Key Points
Question
In suspected acute ischemic stroke with large-vessel occlusion, should thrombolysis-capable stroke centers be bypassed in favor of direct transfer to endovascular-capable stroke centers?
Findings
In this theoretical, conditional probability modeling study, the dominant transport strategy depends on the patient’s distance to both centers and treatment speed. If treatment times are slow at the thrombolysis center, bypass should be considered when the centers are 60 minutes or less apart; with greater transport times between centers, bypass is not always favorable.
Meaning
Regional centralization of stroke triage to endovascular therapy centers will increase positive outcomes after ischemic stroke treatment; immediate alteplase treatment followed by transfer to the endovascular center requires fast treatment and is most relevant for longer transport times.
Abstract
Importance
Ischemic stroke with large-vessel occlusion can be treated with alteplase and/or endovascular therapy; however, the administration of each treatment is time sensitive.
Objective
To identify the optimal triage and transport strategy: direct to the endovascular center (mothership) or immediate alteplase treatment followed by transfer to the endovascular center (drip and ship), for all patients with suspected large-vessel occlusion stroke.
Design Setting, and Participants
This was a theoretical, conditional probability modeling study. Existing data from clinical trials of stroke treatment were used for model generation. The study was conducted from February 1, 2017, to March 1, 2018.
Main Outcomes and Measures
The time-dependent efficacy of alteplase and endovascular therapy and the accuracy of large-vessel occlusion screening tools were modeled to estimate the probability of positive outcome (modified Rankin Scale score, 0-1 at 90 days) for both the drip-and-ship and mothership transport strategies. Based from onset to treatment, the strategy that estimates the greatest probability of excellent outcome is determined in several different scenarios.
Results
The patient’s travel time from both thrombolysis and endovascular therapy centers, speed of treatment, and positive predictive value of the screening tool affect whether the drip-and-ship or mothership strategy estimates best outcomes. With optimal treatment times (door-to-needle time: 30 minutes; door-in-door-out time: 50 minutes; door-to-groin-puncture time: 60 minutes [mothership], 30 minutes [drip and ship]), both options estimate similar outcomes when the centers are 60 minutes or less apart. However, with increasing travel time between the 2 centers (90 or 120 minutes), drip and ship is favored if the patient would have to travel past the thrombolysis center to reach the endovascular therapy center or if the patient would arrive outside the alteplase treatment time window in the mothership scenario. Holding other variables constant, if treatment times are slow at the thrombolysis center (door-to-needle time: 60 minutes; door-in-door-out time: 120 minutes), the area where mothership estimates the best outcomes expands, especially when the 2 centers are close together (60 minutes apart or less). The area where mothership estimates the best outcome also expands as the positive predictive value of the screening tool increases.
Conclusions and Relevance
This study suggests that decision making for prehospital transport can be modeled using existing clinical trial data and that these models can be dynamically adapted to changing realities. Based on current median treatment times to realize the full benefit of endovascular therapy on a population level, the study findings suggest that delivery of the treatment should be regionally centralized. The study modeling suggests that transport decision making is context specific and the radius of superiority of the transport strategy changes based on treatment times at both centers, transport times, and the triaging tool used.
This modeling study examines strategies for optimal triage and transport of patients with suspected large-vessel occlusion stroke to the appropriate center to achieve optimal care.
Introduction
Fast treatment of acute ischemic stroke is essential for disability-free survival.1,2 The evolution of time-dependent therapeutics for ischemic stroke refocuses the need to consider how to triage patients with suspected stroke in the field. Endovascular therapy (EVT), a minimally invasive endovascular procedure, is a more effective reperfusion method than intravenous alteplase for ischemic stroke with large-vessel occlusion (LVO).3 The facilities and expertise needed for EVT are typically limited to urban tertiary hospitals. Conversely, intravenous alteplase is widely available and relevant for patients with ischemic stroke with and without LVO. Both treatments are time sensitive and may be given alone or in combination.4,5
Endovascular therapy has resulted in the new problem of identifying patients with probable LVO such that they could be preferentially moved to an EVT center.6,7,8,9,10 Patients who received EVT with long interhospital transfer delays experienced worse outcomes than those without interhospital transfer.11 Neurovascular imaging is the standard to determine EVT eligibility, but high-quality imaging in the field (eg, a mobile stroke unit capable of computed tomographic angiography12) is not available for most patients. Several clinical scores for use by paramedics modeled after the National Institutes of Health Stroke Scale have been developed.13 Three commonly used scales are the Cincinnati Prehospital Stroke Severity Scale (C-STAT), the Rapid Arterial Occlusion Evaluation (RACE), and the Los Angeles Motor Scale (LAMS), each with varying predictive value.14,15,16 We sought to model the best transport strategies for acute stroke, balancing the benefit of early alteplase treatment, the greater efficacy of EVT, and declining benefit of both treatments over time. The study was conducted from February 1, 2017, to March 1, 2018.
