Abstract
This cohort study evaluates the feasibility of daily customized epidural stimulation configurations targeted for cardiovascular function in individuals with chronic, cervical motor-complete spinal cord injury.
Cervical spinal cord injury (SCI) profoundly disrupts the cardiovascular and autonomic systems, severely diminishing overall health and quality of life.1 Mitigation of unstable blood pressure is a high priority in care for individuals with chronic SCI; however, to our knowledge, no existing therapies have demonstrated the ability to resolve chronic cardiovascular dysfunction.2 Hypotension was acutely resolved in 4 individuals with chronic, cervical motor-complete SCI with customized epidural stimulation configurations targeted for cardiovascular function (CV-scES).3 We evaluated the outcome of CV-scES when used as daily CV-scES training to target chronic orthostatic hypotension.
Methods
This prospective, cohort, early feasibility study conducted from September 24, 2014, to January 22, 2018, included 4 individuals with SCI, presenting with orthostatic hypotension, persistent low resting blood pressure, and symptoms of autonomic dysreflexia. Participants with a spinal cord epidural stimulator (RestoreAdvanced, 16 electrode-array; Medtronic) implanted over segments L1 to S1 were given stimulation parameters that increased systolic blood pressure within 105 to 120 mm Hg.3 Participants completed a mean (SD) of 89 (13) two-hour sessions of daily CV-scES training (Table). Continuous blood pressure was measured using plethysmography (Finometer Pro; FMS) to assess hemodynamic response to orthostatic stress throughout training, with and without stimulation. The study was approved by the University of Louisville institutional review board (ClinicalTrials.gov identifier NCT02037620), and participants provided written informed consent.
Table. Mean Cardiovascular Parameters Before, During, and After Stimulation During Training.
| Parameter | Systolic Blood Pressure, Mean (SD), mm Hg | Diastolic Blood Pressure, Mean (SD), mm Hg | Heart Rate, Mean (SD), Beats/min | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Stimulation | During Stimulation | After Stimulation | Before Stimulation | During Stimulation | After Stimulation | Before Stimulation | During Stimulation | After Stimulation | |
| Participant A41 | |||||||||
| Session 1-39 | 99 (7) | 116 (7) | 114 (13) | 57 (5) | 69 (6) | 66 (10) | 76 (7) | 68 (9) | 70 (10) |
| P value | <.001 | [Reference] | .001 | <.001 | [Reference] | <.001 | <.001 | [Reference] | .04 |
| Session 40-85 | 100 (7) | 114 (7) | 111 (15) | 56 (4) | 67 (5) | 62 (9) | 80 (6) | 70 (9) | 72 (14) |
| P value | <.001 | [Reference] | <.001 | <.001 | [Reference] | <.001 | <.001 | [Reference] | .002 |
| Participant A68 | |||||||||
| Session 1-37 | 90 (5) | 116 (12) | 91 (8) | 54 (3) | 67 (8) | 53 (4) | 59 (5) | 58 (6) | 66 (6) |
| P value | <.001 | [Reference] | <.001 | <.001 | [Reference] | <.001 | .12 | [Reference] | <.001 |
| Session 38-80 | 93 (7) | 118 (12) | 93 (15) | 54 (4) | 66 (8) | 54 (7) | 64 (8) | 63 (6) | 69 (8) |
| P value | <.001 | [Reference] | <.001 | <.001 | [Reference] | <.001 | .42 | [Reference] | <.001 |
| Participant B21 | |||||||||
| Session 1-46 | 104 (4) | 112 (7) | 108 (8) | 62 (3) | 68 (7) | 65 (7) | 70 (8) | 68 (8) | 68 (8) |
| P value | <.001 | [Reference] | <.001 | <.001 | [Reference] | <.001 | <.001 | [Reference] | .83 |
| Session 47-84 | 120 (8) | 113 (8) | 113 (8) | 78 (6) | 71 (8) | 71 (7) | 60 (8) | 64 (9) | 60 (9) |
| P value | <.001 | [Reference] | .68 | <.001 | [Reference] | .001 | <.001 | [Reference] | <.001 |
| Participant A80 | |||||||||
| Session 1-50 | 86 (10) | 106 (9) | 89 (11) | 53 (7) | 66 (8) | 52 (7) | 72 (7) | 74 (9) | 68 (16) |
| P value | <.001 | [Reference] | <.001 | <.001 | [Reference] | <.001 | .41 | [Reference] | <.001 |
| Session 56-108 | 90 (15) | 109 (9) | 89 (10) | 55 (11) | 68 (8) | 55 (7) | 75 (10) | 80 (11) | 68 (16) |
| P value | <.001 | [Reference] | <.001 | <.001 | [Reference] | <.001 | <.001 | [Reference] | <.001 |
We used a linear mixed model on logged values of systolic blood pressure, diastolic blood pressure, and heart rate during orthostatic stress and during daily CV-scES training. Fixed effects included position, intervention, and their interaction. Random intercept and slopes were included as well as random effects of repetition and serial correlation. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
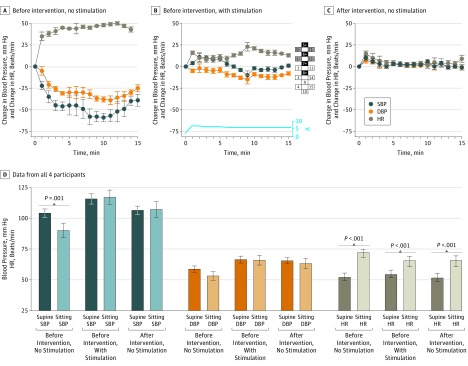
Orthostatic hypotension resolved with CV-scES and after daily CV-scES training (Figure). Before daily CV-scES training, participant A41 experienced a significant decrease in mean systolic and diastolic blood pressure and heart rate increase without stimulation (Figure, A), but with stimulation blood pressure while sitting did not significantly change from values measured while the participant was supine (Figure, B), although heart rate measured while sitting increased significantly, but to lower values. A similar response was observed after daily CV-scES training when presented with orthostatic stress even without stimulation (Figure, C). These results were consistent in all 4 individuals (Figure, D).
