Introduction
Gorham disease, described by Gorham and Stout in 1955, is a type of idiopathic osteolysis of bone1. It is a very rare disorder, about 200 cases having been reported so far2. It most commonly affects the shoulder and pelvic girdles, the pelvis and humerus being the commonest sites. Only 24 cases of Gorham disease of the femur have been reported, the most common site of involvement being the proximal femur3, 4, 5, 6. Initial findings are nonspecific and may mimic infective, neoplastic or degenerative disorders, thus a high index of clinical suspicion is required to make the correct diagnosis3, 6. The outcome of treatment of Gorham disease depends on the stage at which it is diagnosed and the response of the disease to treatment. Various treatment modalities, including hip disarticulation or total hip arthroplasty (THA) combined with radiotherapy have been described for treatment of proximal femur osteolysis7, 8, 9, 10. The type of prosthesis used and the outcome of THA depend on the site and amount of bone resorption. We report here a case of idiopathic osteolysis of the proximal femur in a 54‐year‐old man who we treated with radiotherapy and THA. As far as we know, this is the 10th case report of hip arthroplasty in Gorham disease.
Case Report
A 54‐year‐old man presented to our orthopaedic outpatient department with pain in the left hip and inability to weight‐bear following a minor fall. Radiographs revealed an inter‐trochanteric fracture of the left hip. There was no evidence of any pre‐existing disease in the bone or systemic diseases predisposing to a pathological fracture. Open reduction and internal fixation using a dynamic hip screw (DHS) was performed (Fig. 1). The patient was mobilized with partial weight‐bearing after 4 days.
Figure 1.
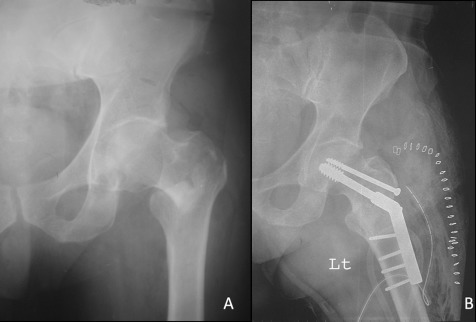
(A) Radiograph showed left intertrochanteric fracture in a 54‐year‐old man after a minor fall. (B) Postoperative radiograph after open reduction and internal fixation of the fracture with a dynamic hip screw and plate.
Sequential radiographs of the hip showed collapse at the fracture site with evidence of bone resorption and no evidence of union. Two months postoperatively, the patient still had pain on weight‐bearing and was unable to perform a straight‐leg‐raise. As bone resorption and osteolysis at the fracture site and the femoral neck progressively worsened, the DHS screw was seen to have cut out superiorly (Fig. 2).
Figure 2.
Radiograph showing bone resorption at the fracture site and superior cut out of the dynamic hip screw.
The DHS was removed via an incision through the previous scar. Blood counts, C‐reactive protein concentration, erythrocyte sedimentation rate and renal function tests were normal. Serum calcium and alkaline phosphatase concentrations were within the normal range. A bone scan showed no significant radioactive tracer uptake in the hip and there was no evidence of any infection. MRI was advised but the patient refused this because of financial constraints. Cultures of intra‐operative specimens were negative. Histopathological examination of the lesion revealed vascularized fibrous tissue between the bony trabeculae. There was endothelial proliferation and capillary formation within the fibrous tissue (Fig. 3). These radiological, biochemical and histological findings were suggestive of Gorham disease. In the meantime, there was gross resorption of the proximal femur involving the neck, head and greater trochanter (Fig. 4).
Figure 3.
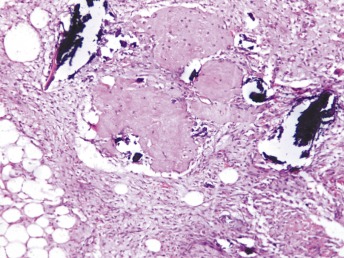
Histopathology of the lesion showing abundant vascularized fibrous tissue with capillary proliferation and intermittent bony trabeculae HE ×40.
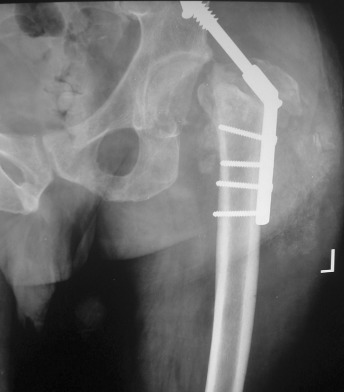
Figure 4.