Methods
Terminology and Simplifying Assumptions
Hospitals are classified as either thrombolysis or EVT centers. A thrombolysis center can administer intravenous alteplase (with onsite stroke expertise or telemedicine services) but does not provide EVT. An EVT center provides both EVT and intravenous alteplase treatment. In this analysis, 2 different treatment paradigms are discussed: the mothership and drip-and-ship treatment paradigms. We assume that treatments are always available. In the mothership paradigm, the patients are transported directly to an EVT center (potentially bypassing closer thrombolysis centers) and in the drip-and-ship paradigm, patients are first treated with intravenous alteplase at a thrombolysis center and then transferred to an EVT center.
This study is an extension of previously published modeling frameworks (eTable 1 in the Supplement).17,18 We assume that stroke onset time is known and transport decisions are made after emergency medical services evaluation using an LVO screening tool and that the decision does not change en route. We assume that patients with occlusions within the guideline treatment time window and without medical contraindications to thrombolysis are eligible for alteplase and that patients with LVOs are eligible for EVT. Last, because the rate of spontaneous early recovery among patients with LVO is low, we assume that patients estimated to have an LVO achieve reperfusion only with treatment. Because this is a modeling study using previously published data in aggregate, ethics board approval was not required at the University of Calgary, Alberta, Calgary, Canada.
Model Components
This model combines conditional probabilities of excellent outcome constructed from clinical trials of stroke treatment and therefore reflects population averages and applies at the population level. We have approached the problem practically using the probability of achieving excellent outcome (modified Rankin Scale [mRS] 0-1 at 90 days) within a given time from stroke onset to treatment.
Patients with LVO (extracranial or intracranial internal carotid artery, middle cerebral artery-M1 segment, or proximal middle cerebral artery-M2 segment occlusion) will receive both alteplase and EVT either at the EVT center or in a drip-and-ship approach. For EVT, the time-dependent probability of excellent outcome was derived from the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke trials collaboration time to treatment analysis.5 For alteplase, the time-dependent probability of excellent outcome was derived from an individual patient data meta-analysis (Table).4 For mothership transport, time from onset to treatment is the sum of time from onset to medical contact, ambulance response and time spent on the scene, travel to the EVT center, and door-to-needle time or door-to-groin-puncture time at the EVT center (alteplase treatment). For drip and ship, transport time from onset to treatment is the sum of time from onset to medical contact, ambulance response and time spent on the scene, travel to the thrombolysis center, and door-to-needle time at the thrombolysis center (alteplase treatment), time from thrombolysis administration to departure for the EVT center, travel from the thrombolysis center to the EVT center, and door-to-groin-puncture time at the EVT center (EVT treatment) (eFigure 1 in the Supplement). Three different time scenarios were used: scenario A describes an optimized system, scenario B assumes slow treatment at the thrombolysis center, and scenario C assumes slow treatment at both centers (eTable 2 in the Supplement).
Table. Conditional Probability Values and Data Sources.
| Probabilitya | Value | Rationale/Data Source |
|---|---|---|
| Large-Vessel Occlusion | ||
| P(mRS 0-1|EVT and OTT = ×) |
0.3394 + 0.00000004×2 – 0.0002×; minimum value = 0.129 |
The exponential common odds ratio decay presented in the HERMES collaboration time to treatment analysis was used.5 This decay was extrapolated to symptom onset to treatment times less than 120 min. Using P(mRS 0-1|control) as the baseline, this was transformed into a second order polynomial function depicting P(mRS 0-1|EVT) over time. This decay is capped at a minimum probability of excellent outcome of 0.129, which is the P(mRS 0-1|control) in the HERMES data. |
| P(mRS 0 – 1|alteplase and OTT = ×) |
0.2359 + 0.0000002×2 – 0.0004×; minimum value = 0.1328 |
The exponential decay presented in the Emberson et al4 effect of treatment delay meta-analysis was used. This decay was extrapolated to onset to treatment times of <60 min. Because this study includes data from patients with both small and large occlusions, we adjusted this decay using NIHSS as a surrogate for occlusion location to estimate outcomes of alteplase treatment over time in patients with LVO. Using P(mRS 0-1|NIHSS 11+ and control) as the baseline value, this was transformed into a second-order polynomial function depicting P(mRS 0-1|alteplase and LVO) over time. At 4.5 h from onset, the function is set to a minimum value of 0.1328, which is the P(mRS 0-1) given no treatment in the patients with NIHSS 11+ in this meta-analysis.4 |
| Non–Large-Vessel Occlusion | ||
| P(mRS 0 – 1|alteplase and OTT = ×) |
0.6343-0.00000005×2 – 0.0005×; minimum value = 0.4622 |
The exponential decay presented in the Emberson et al4 effect of treatment delay meta-analysis was used. This decay was extrapolated to onset to treatment times of <60 min. Because this study includes data from patients with both small and large occlusions, we adjusted this decay using NIHSS as a surrogate for occlusion location to estimate outcomes of alteplase treatment over time in patients with nLVO. Using P(mRS 0-1|NIHSS 0-10 and control) as the baseline value, this was transformed into a second-order polynomial function depicting P(mRS 0-1|alteplase and nLVO) over time. At 4.5 h from onset, the function is set to a minimum value of 0.4622, which is the P(mRS 0-1) given no treatment in the patients with NIHSS 0-10 in this meta-analysis.4 |
