801-1804. doi: 10.1161/01.STR.24.12.1801 [DOI] [PubMed] [Google Scholar]
- 18.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient, 1: a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13-31. [PubMed] [Google Scholar]
- 19.Brott T, Adams HPJ Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. doi: 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 20.Côté R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian Neurological Scale: validation and reliability assessment. Neurology. 1989;39(5):638-643. doi: 10.1212/WNL.39.5.638 [DOI] [PubMed] [Google Scholar]
- 21.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4(4):483-492. doi: 10.1097/00004356-198112000-00001 [DOI] [PubMed] [Google Scholar]
- 22.Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1989;20(6):828. doi: 10.1161/01.STR.20.6.828 [DOI] [PubMed] [Google Scholar]
- 23.Kidd D, Stewart G, Baldry J, et al. The Functional Independence Measure: a comparative validity and reliability study. Disabil Rehabil. 1995;17(1):10-14. doi: 10.3109/09638289509166622 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax. 1984;39(11):818-822. doi: 10.1136/thx.39.11.818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed). 1986;292(6521):653-655. doi: 10.1136/bmj.292.6521.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 28.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0: evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131-2140. doi: 10.1161/01.STR.30.10.2131 [DOI] [PubMed] [Google Scholar]
- 29.Martinsson L, Hardemark HG, Eksborg S. Should amphetamines be given to improve recovery after stroke? Stroke. 2007;38:2400-2401. doi: 10.1161/STROKEAHA.107.484923 [DOI] [Google Scholar]
- 30.Goldstein LB. Amphetamine trials and tribulations. Stroke. 2009;40(3)(suppl):S133-S135. doi: 10.1161/STROKEAHA.108.533703 [DOI] [PubMed] [Google Scholar]
- 31.Goldstein LB. Neurotransmitters and motor activity: effects on functional recovery after brain injury. NeuroRx. 2006;3(4):451-457. doi: 10.1016/j.nurx.2006.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unwin H, Walker-Batson D. No side effects after low-dose amphetamine administration in stroke rehabilitation. Stroke. 2000;31(7):1788-1789. doi: 10.1161/01.STR.31.7.1785-d [DOI] [PubMed] [Google Scholar]
- 33.Martinsson L, Wahlgren NG. Safety of dexamphetamine in acute ischemic stroke: a randomized, double-blind, controlled dose-escalation trial. Stroke. 2003;34(2):475-481. doi: 10.1161/01.STR.0000050161.38263.AE [DOI] [PubMed] [Google Scholar]
- 34.Powers WJ, Rabinstein AA, Ackerson T, et al. American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;67(6):1934. [DOI] [PubMed] [Google Scholar]
- 35.Winstein CJ, Stein J, Arena R, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Stratification of Participants by Stroke Severity and Type
eTable 1. Baseline Demographics and Participant Characteristics
eTable 2. Mean National Institutes of Health Stroke Scale Score (SEM)
eTable 3. Mean Canadian Neurological Scale Score (SEM)
eTable 4. Mean Functional Independence Measure Score (SEM)
eTable 5. Mean Ambulation Distance (6-Minute Walk Test) (SEM)
eTable 6. Mean Ambulation Speed (6-Minute Walk Test) (SEM)
eTable 7. Mean Research Arm Test Score (SEM)
eTable 8. Mean Mini-Mental State Examination Score (SEM)
eTable 9. Mean Beck Depression Index Score (SEM)
eTable 10. Mean Stroke Impact Scale Score (SEM)