Introduction
Giant cell tumors (GCT) of bone rarely occur in the hand. When they do, the metacarpals are the most commonly affected bones. Involvement of carpal bones is very rare. In a review of 1228 cases of GCT of bone, Averill et al. reported that 3% of cases occurred in the hand and only 0.32% in the carpal bones1. Though GCTs most commonly occur in skeletally mature patients, they occasionally affect children2. Most GCTs in the carpal bones are single lesions; multifocal involvement is extremely rare3. However, recurrence is very common after intralesional curettage of multifocal GCT of carpal bones; thus, wide resection of the tumor with wrist arthrodesis or proximal row carpectomy is indicated in such cases4. Movement of the wrist joint is inevitably decreased after these surgeries. We here report a case of multifocal GCT of bone involving the trapezoid, capitate, hamate and base of the third metacarpal in a skeletally immature patient. The tumor was widely resected and the carpal height restored by reconstruction with autologous fibular and iliac crest grafts.
Case Report
A 14‐year‐old male patient presented with a gradually enlarging painful swelling over the right wrist over the previous year without any constitutional symptoms and or preceding trauma. On examination, a tender swelling on the dorsum of wrist joint measuring approximately 3 cm in both horizontal and longitudinal dimensions was noted. Movements at the wrist were significantly restricted. There was no neurovascular impairment distal to the mass. Anteroposterior radiographs revealed a destructive lytic lesion in the capitate bone with probable involvement of surrounding bones and soft tissue (Fig. 1A,B). Three phase bone scan was suggestive of a primary skeletal pathology in the distal carpal row and base of the third metacarpal. There was no evidence of involvement of any other part of the body. Magnetic resonance imaging revealed a multifocal lesion involving the trapezoid, capitate, hamate and base of the third metacarpal up to 2 cm from its proximal articular margin (Fig. 2A,B). Open biopsy of the capitate under general anesthesia was performed and the histopathology reported as suggestive of GCT.
Figure 1.
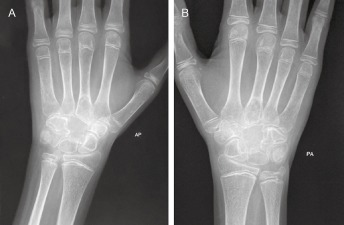
(A) Anteroposterior and (B) posteroanterior radiographs showing a lytic destructive lesion in the capitate with probable involvement of surrounding bones and soft tissue.
Figure 2.
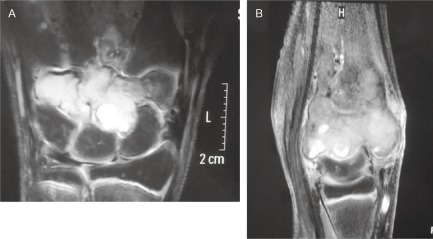
MRI showing a multifocal lesion involving the trapezoid, capitate, hamate and base of third metacarpal. (A) Axial MRI; (B) Sagittal MRI.
The tumor mass was resected en bloc via a dorsal approach and the carpal height restored with a strategically measured autologous fibular graft that included the height of the resected capitate and base of the third metacarpal (Fig. 3). The cartilaginous distal part of the proximal row was removed so that cancellous bone was visible. Two additional small struts of fibular graft and chips from the iliac crest were placed between the proximal row and the proximal resected part of the third metacarpal and base of the second, fourth and fifth metacarpal. The largest fibular graft was fixed in place of the resected third metacarpal with two 2.4 mm locking plates. Another 2.4 mm locking plate was inserted between the proximal row and fourth metacarpal (Fig. 3). After thorough irrigation with normal saline, the rest of the gap was filled with chips of cancellous autograft from the iliac crest. After closure of the wound, a below‐elbow plaster of Paris slab was used to immobilize the wrist in a functional position (Video S1). Histopathological examination of the excised mass demonstrated numerous osteoclast‐like, multinucleated giant cells and spindled to polygonal mononuclear cells (Fig. 4). The slab was removed after 12 weeks, after which the hand was kept in a wrist immobilization splint and intermittent mobilization commenced. Solid union was noted at 16 weeks in anteroposterior, lateral and oblique radiographs (Fig. 5). Strengthening of the forearm muscles was started after 4 months. On physical examination, 40° of extension and 30° of flexion of the wrist was possible on the right side as compared with 90° of extension and 90° of flexion of the left wrist joint. Radial and ulnar deviation was possible up to 10° in the right wrist as compared with 30° on the left side. There was no limitation of supination or pronation and the movements were completely painless. The grip strength of the right hand was 16 kg as compared with 18 kg on the left side. The length of the palm of the hand was found to be 21.8 cm on the right side and 22 cm on the left. Twenty‐two months postoperatively, there was no clinical or radiological evidence of recurrence.
