Abstract
Objective
To determine the rate of curing the infection and mid‐ to long‐term outcomes of using extensively coated non‐modular stems in two‐stage revision for infected total hip arthroplasty (THA).
Methods
The clinical data of 33 patients (33 hips) in whom extensively coated non‐modular stems had been used in two‐stage revision THA for deep infection were retrospectively analyzed. All operations received two‐stage reimplantation, which included resection arthroplasty, thorough debridement, insertion of a hand‐molded antibiotic‐impregnated cement spacer with stainless steel reinforcement, a course of intravenous antibiotics, and delayed reimplantation. Microorganism‐specific antibiotics had been chosen according to the results of microbiological studies performed postoperatively. All patients received i.v. antimicrobial therapy for 4 weeks and oral antibiotics to which their organisms were sensitive for a further 6 weeks. Harris hip score (HHS) and plain X‐ray films were used to perform clinical and radiological evaluations.
Results
During follow‐up for a minimum of 5 years, no reinfection or loosening were found. Cultures of samples taken during the second stage were all negative for infection. The mean HHS improved from 42 preoperative to 89 at the final follow‐up. All granular bones had fused well with the host bones by 12 months after the surgery.
Conclusion
Using extensively coated non‐modular stems combined with intramedullary allografts in two‐stage revision for treating infected THAs can achieve satisfactory outcomes.
Keywords: Infection, Total hip arthroplasty, Two‐stage revision
Introduction
Though the incidence of infection in primary total hip arthroplasty (THA) is extremely low (0.4%–1.0%), it remains a nightmare for joint surgeons1, 2. Because the reported cure rate ranges from 85% to 95%, two‐stage revisions that use a prosthesis with antibiotic bone cement were once accepted as the gold standard for treating infected THAs3, 4, 5, 6.
Cemented fixation, which permits the use of antibiotics, has classically been used in reimplantation of femoral components because it reduces the risk of reinfection4. However, several recent reports have suggested that cemented two‐staged revision has a relatively high mechanical failure rate (25%–33%)6, 7. Many series with mid‐ and long‐term follow‐ups have reported a high incidence of loosening and failure of cement stems used in THA revisions. The main reason is that thinning and sclerosis of the interface between bone and premier prosthesis prevents formation of micro‐lock at that interface6. For these reasons, many surgeons prefer to use uncemented stems for revision. Promising results have been reported with two‐stage cementless revisions, the eradication rates being between 82% and 100% (Table 1).
Table 1.
Results of two‐stage uncemented revision of periprosthetic infection of the hip
| Author | Cases | Follow‐up | Spacer/beads | Local antibiotic | Duration of antibiotics | Interval | Antibiotics after implantation | Implants | Eradication (%) | Aseptic loosening |
|---|---|---|---|---|---|---|---|---|---|---|
| Fehring 15 | 25 | 41 m | Beads | Tobramycin | 6 w | 4.8 m | Nm stem modular | 92 | 0 | |
| Haddad 16 | 50 | 5.8 yrs | Beads cement ball | Gentamicin | iv for 5 d and then oral | 3 w | ≥3 m | Nm stem | 92 | 8% stem |
| Koo 17 | 22 | 41 m | Spacer beads | Vancomycin, Gentamicin, Cefotaxime | 6 w | 6–12 w | Nm stem | 95 |
5% cup 30% stem |
|
| Masri 27 | 29 | ≥2 yrs | PROSTALAC spacer |
Tobramycin Vancomycin Cefuroxime Penicillin |
6 w | 12 w | iv for 5 d | Nm stem | 90 | 0% |
| Fink 29 | 36 | 24 m | Manual spacer |
Vancomycin, Gentamicin Clindamycin |
iv for 2 w oral for 4 w | 6 w | Modular stem | 100 | 0% | |
| Kraay 30 | 33 | ≥24 m | Spacer in 16 cases | Tobramycin | ≥6 w iv | 7.4 m | Nm stem modular | 92 | 9% cup 0% stem | |
| Nestor 31 | 34 | 47 m | Resection | No | ≥4 w | 8 m | Different | Nm stem | 82 | 18% |
| Romanò 32 | 40 | 2 yrs | Spacer | Vancomycin, Gentamicin | 4–6 w | 9–16 m | Modular | 97.5 | 2.5% |
Nm, non‐modular; w, weeks; d, days; m, months.