(A) Radiograph after removal of dynamic hip screw. (B) Six months later, the radiograph shows gross osteolysis in the proximal femur with resorption of neck and calcar; there is also some resorption of the greater trochanter.
The patient received 45 Gy of radiotherapy in two divided doses. The disease process was followed‐up with serial radiographs. There was no further osteolysis. After 18 months radiographs and a CT scan showed massive bone loss with complete resorption of the femoral neck and head sparing a thin shell of articular cartilage and subchondral bone (Fig. 5). The greater trochanter had separated from the proximal femur. The acetabulum, however, remained intact and there was no evidence of any soft tissue extension.
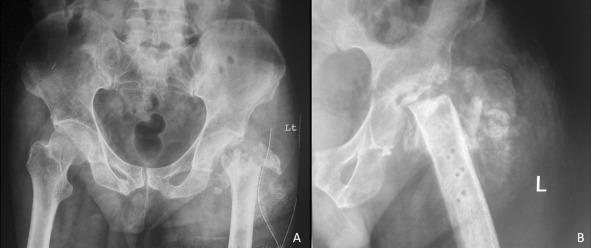
Figure 5.
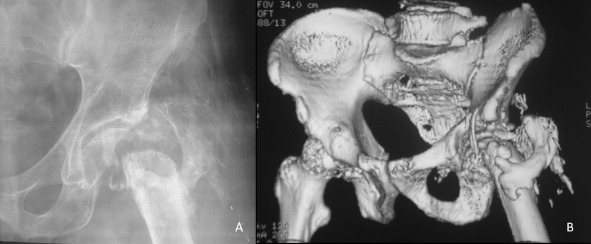
(A) After two doses of radiotherapy and just before surgery, this radiograph shows a few areas of reossification; the fragments of the greater trochanter have united to form a single bone fragment. There is a thin shell of femoral head which remained intact with articular cartilage. (B) Computed tomographic scan of the hip better delineates the proximal femur morphology.
The hip was opened via a lateral approach. The greater trochanter with attached gluteal muscles had separated from the proximal femur and contracted. The overlying soft tissues were fibrosed and contracted. The acetabulum was uninvolved by the disease process. A cemented long stem total hip replacement was performed. The trochanteric fragment was fixed to the proximal femur with tension band wires.
The patient was followed up with clinical examination and radiographs (Fig. 6). At latest follow‐up at 2.5 years, there was no evidence of disease recurrence. The patient had no pain but did have a limp and walked with a cane. There was 100° of hip flexion, 20° of abduction and 2.5 cm of shortening.
Figure 6.
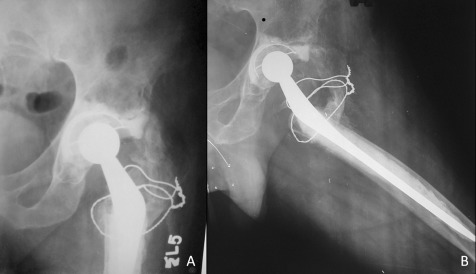
Anteroposterior and lateral radiographs of the left hip 2.5 years after arthroplasty.
Discussion
Gorham disease is a rare, poorly understood disease of unknown cause and pathogenesis. The cells responsible for bone resorption are not known and the role of osteoclasts is controversial1, 5, 6, 11. In our case, there were no osteoclasts at the site of resorption. It has been speculated that endothelial angio‐lymphoblastic tissue causes the osteolysis5, 6. There are no specific laboratory or radiological findings that are diagnostic of Gorham disease. Most blood test results are non‐contributory. Histopathological examination shows growth of vascular and lymphatic tissue with capillary formation and endothelial proliferation in the initial stages and formation of fibrous tissue in the later stages12. The course of the disease is protracted and its diagnosis may be delayed.
Bone pain and pathological fracture are the commonest presenting features in the early stages of the disease3, 4, 5, 6. Treatment of pathological fractures in Gorham disease is challenging. Fractures at the affected site fail to unite and callus is seldom seen because the normal bone architecture has been replaced by angio‐lymphoblastic tissue. What is worse is that the disease is generally not recognized at the time of initial pathological fracture. Characteristically, complications following the initial fracture and massive osteolysis provide clues to the diagnosis6. Increased destruction following fracture may represent a response to the fracture or simply the activity of the disease.