| Intracerebral Hemorrhage | ||
| P(mRS 0-1) | 0.24 | This was generated by combining the overall excellent outcome rate in several trials of intracerebral hemorrhage treatment.19,20,21,22,23 The STICH-II trial in patients with spontaneous ICH of 10-100 mL showed that early surgery had no benefit over conservative treatment.19 The FAST trial showed no difference between recombinant factor VII (at 2 different doses) and placebo There was also no significant interaction found between treatment effect and time from onset to treatment.20 The INTERACT2 trial showed no difference between early intensive BP lowering (SBP <140 mm Hg within 1 h) and guideline-recommended therapy (SBP <180 mm Hg) in the primary outcome (death and disability at 90 d); however, a favorable shift in the overall distribution of mRS scores at 90 d was found.21 The greatest benefit was found in patients who were able to achieve the greatest SBP reductions within 1 h of randomization22; however, randomization occurred a median of 3.7 h after ICH onset. Thus, it remains unknown if this time benefit would persist in the hyperacute window after onset. The INCH trial found no difference between fresh frozen plasma or prothrombin complex concentrate in 90-d clinical outcomes in patients with vitamin K antagonist–related hemorrhages.23 Because none of these trials showed emergency treatment to be superior to standard of care, this probability is considered time invariant. |
| Stroke Mimics | ||
| P(mRS 0-1) | 0.90 | Because most stroke mimics do not have time-dependent treatment options, the probability of excellent outcome for these patients is considered to be time invariant and is set at 0.90 based on the outcomes of stroke-mimic patients in prior studies.24,25,26,27,28 |
Abbreviations: BP, blood pressure; EVT, endovascular therapy; FAST, Factor Seven for Acute Hemorrhagic Stroke; HERMES, Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke; ICH, intracerebral hemorrhage; INCH, International Normalized Ratio Normalization in Coumadin-Associated Intracerebral Hemorrhage; INTERACT2, Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial 2; LVO, large-vessel occlusion; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; nLVO, non–large-vessel occlusion; OTT, onset to treatment time; SBP, systolic BP; STICH, Spontaneous Supratentorial Lobar Intracerebral Haematomas.
In probability notations, P followed by the open and closed parentheses indicates the probability of the statement within the parentheses occurring and the | symbol indicates given in the conditional probability statement.
Because clinical screening is imperfect, patients without LVO (false-positives) will also be identified including (1) ischemic stroke without LVO, (2) intracerebral hemorrhage (ICH), and (3) stroke mimics. Patients with subarachnoid hemorrhage or cerebral venous sinus thrombosis are not considered in this study. Patients with ischemic stroke without LVO within the guideline treatment window will be treated with alteplase. For this, the time-dependent probability of excellent outcome was derived from an individual patient data meta-analysis (Table).4
Patients with ICH may eventually require a higher level of care; however, there is currently indeterminate evidence on the efficacy of emergency medical or surgical treatment.19,20,21,22,23 By combining the excellent outcome rates from several trials of emergency ICH treatment, the probability of excellent outcome for ICH is estimated to be 0.24 and is assumed to be time invariant (Table).19,20,21,22,23 Because most stroke mimics are not immediately life threatening and do not have time-dependent treatment options, the probability of excellent outcome for these patients is considered time invariant (Table).24,25,26,27,28
Patient Diagnoses
Three prehospital LVO screening tools were modeled. The Los Angeles Motor Scale, a 5-point scale in which higher scores indicate ischemic stroke with LVO16; RACE, a 9-point scale in which higher scores indicate ischemic stroke with LVO15; and C-STAT, a 3-item scale, originally developed to detect thrombolysis candidates, where scores of 2 or higher are indicative of LVO.14 In a recent study of 565 consecutive paramedic-initiated code strokes in Melbourne, Australia, these scales were evaluated.29 It was found that LAMS scores of 4 or greater, RACE scores of 5 or greater, and C-STAT scores of 2 or greater had positive predictive values for identifying LVO of 0.4538, 0.5294, and 0.4000, respectively (Henry Zhao, MBBS, email personal correspondence, April 21, 2017). The prevalence of proximal anterior circulation LVO among these patients was 14.5%.29 See the eAppendix in the Supplement for detailed explanation of model components.
Visualizations
Results are visualized using 2-dimensional (2-D) temporospatial diagrams. These diagrams depict a single thrombolysis center in the middle of the figures and a single EVT center at varying transport times below it. Concentric circles representing 5-minute increments of travel time radiate from the thrombolysis center. Color coding is used to represent the transport option with the greatest predicted probability of excellent outcome. Red and green indicate that drip and ship and mothership, respectively, estimate the best probability of excellent outcome. Areas where the 2 options estimate near-equivalent outcomes (probabilities within 0.01 of each other) are indicated using white stippling. Color intensity increases as the probability of achieving excellent outcome increases.