It is difficult to achieve “fit and fill” because the diameters of the medullary canal of the metaphysis and diaphysis bear little relationship to each other. This problem can be overcome by using a modular stem or by having a very large variety of monolithic stems. A modular stem allows independent metaphyseal/diaphyseal sizing, stem‐to‐neck length options, and adjustable offset and version. Modularity can also be advantageous in helping to offset leg length in situations where there are major discrepancies8. However, numerous potential concerns about all modular stems have been raised, including corrosion at the taper causing lysis, fear of breakage and taper disengagement9, 10, 11. Extensively porous coated revision stems are designed to bypass the regions of proximally deficient bone and achieve stability and fixation in more distal femoral bone. Such stems have produced encouraging results12, 13. Many published reports have described that good mid‐ and long‐term results after using uncemented prostheses in two‐stage revisions for treating infected THAs7, 14, 15, 16, 17. However, both modular and non‐modular prosthesis are mentioned in these reports. Favorable features of extensively coated non‐modular stems include that they are easy to use, have a high friction coefficient and achieve stable initial fixation. Long‐term evaluation has shown that single prostheses are not very effective.
The purpose of the present retrospective study was to determine the rate of cure of infection and mid‐ to long‐term outcomes (minimum 5‐year follow‐up) of extensively coated non‐modular stems used in two‐stage revision for infected THAs.
Materials and Methods
General Data
Thirty‐three patients (20 men and 13 women) with 33 infected THAs who had been treated surgically from March 2005 to December 2006 were retrospectively reviewed. All patients had been subjected to a two‐stage reimplantation protocol for deep chronic regional infection after THA. The underlying diagnoses leading to the index THA were osteoarthritis in 16 hips, avascular necrosis of the femoral head in 12 hips, rheumatoid arthritis in 4 hips and femoral neck fracture in one hip. Of the 33 patients, 19 had originally undergone fixation without cement, 13 patients fixation with cement and one hip was hybrid. Eighteen of the 33 patients (54.5%) had specific comorbidities including diabetes mellitus, hypertension and chronic obstructive pulmonary disease. The median age at the time of initial surgical treatment was 65 years (range, 52–79 years), and the mean follow‐up was 6 years (range, 5–8 years). The period of time from the initial, clean implantation to the diagnosis of infection was 12–42 months, the average being 18 months. The most sources for the infection in our study may be hematogenous spread (dental infection, endoscopy) or contamination at the time of surgery.
All operations had been performed by a single surgeon according to a two‐stage reimplantation protocol, which included resection arthroplasty, thorough debridement, insertion of a hand‐molded antibiotic‐impregnated cement spacer with stainless steel reinforcement, a course of intravenous antibiotics, and delayed reimplantation.
First‐stage Procedure
The first‐stage procedure consisted of complete removal of all foreign materials involving the prosthesis and cement, the fibrous membrane, sinus tracts, and all devitalized bone and soft tissues. This was performed via a posterolateral approach. The infected prosthetic stem was removed by a transfemoral approach in 17 patients. When removing solidly fixed cemented, cementless femoral stems or cement mantles, extended trochanteric osteotomy was performed in 9 hips and cortical windowing of the femoral diaphysis in 8 hips. Sinus tracts were present in 15 hips; these were completely excised using methylene blue. Postoperatively, the patients were encouraged to walk with toe‐touch down weight bearing.