Figure 3.
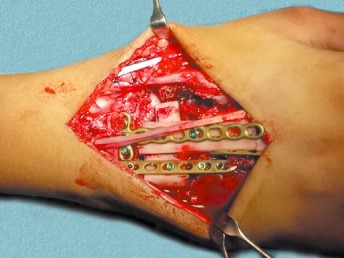
The final construct with strategically measured fibular graft and 2.4 mm locking plates.
Figure 4.
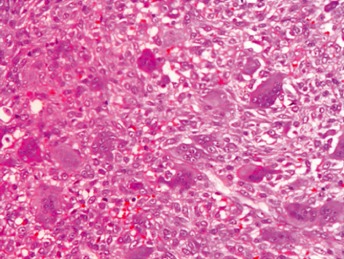
Histopathology examination of the excised mass demonstrated numerous multinucleated giant cells and spindled to polygonal mononuclear cells (HE stain; ×100).
Figure 5.
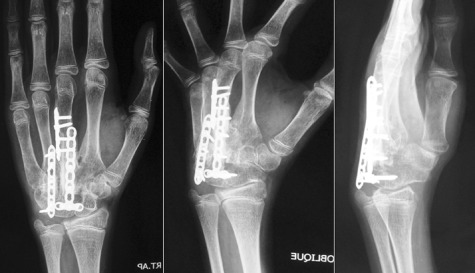
Post‐operative radiographs after 16 weeks showing solid union.
Discussion
Giant cell tumors of bone, which are benign but locally aggressive, account for approximately 5% of all bone tumors5. They are most frequently seen in adults, the peak prevalence being in the third decade. There are only a few reports of their occurrence in skeletally immature patients6, 7. They mostly involve the long bones of the lower extremities and very uncommonly occur in the carpal bones. Most reported carpal bone GCTs have involved a single bone; there are very few reports of multifocal GCTs of carpal bones and all have occurred in skeletally mature patients4, 8.
The clinical characteristics of GCTs in the hand differ distinctly from those of GCTs in the long bones1, 9, 10. The mean age for GCTs in the hand is 10 years younger than that of GCTs in long bones, which is 32 years. Pain and swelling occur earlier in the hand, probably because of its superficial character and the mobility of the fingers. Radiographs of GCTs of the hand usually show a central epiphyseal location, rather than the typical eccentric location. GCTs of the hand are considered more aggressive, their local recurrence rate after curettage being approximately 70%. Therefore, wide local resection is the optimal treatment. They can be a component of multicentric GCT with involvement of other skeletal sites. They are associated with a higher risk of pulmonary metastasis than those arising in other anatomic sites10, 11.
These distinctive clinical features of GCT of the hand demand a thorough evaluation of these patients on presentation. A chest radiograph is indicated to look for evidence of pulmonary metastases. A bone scan should be performed to look for multifocal skeletal involvement because multicentricity is more common with GCTs of hand than with those in other anatomic sites. In our case, the chest radiograph was clear and a bone scan showed no evidence of lesions other than those involving the distal carpal row and base of the third metacarpal.
On radiographs, these tumors typically appear as osteolytic and expansile lesions3. Computed tomography and magnetic resonance imaging (MRI) can help in assessing extent of mineralization, cortical integrity and soft tissue involvement. MRI can further help to define the relationship of the tumor mass to neurovascular structures, which helps with pre‐operative planning. The differential diagnoses of chondroblastoma and aneurysmal bone cyst should be considered because these tumors have similar clinical and radiological characteristics. Chondroblastoma can be easily ruled out by histopathological examination. However, it is quite difficult to differentiate a GCT from an aneurysmal bone cyst, because both tumors show proliferative fibroblast, spindle cells, areas of necrosis and hemorrhage on histological examination.
Treatment modalities for managing carpal GCTs include curettage with bone grafting, cryosurgical ablation and drilling with high speed burr: these have all been shown to be more effective in cases of single bone involvement without cortical destruction. Because multifocal GCTs of carpal bones treated by curettage have a high recurrence rate, the optimal treatment is wide excision with wrist arthrodesis or proximal row carpectomy4, 12. However, such procedures disturb the anatomy of the radiocarpal joint, compromising its function. There has been only one case report of multifocal carpal GCT treated with preservation of wrist joint function in which there was no subsequent recurrence8. In that case, the patient was skeletally mature; hence the authors used a modified bone plate technique with tri‐cortical iliac crest graft and screws to fill the gap created by en bloc excision of the capitate, trapezium, trapezoid and distal scaphoid.