To show geographic context, results in California are also visualized. For the purposes of this illustration we have used data from The Joint Commission Quality Check Stroke Certification program as a surrogate for EVT capability.30 We considered acute stroke-ready and advanced primary stroke centers to be thrombolysis centers and advanced comprehensive stroke centers to be EVT centers. Maps were generated using a desktop application developed for this research (DESTINE; Apple Inc). Esri’s ArcGIS Software Development Kit was used to access a map of California. A 3 × 3-km grid was overlaid on the state and the geographic coordinates of the center of each grid section was passed through Google’s Distance Matrix API (Google Inc) to estimate the ground transport time to each hospital under optimal driving conditions. These travel times were fed into the conditional probability models and the probability of excellent outcome for each strategy in each grid section was calculated. The grid sections were color coded in the same manner as the 2-D temporospatial diagrams.
Results
We modeled the probability of excellent outcome for both the drip-and-ship and mothership transport models, with varying transport times to and between centers (eAppendix and eFigure 1 in the Supplement). This model differs from prior published models.17,18 The earlier models assumed that patients were known to have an acute ischemic stroke with LVO. In this model, patients are suspected to have an LVO based on an LVO screening tool. The treatment options for other possible diagnoses (non-LVO, ICH, and stroke mimics) were also included. eTable 1 in the Supplement provides a comparison of this and prior models.
Several scenarios were created illustrating the association of varying transport times, treatment times, and screening-tool positive predictive values with decision making (eTable 3 in the Supplement). Each scenario was visualized using 2-D temporospatial diagrams. These scenarios (eTable 2 in the Supplement) were also visualized in California.
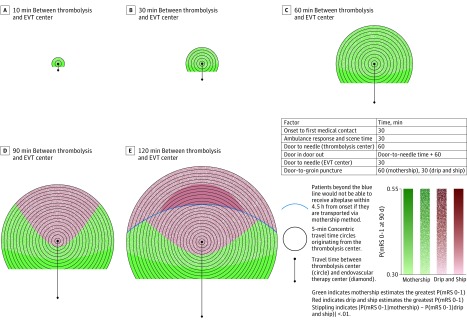
When the patient is closest to the EVT center, mothership always estimates the greatest probability of excellent outcome. Patients with known contraindications to thrombolysis, including those beyond 4.5 hours from onset, should also be transported directly to an EVT center. When the patient is closest to the thrombolysis center, outcomes vary by transport time and treatment efficiency. With optimal treatment times (scenario A, eTable 2 in the Supplement) (door-to-needle time: 30 minutes; door-in-door-out time: 50 minutes; door-to-groin-puncture time: 60 minutes [mothership], 30 minutes [drip and ship]), when the thrombolysis and EVT centers are less than 60 minutes travel time apart, both strategies estimate near-equivalent probabilities of excellent outcome (Figure 1). As the transport time between centers lengthens (90 or 120 minutes), a region where drip and ship clearly outweighs mothership appears; this includes locations close to the thrombolysis center and the narrow corridor in which patients would have to travel past the thrombolysis center en route to the EVT center. This drip-and-ship area expands as the centers are moved further apart and is especially pronounced when the centers are 120 minutes apart. In this instance, there is an area in the temporospatial plane where, if transported by mothership route, the patient foregoes the opportunity for treatment with alteplase under current guidelines as onset to needle time would exceed 4.5 hours.
Figure 1. Transport Decision Making in an Optimally Performing System.
Two-dimensional temporospatial diagrams depicting transport decision making for patients with suspected ischemic stroke with large-vessel occlusion, defined as Los Angeles Motor Scale Score 4 or higher, in an optimally performing system. The diagrams depict a single thrombolysis center in the middle of the Figure, depicted with a circle, and an endovascular therapy center (EVT), depicted by a diamond, at travel times of 10 (A), 30 (B), 60 (C), 90 (D), and 120 (E) minutes below it. There are 5-minute concentric travel time circles radiating from the thrombolysis center. Red indicates areas where drip and ship estimates the greatest probability of excellent outcome and green indicates areas where mothership estimates the greatest probability of excellent outcome. White stippling indicates areas where the optimal transport method supersedes the other areas by 1% or less. Area where the patient is closest to the EVT center is not shown because the mothership option is always best in this scenario. The degree of color saturation reflects the value of the probability of excellent outcome. The blue line represents the point where the onset to needle time in the mothership scenario is more than 270 minutes.
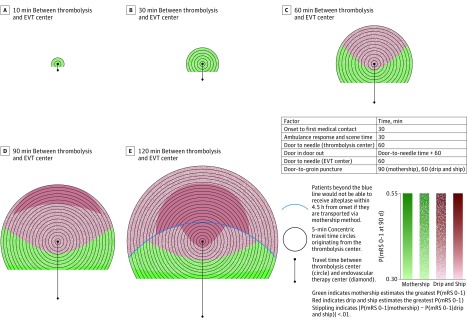
In scenario B (eTable 2 in the Supplement) (door-to-needle time: 60 minutes [thrombolysis center], 30 minutes [EVT center]; door-in-door-out time: 120 minutes; door-to-groin-puncture time 60 minutes [mothership], 30 minutes [drip-and-ship]), the outcome of slowing treatment at thrombolysis centers is shown; drip and ship is no longer associated with the greatest probability of excellent outcome when the travel time between centers is 60 minutes or less (Figure 2). Even as the travel time between centers increases, drip and ship clearly outweighs mothership only when travel time would preclude patients from receiving alteplase in the mothership model.