Cup‐shaped acetabulum spacers had been used to separate the antibiotic‐loaded cement from the prostheses. For the stem components, the spacers were created by placing antibiotic‐loaded cement around stainless steel in the shape of the medullary cavity. The antibiotics used were a combination of 2 g of vancomycin and 1 g of aminoglycoside per package (40 g) of cement.
During resection arthroplasty, samples for microbiological and histopathological studies were always obtained from joint fluid and tissues suspected to be infected. Deep periprosthetic infection was diagnosed if two or more cultures of intraoperative specimens yielded the same microorganism, there was frank purulence surrounding the prosthesis at the time of resection, there was evidence of acute inflammation on intraoperative histopathologic examination, or there was a sinus tract communicating with the prosthesis. Diagnoses of deep periprosthetic infection were based on positive cultures from operative specimens in 12 hips, intraoperative pathology in 23 hips and presence of sinus tracts in 15 hips (more than one of these characteristics was present in some cases). Of the 21 hips where cultures of operative specimens were negative, the diagnosis of infection was based on positive intraoperative pathology in 11 hips, frank purulence in 6 hips (with positive pathology) and sinus tracts in 4 hips. Causative infective organisms included Staphylococcus aureus in eight hips, Staphylococcus epidermidis in three hips and streptococcus in one hip.
Microorganism‐specific antibiotics were chosen according to the results of microbiological studies performed postoperatively. All patients received i.v. antimicrobial therapy for 4 weeks and oral antibiotics (chosen according to known sensitivities of infecting microorganisms) for 6 weeks after the course of i.v. antibiotics. Patients in whom no causative organism had been identified by culture received i.v. vancomycin and cefuroxime for 4 weeks and oral rifampin for a further 6 weeks.
Second‐stage Procedure
Second‐stage reimplantation procedures were considered only after the following criteria had been fulfilled; (i) persistent normalization of C‐reactive protein concentration; (ii) persistent normalization of blood leukocyte count; and (iii) absence of clinical symptoms of infection for at least 2 weeks after discontinuation of antimicrobial therapy. Because the erythrocyte sedimentation rate has shown to be high following infection, this variable was not considered when making reimplantation decisions. Aspiration before reimplantation was not routinely performed because many patients refused aspiration and false‐negative cultures can occur. During the second stage of the two‐stage reimplantation procedure, samples for microbiological and histopathological studies were always taken from joint fluid and deep tissues before administration of any further antibiotics. When any evidence of infection was detected histologically and clinically, the first‐stage procedure was repeated and the second‐stage procedure postponed. The mean time from first‐stage resection arthroplasty to reimplantation was 20 weeks (range, 12–36 weeks).
Reimplantation was performed without extended trochanteric osteotomy via a posterolateral approach in 24 patients. In the nine patients whose implants were removed via a transfemoral approach, extended trochanteric osteotomy was opened to remove the spacer. Early healing of the osteotomy was observed in all patients. Uncemented press‐fit acetabular cups were used in the revisions and screws were used to assist fixation in 26 hips. Extensively coated non‐modular stems (anatomic medullary locking) were used on the femoral side and impaction bone grafting technique in 11 hips on the acetabular side. Cortical bone grafting was used in 10 hips and intramedullary impaction bone grafting on all femoral sides. Postoperatively, the patients were allowed to walk with the aid of two crutches for 3 months.
Evaluation Index
Treatment was considered successful in patients whose reimplanted prostheses remained infection‐free state at final follow‐up. Treatment was considered a failure in patients who (i) required permanent resection arthroplasty because of persistent infection after the first‐stage procedure; (ii) had recurrent periprosthetic infection resulting from the original or a different microorganism after second‐stage reimplantation; or (iii) required reoperation for mechanical loosening of the reimplanted prostheses. Outcomes were determined at last documented visits or when clinical failure occurred. Serial radiographs were examined for component stability, migration and loosening. The Harris hip score (HHS) was used to assess functional outcome in all 33 patients; there enough data to calculate this score in all cases. Reinfection was defined by the same criteria as were used to diagnose the index infection.