The diagnosis of Gorham disease is essentially one of exclusion. The diagnosis can be made only after a thorough biochemical screen, exclusion of infective, neoplastic and traumatic disorders, and exclusion of other causes of massive osteolysis12. The disease is generally progressive, leading to advanced bone osteolysis. The bone appears to dissolve away giving the disease the name “phantom bone” or “vanishing bone disease”. The natural history of the disease is variable. After several years of bone destruction, the disease may enter a quiescent stage and stabilize with no further bone destruction13. A partial reconsolidation of destroyed bone has also been seen in a few cases. There are only a few reports that describe spontaneous re‐ossification following Gorham's osteolysis13, 14, 15. More commonly, the bony architecture does not reform in the quiescent phase.
Local complications of Gorham disease may increase morbidity and mortality, particularly when it involves the axial skeleton. The disease process may cross anatomical barriers to infiltrate surrounding bones and soft tissue. When this disease involves the axial skeleton, there may be involvement of the pleural cavity with chylous effusion16, 17. Other than through visceral involvement, Gorham disease as such characteristically does not reduce the overall life span.
Various medical treatment modalities have been described. Anti‐resorptive drugs like bisphosphonate and anti‐angiogenic drugs like interferon‐α have shown variable results6, 18. A few reports have documented spontaneous resolution of the disease3, 6. Radiotherapy has had some success in arresting the osteolytic process3, 4, 19, 20. Use of this modality is mainly based on the fact that this disease involves anomalous growth of endothelial tissue and the conjecture that this process may be analogous to neoplasia, in which case radiotherapy might control the growth of the endothelial tissue. Since the course of the disease is poorly understood and non‐responsiveness to radiotherapy has been reported, it is difficult to say whether it does respond to radiotherapy or whether apparent responses merely reflect the natural history of the disease.
Resection of the lesion and reconstruction offer a good chance of remission. Incomplete or marginal resection may cause disease recurrence. Use of bone grafts has not been very successful because of the strong probability of resorption, especially in the active stages of the disease. Due to limitations in the treatment options available and lack of evidence about the efficacy of any particular treatment, radiotherapy followed by excision, with or without further radiotherapy, is the most commonly used treatment approach. In the femur, this involves the need for reconstruction with a prosthesis or cortical strut grafts3, 4, 5, 6.
Whereas radiotherapy is generally recommended early in the course of the disease, surgery should preferably be reserved till the disease is quiescent21. This is important for two reasons. Firstly, it helps the surgeon to determine the optimal extent of resection. Complete excision is essential to reduce the chances of recurrence and possibly achieve a cure. Because trauma possibly exacerbates the disease, surgical trauma may lead to a flare up if the disease is in the active stage. Secondly, though rare, spontaneous re‐ossification does occur. It is very difficult to predict when the disease will become quiescent22.
In the presence of massive osteolysis in the proximal femur, THA is a good option for joint reconstruction. However, the outcome may be less rewarding than that of THA following arthritis and fracture of the neck of the femur. Preoperative radiation therapy may lead to fibrosis of surrounding soft tissues. Bone resorption may be erratic, involving variable parts of the trochanter and calcar. Performing a THA in such circumstances may be challenging. In our case, the greater trochanter was involved and trochanteric fragments had detached along with the abductor muscles. Extensive fibrosis and contractures of affected muscles increased surgical morbidity. The trochan teric fragments were attached to the femur with tension band wires. After two and half‐years follow‐up, the patient was able to walk, though with a limp and the support of a cane.
Early diagnosis and treatment of Gorham disease is essential. Radiotherapy will only result in involution of the angiomatous tissue if the endothelial cells of the proliferating capillary‐like or lymphatic‐like cells are radiosensitive. Surgical intervention is indicated once the disease is inactive. If this disease is diagnosed early, THA is a viable option for joint reconstruction after proximal femur osteolysis. However, because of the characteristically irregular resorption and local soft tissue fibrosis, the outcome of THA may not be rewarding.