Figure 2. Transport Decision Making in a System With Slow Treatment Times at the Thrombolysis Center.
Two-dimensional temporal spatial diagrams depicting transport decision making for patients with suspected ischemic stroke with large-vessel occlusion, defined as Los Angeles Motor Scale Score of 4 or higher, in a system with slow treatment times at the thrombolysis center. The diagrams depict a single thrombolysis center in the middle of the Figure, depicted with a circle, and an endovascular therapy (EVT) center, depicted by a diamond, at travel times of 10 (A), 30 (B), 60 (C), 90 (D), and 120 (E) minutes below it. There are 5-minute concentric travel time circles radiating from the thrombolysis center. Red indicates areas where drip and ship estimates the greatest probability of excellent outcome and green indicates areas where mothership estimates the greatest probability of excellent outcome. White stippling indicates areas where the optimal transport method supersedes the other by 1% or less. Area where the patient is closest to the EVT center is not shown because the mothership option is always best in this scenario. The degree of color saturation reflects the value of the probability of excellent outcome. The blue line represents the point where the onset to needle time in the mothership scenario is more than 270 minutes.
In scenario C (eTable 2 in the Supplement) (door-to-needle time: 60 minutes; door-in-door-out time: 120 minutes; door-to-groin-puncture time: 90 minutes [mothership], 60 minutes [drip-and-ship]), we consider slow treatment times at both centers. Here, drip and ship outweighs mothership only when travel time would preclude alteplase administration in the mothership scenario or if the centers are 120 minutes apart and the patient is in the immediate vicinity of the thrombolysis center (Figure 3).
Figure 3. Transport Decision Making in a System With Slow Treatment Times at the Thrombolysis Center and Endovascular Therapy Center.
Two-dimensional temporal spatial diagrams depicting transport decision making for patients with suspected ischemic stroke with large-vessel occlusion, defined as Los Angeles Motor Scale Score of 4 or higher, in a system with slow treatment times at the thrombolysis center and endovascular therapy (EVT) center. The diagrams depict a single thrombolysis center in the middle of the Figure, depicted with a circle, and an EVT center, depicted by a diamond, at travel times of 10 (A), 30 (B), 60 (C), 90 (D), and 120 (E) minutes below it. There are 5-minute concentric travel time circles radiating from the thrombolysis center. Red indicates areas where drip and ship estimates the greatest probability of excellent outcome and green indicates areas where mothership estimates the greatest probability of excellent outcome. White stippling indicates areas where the optimal transport method supersedes the other by 1% or less. Area where the patient is closest to the EVT center is not shown because the mothership option is always best in this scenario. The degree of color saturation reflects the value of the probability of excellent outcome. The blue line represents the point where the onset to needle time in the mothership scenario is more than 270 minutes.
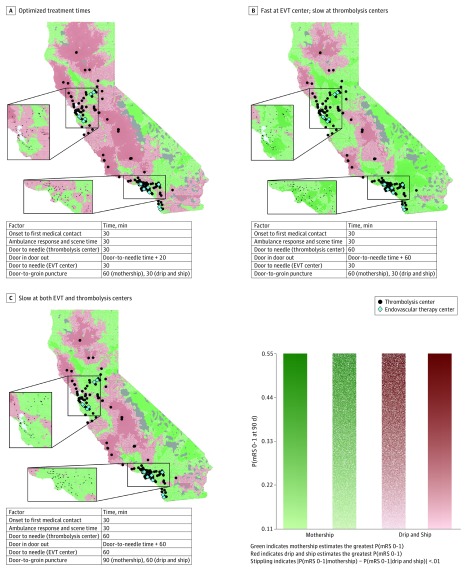
The results for all 3 time scenarios, using the LAMS screening tool, in California are shown in Figure 4 (enlarged maps of Los Angeles and San Francisco in eFigures 2 and 3 in the Supplement). Consistent with the 2-D temporospatial diagrams in an optimal scenario, drip and ship outweighs mothership only when the thrombolysis center is far from EVT centers. In Figure 4A, both strategies estimate equivalent outcomes in the greater Los Angeles area. However, drip and ship is the best option for patients in Bakersfield, which is an approximate 2-hour drive from Los Angeles. In Figure 4B, where treatment at the thrombolysis center is slow, the areas where drip and ship clearly outweigh mothership have shrunk and are now the best option only for patients near Fresno, San Luis Obispo/Santa Maria, Redding, and a portion of Mendocino County. When treatment times are slow at both thrombolysis and EVT centers (Figure 4C), areas where drip and ship clearly outweigh mothership have decreased in size compared with Figure 4A, but remain larger than those in Figure 4B.
Figure 4. Best Estimated Transport Strategy With Probability of Excellent Outcome .
Maps depicting the probability of excellent outcome and best predicted transport strategy for patients with suspected ischemic stroke with large-vessel occlusion, defined as Los Angeles Motor Scale Score of 4 or higher in California. In the maps, thrombolysis centers are depicted by black dots and endovascular therapy (EVT) centers are depicted by blue diamonds. A, System with optimized treatment times. B, System with fast treatment at EVT centers but slow treatment at thrombolysis centers. C, System with slow treatment at both thrombolysis and EVT centers. See the caption to Figure 1 for color guide. Gray areas indicate a lack of road infrastructure data; thus, transport times and optimal transport method could not be determined.