Assessment of radiological evidence of loosening after reconstruction was performed by one of the authors (Z‐k.Z.) who was blinded for this purpose. Anterior‐posterior radiographs of the pelvis and lateral radiographs of the hip were evaluated at every follow‐up visit. Acetabular bone defects were assessed according to the classification of D′Antonio et al.18 and the stem according to that of Pak et al.19 (Table 2). In the area of the acetabular component, radiolucency was classified according to the criteria of DeLee and Charnley20, and in that of the stem those of Gruen et al.21. Migration of the implants was assessed according to the criteria of Nunn et al.22 and Wetherell et al.23. After correction for the magnification factor and pelvic rotation, horizontal and vertical cup migrations were determined. Any deviation greater than the accuracy of the measurement technique (determined to be 2 mm) was defined as a definite migration. Definite radiographic loosening of the cup or the stem was defined as migration of 5 mm. Fixation of the femoral stem was assessed radiographically using the criteria ofEngh et al.24 (bone‐ingrowth fixation, stable fibrous fixation, unstable fixation).
Table 2.
Distribution of the bone defects (hips)
Statistical Analysis
Statistical analysis was performed with SPSS 16.0 for Windows. The means, standard deviations, medians, and minimum and maximum were determined. Both the time course of all patients' variables and differences between the study groups at each follow‐up were calculated. A level of significance of P ≤ 0.05 was specified for all statistical test methods.
Results
At a minimum of 5 years of follow‐up (range, 5–8 years) of 33 patients who had been treated by two‐stage revision for infected THAs, no reinfection and loosening were found. Cultures of samples (three samples/patient) taken during the second stage were all negative for infection. The mean HHS improved from 42 preoperatively to 89 at the final follow‐up. At final follow‐up, two patients had dull pain when they moved and three patients had mild limps. According to the system of DeLee and Charnley20, a radiolucent line adjacent to the acetabular component was seen in zone 1 on three hips and in zone 2 on four hips; no radiolucent lines were seen in zone 3. All radiolucent lines were less than 2 mm and there was no evidence of progressive widening. The range of motion was well improved at the final follow‐up. Leg‐length discrepancy more than 2 cm was found in 3 patients.
According to the method described by Engh et al.24, 30 patients achieved stable bone ingrowth and the remaining three had stable fibrous ingrowth. Allogeneic cortical bone plate grafts were used in 10 hips on the femoral side. Twelve months after revision, nine of these cortical bone plates had fused with the host bones (Figs 1,2), whereas one remained unfused, but was found to have fused by the 24 month follow‐up. Impaction bone allografts were used in 11 hips for acetabular bone deficiency and in all femoral canals. All morselized bone allografts had fused well with the host bones 12 months postoperatively.
Figure 1.
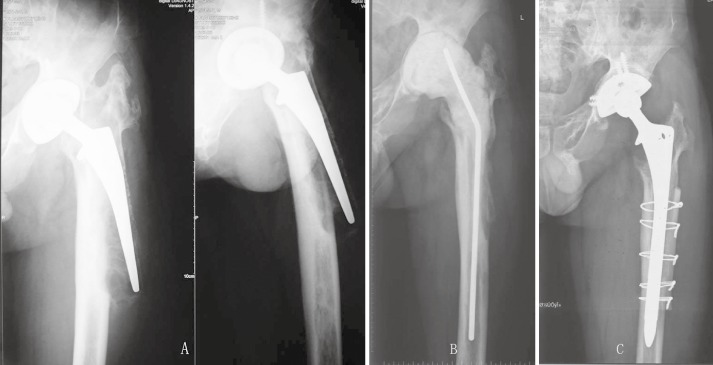
A 39‐year‐old man 7 years after THA complicated by infection with femoral bone defect Paprosky IIIa.
(A) X‐ray films before revision.