Disclosure: No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1. Gorham's LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am, 1955, 37: 985–1004. [PubMed] [Google Scholar]
- 2. Papadakis SA, Khaldi L, Babourda EC, et al Vanishing bone disease: review and case reports. Orthopedics, 2008, 31: 278. [DOI] [PubMed] [Google Scholar]
- 3. Baba AN, Bhat YJ, Paljor SD, et al Gorham's disease of femur. Indian J Orthop, 2011, 45: 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Browne JA, Shives TC, Trousdale RT. Thirty‐year follow‐up of patient with Gorham's disease (massive osteolysis) treated with hip arthroplasty. J Arthroplasty, 2011, 26: 339.e7–e10. [DOI] [PubMed] [Google Scholar]
- 5. Tripathy SK, Sen RK, Agarwal A. Fibular grafting in diaphyseal Gorham's disease: report of two cases and review of literature. Eur J Orthop Surg Traumatol, 2009, 19: 485–490. [Google Scholar]
- 6. Van der Linden‐van der Zwaag H, Onvlee GJ. Massive osteolysis (Gorham's disease) affecting the femur. Acta Orthop Belg, 2006, 72: 261–268. [PubMed] [Google Scholar]
- 7. Möller G, Priemel M, Amling M, et al The Gorham‐Stout syndrome (Gorham's massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br, 1999, 81: 501–506. [DOI] [PubMed] [Google Scholar]
- 8. Shives TC, Beabout JW, Unni KK. Massive osteolysis. Clin Orthop Relat Res, 1993, 294: 267–276. [PubMed] [Google Scholar]
- 9. Poirier H. Massive osteolysis of the humerus treated by resection and prosthetic replacement. J Bone Joint Surg Br, 1968, 50: 158–160. [PubMed] [Google Scholar]
- 10. Stavlas P, Katsiva V, Kouvaras Y. Massive osteolysis of the hip: Gorham's disease. Orthopedics, 2007, 30: 1059–1060. [DOI] [PubMed] [Google Scholar]
- 11. Spieth ME, Greenspan A, Forrester DM, et al Gorham's disease of the radius: radiographic, scintigraphic, and MRI findings with pathologic correlation. A case report and review of the literature. Skeletal Radiol, 1997, 26: 659–663. [DOI] [PubMed] [Google Scholar]
- 12. Heffez L, Doku HC, Carter BL, et al Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol, 1983, 55: 331–343. [DOI] [PubMed] [Google Scholar]
- 13. Choma ND, Biscotti CV, Bauer TW, et al Gorham's syndrome: a case report and review of the literature. Am J Med, 1987, 83: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 14. Chattopadhyay P, Bandyopadhyay A, Das S, et al Gorham's disease with spontaneous recovery. Singapore Med J, 2009, 50: e259–e263. [PubMed] [Google Scholar]
- 15. Campbell J, Almond HG, Johnson R. Massive osteolysis of the humerus with spontaneous recovery. J Bone Joint Surg Br, 1975, 57: 238–240. [PubMed] [Google Scholar]
- 16. Chavanis N, Chaffanjon P, Frey G, et al Chylothorax complicating Gorham's disease. Ann Thorac Surg, 2001, 72: 937–939. [DOI] [PubMed] [Google Scholar]
- 17. Bode‐Lesniewska B, von Hochstetter A, Exner GU, et al Gorham‐Stout disease of the shoulder girdle and cervico‐thoracic spine: fatal course in a 65‐year‐old woman. Skeletal Radiol, 2002, 31: 724–729. [DOI] [PubMed] [Google Scholar]
- 18. Lehmann G, Pfeil A, Böttcher J, et al Benefit of 17‐year long‐term bisphosphonate therapy in a patient with Gorham‐Stout syndrome. Arch Orthop Trauma Surg, 2009, 129: 967–972. [DOI] [PubMed] [Google Scholar]
- 19. Handl‐Zeller L, Hohenberg G. Radiotherapy of Morbus Gorham‐Stout: the biological value of low irradiation dose. Br J Radiol, 1990, 63: 206–208. [DOI] [PubMed] [Google Scholar]
- 20. Dunbar SF, Rosenberg A, Mankin H, et al Gorham's massive osteolysis: the role of radiation therapy and a review of the literature. Int J Radiat Oncol Biol Phys, 1993, 26: 491–497. [DOI] [PubMed] [Google Scholar]
- 21. Aizawa T, Sato T, Kokubun S. Gorham disease of the spine: a case report and treatment strategies for this enigmatic bone disease. Tohoku J Exp Med, 2005, 205: 187–196. [DOI] [PubMed] [Google Scholar]
- 22. Tripathy SK, Sen RK, Goyal T. Gorham's disease of femur—A response. Indian J Orthop, 2012, 46: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]