When using RACE scores of 5 or higher (higher positive predictive values than LAMS scores ≥4) to identify patients with probable LVO, a similar pattern of results is obtained. However, the area where mothership estimates the best outcomes enlarges slightly (eFigures 4-6 in the Supplement). Overall, the probability of excellent outcome decreases because a greater proportion of patients with LVO, who have inherently poorer outcomes, is identified. When using C-STAT scores of 2 or higher (lower positive predictive value than LAMS scores ≥4) to identify probable LVO, the drip-and-ship area expands (eFigures 7-9 in the Supplement), and the overall probability of excellent outcome increases as fewer patients with LVO are identified. Overall, the choice of prehospital scale does not substantively change the transport decision, as these scales have similar positive predictive values and the prevalence of LVO is low. The scenarios outlined deal with the complex interaction of several factors. The outcome of varying a single factor on the models is detailed in eTable 4 in the Supplement.
Discussion
We have modeled and visualized a prehospital transport system for patients with acute ischemic stroke with suspected LVO using clinical trial data. The transport time threshold for bypass varies depending on treatment speed at the thrombolysis and EVT centers. This threshold is especially pronounced in scenario B, where door-to-needle time at thrombolysis centers is 60 minutes (door-in-door-out time, 120 minutes). This is the current reality in many stroke systems. Among hospitals in the Get With The Guidelines Target Stroke program, the postintervention median door-to-needle time was 67 minutes (interquartile range, 51-87 minutes).31 Our results imply that, based on current treatment times, delivery should be regionally centralized to realize the full benefit of EVT on a population basis.
Transport decision making is context specific. The radius of superiority for mothership changes based on the relative location of centers to each other and the treatment times at these centers; thus, model inputs need to be customized regionally. This need has potential implications for current accreditation standards and time metrics for quality stroke care. One way to drive change is to accredit centers that cannot meet efficiency targets and are within a short travel time to centers that can at a lower level than those meeting targets and to use such accreditation to guide bypass decisions for emergency medical services. The same considerations on efficient treatment times apply equally to thrombolysis and EVT centers. Population density and distribution, not modeled here, are also important when establishing regional transport and triage policy. Areas where both transport options produce near-equivalent outcomes may be treated differently jurisdiction to jurisdiction owing to economics, staffing, and/or redundancy in resources.
An ongoing randomized clinical trial is addressing the question of transport strategy in Barcelona (RACECAT [Direct Transfer to an Endovascular Center Compared to Transfer to the Closest Stroke Center in Acute Stroke Patients With Suspected Large Vessel Occlusion]).32 Owing to context-specific factors having an association with decision making, the results of RACECAT may not be generalizable to other jurisdictions with different geographic constraints. However, empirical data from RACECAT may be combined with this modeling approach to estimate the ideal strategy in regions where a randomized comparison is not feasible.
The benefits of prehospital screening tools on transport decision making appear to be modest. Given the need to keep things simple in the prehospital environment, the most easily taught of these scales is likely to gain the most traction with emergency medical services. The merits of each tool should be considered when choosing one for implementation. Any intervention that would speed triage and transport in the prehospital environment may change the transport strategy estimated to be most favorable. However, this change would benefit all patients because time from onset to treatment would be shortened.
In taking a population perspective, we have not taken into consideration the political and economic realities that sometimes govern system design. Stroke due to suspected LVO accounts for a minority of the total stroke population. In the Melbourne triaging study, LVO prevalence was only 14.5%. Depending on the screening tool used, anywhere from 18.4% to 21.4% of patients would have screened positive for LVO and therefore be guided by this model.29 The remaining approximately 80% of patients would not be considered potential bypass candidates, and thus would require treatment at thrombolysis centers. Strategies need to be in place for urgent drip-and-ship transport for patients identified to have an LVO at the thrombolysis center but missed by the prehospital screen. Another consideration is the level of ambulance redundancy in each jurisdiction because mothership transport could leave ambulances out of their home region for longer than typical times. Although beyond the scope of this analysis, the potential volume increase at EVT centers, especially regarding patients with false-positive stroke, should be considered when implementing a transport protocol.
Limitations
There are limitations to the model due to assumptions and available data. We have assumed the ICH treatment outcomes are time invariant; however, patients with ICH may require the higher level of care available at EVT centers (eg, neurosurgical teams and neurointensive care units) and that care may not be time invariant. Conversely, although unproven to date, it remains plausible that hyperacute medical treatment (eg, procoagulant drug <120 minutes from onset) could improve outcomes and, as such, patients might benefit from transport to the nearest stroke center. We have assumed that all patients with LVO will be eligible for EVT. However, this may not be the case and, as further data become available on the association between time and EVT eligibility, the models can be updated.