(B) X‐ray film 1 month after removal of component and placement of an antibiotic‐impregnated cement spacer.
(C) X‐ray film showing a well‐fixed extensively coated non‐modular stem 5 years after reimplantation.
Figure 2.
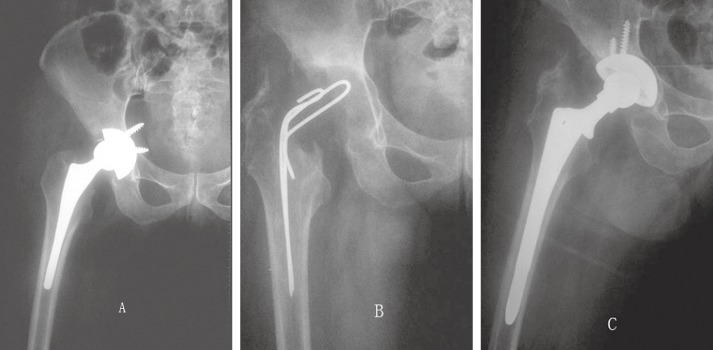
A 54‐year‐old man 10 years after THA complicated by infection.
(A) X‐ray films before revision.
(B) X‐ray film 2 months after removal of component and placement of an antibiotic‐impregnated cement spacer.
(C) X‐ray film showing a well‐fixed extensively coated non‐modular stem 5 years after reimplantation.
Discussion
In the present investigation, two‐stage surgery using extensively coated non‐modular stem was chosen for treating patients with infectious hip arthroplasties. We achieved equivalent or even better clinical and radiological outcomes than studies reporting similar procedures with modular stems.
This study has a number of limitations. The sample size was small and the rate of positive cultures low (12/33). We believe there were two reasons for the latter. First, bacteria that cause periprosthetic infection usually occur in very small numbers in the form of a biofilm, which means they are often sessile, and are characterized by a slow rate of reproduction25, 26. Second, bacterial cultures of specimens from patients who have been managed with oral or parenteral antibiotics before the diagnostic aspiration is performed are frequently negative. The reported rates of negative culture of preoperative aspirates range from 7% to 50%. The phenomenon of abuse of antibiotics is very common. We believe that rigorous removal of all foreign material and radical debridement of inflamed and necrotic tissues are essential for the success of any form of septic prosthesis revision27, 28.
During the last three decades, cementless revision stems have become an appealing option for the arthroplasty surgeon treating patients with deficient femurs. These stems are suitable for use with various types of bone deficiency and are compatible with extended trochanteric osteotomies. Modular stems offer the advantage of adjustment and restoration of joint kinematics including leg length, version and offset, regardless of the exact position of the distal part of the stem. However, modular prostheses are costly and concerns about the potential complications of fretting and fracture at the modular junction have been raised. Lakstein et al. reported six patients with fractures at the mid‐stem junctions of modular revision hip implants in a database of patients who had undergone revision arthroplasty11. Risk factors for fractures of the modular junction include excessive body weight, inadequate proximal osseous support consequent to trochanteric osteotomy, reduced preoperative bone stock, osteolysis, loosening and/or undersized implants. Barrack believes that modular stems introduce increased complexity, cost and potential complications and are unnecessary in revision total hip arthroplasty3. Using modular cementless prostheses, Fink et al. achieved stable bone‐ingrowth fixation in 94% of their patients and two subsidences. In our study we used non‐modular stems: there was no reinfection, loosening or subsidence29. Thus, using extensively coated non‐modular stems in two‐stage revision for treating infected THAs can achieve satisfactory outcomes.