Figure. Cardiovascular Response to Orthostatic Stress With Stimulation and After Cardiovascular Stimulation Intervention.
A, Exemplary data from participant A41 before the intervention without stimulation demonstrates a significant decrease (P < .001) in mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) and increase (P < .001) in heart rate (HR) during a sit-up test. B, Before the intervention with stimulation, there was no significant decrease (P = .72) in mean SBP and DBP, while HR increased significantly (P < .001) during a sit-up test. Amplitude of stimulation is represented in volts; configuration of the numbered electrode array is illustrated at right: black is anode, gray is cathode, and white is inactive. C, After the intervention without stimulation, there was no significant decrease (P = .42) in SBP and DBP, while HR increased significantly (P = .002) during the sit-up test. D, Data from all 4 participants are averaged for these conditions; stimulation is depicted by diagonal bars. In all participants, before intervention without stimulation, mean SBP decreased significantly from supine to a sitting position (P = .001). The decrease in mean DBP from supine to a sitting position was not significant (P = .64). Mean HR increased significantly (P < .001) from supine to a sitting position. Before intervention with stimulation, there was no significant difference in SBP and DBP from supine to a sitting position (SBP, P = .15; DBP, P = .16); HR increased significantly (P < .001). After intervention without stimulation, SBP and DBP was not significantly different from supine to a sitting position (SBP, P = .36; DBP, P = .87); HR while sitting increased significantly from supine to a sitting position (P < .001). Error bars indicate SD.
Discussion
Orthostatic hypotension was alleviated in 4 individuals with chronic cervical motor-complete SCI when lumbosacral CV-scES was used during orthostatic stress. The improved cardiovascular response was observed after daily CV-scES training without stimulation. These results suggest that there is immediate cardiovascular responsiveness with stimulation that persists after training, indicating long-term adaptation. A potential mechanism consistent with these results is activation of sympathetic vasomotor efferents, causing vasoconstriction and an increase in both blood pressure and venous return.4 Decreased heart rate during orthostatic stress would be consistent with increased parasympathetic tone and attenuation of vagal withdrawal1 subsequent to baroreceptor stimulation.2 These mechanisms have been shown in a preliminary proof-of-principal, non-SCI human study of epidural stimulation.4
Improved orthostatic tolerance indicates that CV-scES had outcomes associated with adaptive plasticity that stabilized cardiovascular and autonomic regulatory systems. Chronic SCI leads to deconditioning of the baroreflex and vagal control mechanisms despite intact neural circuitry1,2 and daily CV-scES training can have a positive outcome. Epidural stimulation applied to individuals with motor-complete SCI demonstrates significant potential for not only motor recovery,5,6 but autonomic recovery as well3: physiological and motor behavior can respond to stimulation of the lumbosacral cord when targeted to a physiological response or motor task. Long-term CV-scES could be a viable therapeutic approach for paralysis and secondary consequences that cause hospitalizations, accrual of costs, and diminished quality of life in individuals with chronic motor-complete SCI. Additional long-term investigation of heart rate and blood pressure variability, cardiac and vessel function and structure, and poststimulation effects are needed to understand the long-term safety and feasibility of epidural stimulation as a viable therapy.
References
- 1.Wecht JM, Bauman WA. Implication of altered autonomic control for orthostatic tolerance in SCI. Auton Neurosci. 2018;209:51-58. doi: 10.1016/j.autneu.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 2.Draghici AE, Taylor JA. Baroreflex autonomic control in human spinal cord injury: physiology, measurement, and potential alterations. Auton Neurosci. 2018;209:37-42. doi: 10.1016/j.autneu.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 3.Harkema SJ, Wang S, Angeli CA, et al. Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front Hum Neurosci. 2018;12:83. doi: 10.3389/fnhum.2018.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamasaki F, Ushida T, Yokoyama T, Ando M, Yamashita K, Sato T. Artificial baroreflex: clinical application of a bionic baroreflex system. Circulation. 2006;113(5):634-639. doi: 10.1161/CIRCULATIONAHA.105.587915 [DOI] [PubMed] [Google Scholar]
- 5.Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938-1947. doi: 10.1016/S0140-6736(11)60547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grahn PJ, Lavrov IA, Sayenko DG, et al. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017;92(4):544-554. doi: 10.1016/j.mayocp.2017.02.014 [DOI] [PubMed] [Google Scholar]