The treatment of EVT patients will evolve33 and changes in technology, treatment paradigms, or the use of mobile stroke units may affect the organization of stroke care. As further data become available, these models can be updated. The temporal spatial diagrams are transport-modality agnostic because they are based on transport time—not distance. However, the map of California was generated using ground transport times, and including air transport could change the results. Ground transport times are dependent on time of day and weather patterns. Thus, for jurisdictional planning, health systems may wish to evaluate several scenarios, including some in nonoptimal driving conditions, before deciding on a transport strategy. We used average times for time from onset to first-medical contact and time on-scene, changing these will influence the model results. Finally, we have defined excellent outcome as an mRS score of 0 to 1 at 90 days; using another definition may affect model results.
Conclusions
Decision making for prehospital transport can be modeled using existing clinical trial data. These models are dynamic and can be adapted to different geographies or changing treatment realities. For ischemic stroke with suspected LVO, regional centralization of care is estimated to result in the best outcomes given current average treatment times.
eAppendix. Supplemental Equations: Breakdown of Model Components
eFigure 1. Diagram Showing Model Components
eFigure 2. Enlarged Maps of the Greater Los Angeles Area
eFigure 3. Enlarged Maps of the Greater San Francisco Area
eFigure 4. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Rapid Arterial Occlusion Evaluation Score >=5 in an Optimally Performing System
eFigure 5. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Rapid Arterial Occlusion Evaluation Score >=5 in a System With Slow Thrombolysis Centres
eFigure 6. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Rapid Arterial Occlusion Evaluation Score >=5 in a Slow System
eFigure 7. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Cincinnati Stroke Triage Assessment Tool >=2, in an Optimally Performing System.
eFigure 8. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Cincinnati Stroke Triage Assessment Tool >=2, in a System With Slow Thrombolysis Centres
eFigure 9. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Cincinnati Stroke Triage Assessment Tool >=2, in a Slow System
eTable 1. Comparison of Prior and Current Models
eTable 2. Time Parameters Used in Analyses
eTable 3. Modelling Scenarios
eTable 4. Impact of Varying Each Model Parameter on Model Results
References
- 1.Menon BK, Sajobi TT, Zhang Y, et al. . Analysis of workflow and time to treatment on thrombectomy outcome in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) randomized, controlled trial. Circulation. 2016;133(23):2279-2286. doi: 10.1161/CIRCULATIONAHA.115.019983 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL. Time is brain—quantified. Stroke. 2006;37(1):263-266. doi: 10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 4.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 6.Southerland AM, Johnston KC, Molina CA, Selim MH, Kamal N, Goyal M. Suspected large vessel occlusion: should emergency medical services transport to the nearest primary stroke center or bypass to a comprehensive stroke center with endovascular capabilities? Stroke. 2016;47(7):1965-1967. doi: 10.1161/STROKEAHA.115.011149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park M-S, Yoon W, Kim J-T, et al. Drip, ship, and on-demand endovascular therapy for acute ischemic stroke. PLoS ONE 2016;11(3):e0150668. doi: 10.1371/journal.pone.0150668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamad NF, Hastrup S, Rasmussen M. Bypassing primary stroke centre reduces delay and improves outcomes for patients with large vessel occlusion. Eur Stroke J. 2016;1(2):85-92. doi: 10.1177/2396987316647857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan LR. Primary stroke centers vs comprehensive stroke centers with interventional capabilities: which is better for a patient with suspected stroke? JAMA Neurol. 2017;74(5):504-506. doi: 10.1001/jamaneurol.2017.0006 [DOI] [PubMed] [Google Scholar]
- 10.Mocco J, Fiorella D, Albuquerque FC. The mission lifeline severity-based stroke treatment algorithm: we need more time. J Neurointerv Surg. 2017;9(5):427-428. doi: 10.1136/neurintsurg-2017-013093 [DOI] [PubMed] [Google Scholar]
- 11.Froehler MT, Saver JL, Zaidat OO, et al. . Interhospital transfer prior to thrombectomy is associated with delayed treatment and worse outcome in the STRATIS Registry. Circulation. 2017;136(24):2311-2321. doi: 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fassbender K, Grotta JC, Walter S, Grunwald IQ, Ragoschke-Schumm A, Saver JL. Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. Lancet Neurol. 2017;16(3):227-237. doi: 10.1016/S1474-4422(17)30008-X [DOI] [PubMed] [Google Scholar]
- 13.Pérez de la Ossa N, Ribó M, Jiménez X, Abilleira S. Prehospital scales to identify patients with large vessel occlusion: it is time for action. Stroke. 2016;47(11):2877-2878. doi: 10.1161/STROKEAHA.116.014911 [DOI] [PubMed] [Google Scholar]