The survival rate of cementless implants in aseptic hip revisions is believed by some to be higher than that of cemented implants10, 12, 30, 31. A few reports have described the stability of cementless fixation after septic revision using mostly non‐modular implants. Hofmann et al. achieved stable bone‐ingrowth fixation in 96% of their cases using non‐modular cementless prostheses32. Fehring et al. achieved stable bone‐ingrowth fixation in 96% of their cases using non‐modular and modular cementless prostheses with proximal fixation15. Masri et al. achieve 95% bone‐ingrowth fixation after more than two years using cementless non‐modular stems. The rate of early loosening of cementless revisions stems reportedly varies from 0% to 18%27. Sanchez‐Sotelo et al. ascribed the relatively high rate of mechanical failure to the large proportion of proximal fixation uncemented femoral stems used in their study33. It has been suggested that using cementless components designed for distal fixation may decrease the rates of mechanical failure after reimplantation. In our opinion, the low rates of subsidence (0%), loosening (0%) and high rate of bone‐ingrowth fixation (91%) of the cementless non‐modular revision stem system we used are attributable to the distal fixation procedures in viable bone.
Our data lend support to the contention that two‐stage cementless revisions of infected total hip arthroplasties using non‐modular stem combined with local and systemic antibiotic therapy regimens lead to high rates of eradication of infection comparable to the rates achieved by two‐stage cemented revisions with antibiotic‐loaded cement. We believe four factors contribute to the success of our procedure. First, we believe rigorous removal of all foreign material and radical debridement of inflamed and necrotic tissues are essential for the success of any form of septic prosthesis revision26, 27, 28. Second, we knew the nature of the infecting microorganism and its antibiotic susceptibility in many of our cases. Third, we administered specific systemic therapy with antibiotics of high bioavailability to which the bacteria were likely highly sensitive (or definitely sensitive in patients from whom positive cultures had been obtained) coupled with high doses of vancomycin and gentamicin as local antibiotics on a regular basis. Although the rate of positive cultures was low, vancomycin and gentamicin have a broad spectrum of activity15, 30. Four, the treatment regime is effective. The 4‐week duration of parenteral antibiotics we used seems short. However, it is consistent with the recommendations of Nestor et al.31 and Romanò et al.34. Also, the total duration of antibiotic treatment of 3 months in our patients is consistent with the recommendations of Zimmerli35 and Trampuz and Zimmerli36. The spacer period we used is also short, but has been used by Fehring et al.15 and Nestor et al.31. Moreover, the 100% rate of eradication suggests our protocol is adequate.
It is widely accepted that two‐stage revision surgery further depletes bone stock. In the face of this bone loss, allografts are frequently necessary to help with final reconstruction. The use of allograft bone in total hip revision for septic failure remains controversial37. Because allografts act as potential sequestra, they may lead to a high rate of recurrence of infection in infected two‐stage revisions. Previous studies have examined the use of allografts in the final reconstructions after infection, and there does not appear to be an increase in reinfection rate. Berry et al. used various combinations of morselized and bulk allografts in the second stage of revision for infection, and reported only two recurrent infections in 11 patients at a mean follow‐up of 4.2 years38. Alexeeff et al. used massive structural allografts in the second stage of a two‐stage procedure in 11 patients. They reported no further sepsis at a mean follow‐up of four years39. We used morselized allografts from a femoral head for acetabular reconstruction in 11 patients. We used cortical bone grafting in 10 hips and intramedullary impaction bone grafting on all femoral sides, none of which developed subsequent reinfection. We conclude that, provided the infection is well controlled, two‐stage revision with uncemented prostheses allows the use of allografts and is safe and effective.
With good control of the infection during the first‐stage, using extensively coated non‐modular stem combined with allograft in two‐stage revision for treating infected THAs can achieve satisfactory outcomes.
Acknowledgments
We thank Ming‐yao Sun for identifying relevant published reports to cite.
Disclosure: The authors have no conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript. This research was funded by the China Health Ministry Program (201302007).