In our case, though the lesion was multifocal with cortical destruction and soft tissue involvement, the proximal carpal row was fortunately disease‐free. However, there was involvement of the base of the third metacarpal in addition to the carpal bones. Thus, the pathology involved both the mid‐carpal and carpometacarpal joints. Because the patient was skeletally immature and because there was a large gap after tumor resection, it was not possible to use a tri‐cortical iliac crest graft. There was a huge gap after en bloc excision of the tumor mass and we aimed to fill this gap with minimal compromise of wrist function. We considered that a T shaped 2.4 mm locking plate (AO Synthes, Solothurn, Switzerland) was the most appropriate implant for achieving rigid fixation between the metacarpals and proximal row. We placed a strategically measured autologous fibular graft between the proximal row and distal part of the third metacarpal and then fixed it with a 2.5 mm T shaped locking plate with its base fixed to the proximal row with two screws and the vertical column fixed to the graft with two more screws. We then used another 2.4 mm locking plate to fix the graft to the distal part of the third metacarpal and placed it on the ulnar aspect. We used the third locking plate to fix the proximal row to the fourth metacarpal and filled the rest of the gap with chips of autologous cancellous iliac crest graft. This strategy helped us to achieve our goals of maintaining carpal height and preserving radiocarpal joint anatomy and function. The use of other implants or K wires would have led to inadequate fixation and loss of integrity of the radiocarpal joint and its function. Fortunately, there was no evidence of recurrence in spite of the cortical destruction and soft tissue involvement; this can be attributed to the wide excision of the tumor mass.
The trio of rigid fibular graft, cancellous iliac crest graft and an appropriate locking plate enabled achievement of solid union after 16 weeks with maintenance of carpal height and radiocarpal joint function. This also enabled us to start a rehabilitation program early, thus reducing the risk of stiffness in the surrounding joints. The patient had achieved a good range of motion by the completion of his rehabilitation program. A partial loss of motion was attributable to midcarpal fusion. To our knowledge, this is the first reported case that describes the management of multifocal GCT involving carpal and metacarpal bones in a skeletally immature patient that was managed by en bloc excision and preservation of radiocarpal joint function without any clinical or radiological evidence of recurrence after 22 months of follow‐up.
Supporting information
Video S1 Wrist‐preserving surgery for multifocal giant cell tumor of carpal bones.
Disclosure: All named authors hereby declare that they have no conflicts of interest to disclose. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
References
- 1. Averill RM, Smith RJ, Campbell CJ. Giant‐cell tumors of the bones of the hand. J Hand Surg Am, 1980, 5: 39–50. [DOI] [PubMed] [Google Scholar]
- 2. Gupta GG, Lucas GL, Pirela‐Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am, 1995, 20: 1003–1006. [DOI] [PubMed] [Google Scholar]
- 3. Schutte HE, Taconis WK. Giant cell tumor in children and adolescents. Skeletal Radiol, 1993, 22: 173–176. [DOI] [PubMed] [Google Scholar]
- 4. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant‐cell tumors of the carpus. J Hand Surg Am, 2006, 31: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 5. Dahlin DC. Giant cell tumor of bone: highlights of 407 cases. AJR Am J Roentgenol, 1985, 144: 955–960. [DOI] [PubMed] [Google Scholar]
- 6. Picci P, Manfrini M, Zucchi V, et al Giant‐cell tumor of bone in skeletally immature patients. J Bone Joint Surg Am, 1983, 65: 486–490. [PubMed] [Google Scholar]
- 7. Schütte HE, Taconis WK. Giant cell tumor in children and adolescents. Skeletal Radiol, 1993, 22: 173–176. [DOI] [PubMed] [Google Scholar]
- 8. Tarng YW, Yang SW, Hsu CJ. S Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am, 2009, 34: 262–265. [DOI] [PubMed] [Google Scholar]
- 9. López‐Barea F, Rodríguez‐Peralto JL, García‐Girón J, Guemes‐Gordo F. Benign metastasizing giant‐cell tumor of the hand. Report of a case and review of the literature. Clin Orthop Relat Res, 1992, 274: 270–274. [PubMed] [Google Scholar]
- 10. Wold LE, Swee RG. Giant cell tumor of the small bones of the hands and feet. Semin Diagn Pathol, 1984, 1: 173–184. [PubMed] [Google Scholar]
- 11. Cavender RK, Sale WG 3rd. Giant cell tumor of the small bones of the hands and feet: metatarsal giant cell tumor. W V Med J, 1992, 88: 342–345. [PubMed] [Google Scholar]
- 12. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am, 1984, 9: 272–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Wrist‐preserving surgery for multifocal giant cell tumor of carpal bones.