- 14.Katz BS, McMullan JT, Sucharew H, Adeoye O, Broderick JP. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;46(6):1508-1512. doi: 10.1161/STROKEAHA.115.008804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez de la Ossa N, Carrera D, Gorchs M, et al. . Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45(1):87-91. doi: 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 16.Nazliel B, Starkman S, Liebeskind DS, et al. . A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke. 2008;39(8):2264-2267. doi: 10.1161/STROKEAHA.107.508127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holodinsky JK, Williamson TS, Kamal N, Mayank D, Hill MD, Goyal M. Drip and ship versus direct to comprehensive stroke center: conditional probability modeling. Stroke. 2017;48(1):233-238. doi: 10.1161/STROKEAHA.116.014306 [DOI] [PubMed] [Google Scholar]
- 18.Milne MSW, Holodinsky JK, Hill MD, et al. . Drip 'n ship versus mothership for endovascular treatment: modeling the best transportation options for optimal outcomes. Stroke. 2017;48(3):791-794. doi: 10.1161/STROKEAHA.116.015321 [DOI] [PubMed] [Google Scholar]
- 19.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM; STICH II Investigators . Early surgery versus initial conservative treatment in patients with Spontaneous Supratentorial Lobar Intracerebral Haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397-408. doi: 10.1016/S0140-6736(13)60986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer SA, Brun NC, Begtrup K, et al. ; FAST Trial Investigators . Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-2137. doi: 10.1056/NEJMoa0707534 [DOI] [PubMed] [Google Scholar]
- 21.Anderson CS, Huang Y, Arima H, et al. ; INTERACT Investigators . Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41(2):307-312. doi: 10.1161/STROKEAHA.109.561795 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Arima H, Heeley E, et al. ; INTERACT2 Investigators . Magnitude of blood pressure reduction and clinical outcomes in acute intracerebral hemorrhage: intensive blood pressure reduction in acute cerebral hemorrhage trial study. Hypertension. 2015;65(5):1026-1032. doi: 10.1161/HYPERTENSIONAHA.114.05044 [DOI] [PubMed] [Google Scholar]
- 23.Steiner T, Poli S, Griebe M, et al. . Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol. 2016;15(6):566-573. doi: 10.1016/S1474-4422(16)00110-1 [DOI] [PubMed] [Google Scholar]
- 24.Nguyen PL, Chang JJ. Stroke mimics and acute stroke evaluation: clinical differentiation and complications after intravenous tissue plasminogen activator. J Emerg Med. 2015;49(2):244-252. doi: 10.1016/j.jemermed.2014.12.072 [DOI] [PubMed] [Google Scholar]
- 25.Zinkstok SM, Engelter ST, Gensicke H, et al. . Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke. 2013;44(4):1080-1084. doi: 10.1161/STROKEAHA.111.000126 [DOI] [PubMed] [Google Scholar]
- 26.Giraldo EA, Khalid A, Zand R. Safety of intravenous thrombolysis within 4.5 h of symptom onset in patients with negative post-treatment stroke imaging for cerebral infarction. Neurocrit Care. 2011;15(1):76-79. doi: 10.1007/s12028-011-9523-x [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Bogosavljevic V, Leys D, Jovanovic D, Beslac-Bumbasirevic L, Lucas C. Intravenous thrombolytic therapy in patients with stroke mimics: baseline characteristics and safety profile. Eur J Neurol. 2011;18(10):1246-1250. doi: 10.1111/j.1468-1331.2011.03367.x [DOI] [PubMed] [Google Scholar]
- 28.Winkler DT, Fluri F, Fuhr P, et al. . Thrombolysis in stroke mimics: frequency, clinical characteristics, and outcome. Stroke. 2009;40(4):1522-1525. doi: 10.1161/STROKEAHA.108.530352 [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Coote S, Pesavento L, et al. . Large vessel occlusion scales increase delivery to endovascular centers without excessive harm from misclassifications. Stroke. 2017;48(3):568-573. doi: 10.1161/STROKEAHA.116.016056 [DOI] [PubMed] [Google Scholar]
- 30.The Joint Commission Certification for primary stroke centers. https://www.jointcommission.org/certification/primary_stroke_centers.aspx. Accessed August 1, 2018.
- 31.Fonarow GC, Zhao X, Smith EE, et al. . Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311(16):1632-1640. doi: 10.1001/jama.2014.3203 [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov. Direct Transfer to an Endovascular Center Compared to Transfer to the Closest Stroke Center in Acute Stroke Patients With Suspected Large Vessel Occlusion. NCT02795962. https://clinicaltrials.gov/ct2/show/ NCT02795962. Accessed July 14, 2018.
- 33.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 Hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Equations: Breakdown of Model Components
eFigure 1. Diagram Showing Model Components
eFigure 2. Enlarged Maps of the Greater Los Angeles Area
eFigure 3. Enlarged Maps of the Greater San Francisco Area
eFigure 4. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Rapid Arterial Occlusion Evaluation Score >=5 in an Optimally Performing System
eFigure 5. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Rapid Arterial Occlusion Evaluation Score >=5 in a System With Slow Thrombolysis Centres
eFigure 6. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Rapid Arterial Occlusion Evaluation Score >=5 in a Slow System
eFigure 7. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Cincinnati Stroke Triage Assessment Tool >=2, in an Optimally Performing System.
eFigure 8. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Cincinnati Stroke Triage Assessment Tool >=2, in a System With Slow Thrombolysis Centres
eFigure 9. Two Dimensional Temporal Spatial Diagrams Depicting Transport Decision for Patients With Suspected Ischemic Stroke With Large Vessel Occlusion, Defined as Cincinnati Stroke Triage Assessment Tool >=2, in a Slow System
eTable 1. Comparison of Prior and Current Models
eTable 2. Time Parameters Used in Analyses
eTable 3. Modelling Scenarios
eTable 4. Impact of Varying Each Model Parameter on Model Results