References
- 1. Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect, 1999, 48: 111–122. [PubMed] [Google Scholar]
- 2. Hanssen AD, Spangehl MJ. Treatment of the infected hip replacement. Clin Orthop Relat Res, 2004, 420: 63–71. [DOI] [PubMed] [Google Scholar]
- 3. Barrack RL. Rush pin technique for temporary antibiotic‐impregnated cement prosthesis for infected total hip arthroplasty. J Arthroplasty, 2002, 17: 600–603. [DOI] [PubMed] [Google Scholar]
- 4. Duncan CP, Masri BA. The role of antibiotic‐loaded cement in the treatment of an infection after a hip replacement. Instr Course Lect, 1995, 44: 305–313. [PubMed] [Google Scholar]
- 5. Durbhakula SM, Czajka J, Fuchs MD, Uhl R. Spacer endoprosthesis for the treatment of infected total hip arthroplasty. J Arthroplasty, 2004, 19: 760–767. [DOI] [PubMed] [Google Scholar]
- 6. Davis CM 3rd, Berry DJ, Harmsen WS. Cemented revision of failed uncemented femoral components of total hip arthroplasty. J Bone Joint Surg Am, 2003, 85: 1264–1269. [DOI] [PubMed] [Google Scholar]
- 7. Strömberg CN, Herberts P, Palmertz B. Cemented revision hip arthroplasty: a multicenter 5–9‐year study of 204 first revisions for loosening. Acta Orthop Scand, 1992, 63: 111–119. [DOI] [PubMed] [Google Scholar]
- 8. Sporer SM, Paprosky WG. Femoral fixation in the face of considerable bone loss: the use of modular stems. Clin Orthop Relat Res, 2004, 429: 227–231. [DOI] [PubMed] [Google Scholar]
- 9. Bobyn JD, Tanzer M, Krygier JJ, Dujovne AR, Brooks CE. Concerns with modularity in total hip arthroplasty. Clin Orthop Relat Res, 1994, 298: 27–36. [PubMed] [Google Scholar]
- 10. Brown SA, Flemming CA, Kawalec JS, et al Fretting corrosion accelerates crevice corrosion of modular hip tapers. J Appl Biomater, 1995, 6: 19–26. [DOI] [PubMed] [Google Scholar]
- 11. Lakstein D, Eliaz N, Levi O, et al Fracture of cementless femoral stems at the mid‐stem junction in modular revision hip arthroplasty systems. J Bone Joint Surg Am, 2011, 93: 57–65. [DOI] [PubMed] [Google Scholar]
- 12. Krishnamurthy AB, MacDonald SJ, Paprosky WG. 5‐ to 13‐year follow‐up study on cementless femoral components in revision surgery. J Arthroplasty, 1997, 12: 839–847. [DOI] [PubMed] [Google Scholar]
- 13. Moreland JR, Bernstein ML. Femoral revision hip arthroplasty with uncemented, porous‐coated stems. Clin Orthop Relat Res, 1995, 319: 141–150. [PubMed] [Google Scholar]
- 14. Dohmae Y, Bechtold JE, Sherman RE, Puno RM, Gustilo RB. Reduction in cement‐bone interface shear strength between primary and revision arthroplasty. Clin Orthop Relat Res, 1988, 236: 214–220. [PubMed] [Google Scholar]
- 15. Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2‐stage reimplantation for periprosthetic sepsis. J Arthroplasty, 1999, 14: 175–181. [DOI] [PubMed] [Google Scholar]
- 16. Haddad FS, Muirhead‐Allwood SK, Manktelow AR, Bacarese‐Hamilton I. Two‐stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br, 2000, 82: 689–694. [DOI] [PubMed] [Google Scholar]
- 17. Koo KH, Yang JW, Cho SH, et al Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two‐stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty, 2001, 16: 882–892. [DOI] [PubMed] [Google Scholar]
- 18. D'Antonio JA, Capello WN, Borden LS, et al Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res, 1989, 243: 126–137. [PubMed] [Google Scholar]
- 19. Pak JH, Paprosky WG, Jablonsky WS, Lawrence JM. Femoral strut allografts in cementless revision total hip arthroplasty. Clin Orthop Relat Res, 1993, 295: 172–178. [PubMed] [Google Scholar]
- 20. DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res, 1976, 121: 20–32. [PubMed] [Google Scholar]
- 21. Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem‐type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res, 1979, 141: 17–27. [PubMed] [Google Scholar]
- 22. Nunn D, Freeman MA, Hill PF, Evans SJ. The measurement of migration of the acetabular component of hip prostheses. J Bone Joint Surg Br, 1989, 71: 629–631. [DOI] [PubMed] [Google Scholar]
- 23. Wetherell RG, Amis AA, Heatley FW. Measurment of acetabular erosion. The effect of pelvic rotation on common landmarks. J Bone Joint Surg Br, 1989, 71: 447–451. [DOI] [PubMed] [Google Scholar]
- 24. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long‐term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res, 2001, 393: 137–146. [DOI] [PubMed] [Google Scholar]
- 25. Costerton JW. Biofilm theory can guide the treatment of device‐related orthopaedic infections. Clin Orthop Relat Res, 2005, 437: 7–11. [DOI] [PubMed] [Google Scholar]
- 26. Gallo J, Kolář M, Novotný R, Riháková P, Tichá V. Pathogenesis of prosthesis‐related infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 2003, 147: 27–35. [DOI] [PubMed] [Google Scholar]
- 27. Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP. Cementless two‐stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty, 2007, 22: 72–78. [DOI] [PubMed] [Google Scholar]
- 28. Steinbrink K, Frommelt L. Treatment of periprosthetic infection of the hip using one‐stage exchange surgery. Orthopade, 1995, 24: 335–343. [PubMed] [Google Scholar]
- 29. Fink B, Grossmann A, Fuerst M, Schäfer P, Frommelt L. Two‐stage cementless revision of infected hip endoprostheses. Clin Orthop Relat Res, 2009, 467: 1848–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two‐staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res, 2005, 441: 243–249. [DOI] [PubMed] [Google Scholar]
- 31. Nestor BJ, Hanssen AD, Ferrer‐Gonzalez R, Fitzgerald RH Jr. The use of porous prostheses in delayed reconstruction of THA that have failed because of infection. J Bone Joint Surg Am, 1994, 76: 349–359. [DOI] [PubMed] [Google Scholar]
- 32. Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten‐year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty, 2005, 20: 874–879. [DOI] [PubMed] [Google Scholar]
- 33. Sanchez‐Sotelo J, Berry DJ, Hanssen AD, Cabanela ME. Midterm to long‐term followup of staged reimplantation for infected hip arthroplasty. Clin Orthop Relat Res, 2009, 467: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Romanò CL, Romanò D, Logoluso N, Meani E. Septic versus aseptic hip revision: how different? J Orthop Traumatol, 2010, 11: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zimmerli W. Infection and musculoskeletal conditions: prosthetic‐joint‐associated infections. Best Pract Res Clin Rheumatol, 2006, 20: 1045–1063. [DOI] [PubMed] [Google Scholar]
- 36. Trampuz A, Zimmerli W. New strategies for the treatment of infections associated with prosthetic joints. Curr Opin Investig Drugs, 2005, 6: 185–190. [PubMed] [Google Scholar]
- 37. Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two‐stage reimplantation protocol. Clin Orthop Relat Res, 1994, 301: 205–212. [PubMed] [Google Scholar]
- 38. Berry DJ, Chandler HP, Reilly DT. The use of bone allografts in two‐stage reconstruction after failure of hip replacements due to infection. J Bone Joint Surg Am, 1991, 73: 1460–1468. [PubMed] [Google Scholar]
- 39. Alexeeff M, Mahomed N, Morsi E, Garbuz D, Gross A. Structural allograft in two‐stage revision for failed septic hip arthroplasty. J Bone Joint Surg Br, 1996, 78: 213–216. [PubMed] [Google Scholar]


