Key Points
Question
Is the monoclonal antibody fremanezumab effective in preventing episodic migraine?
Findings
In this randomized clinical trial that included 875 adults with episodic migraine in whom multiple medication classes had not previously failed, fremanezumab compared with placebo resulted in significantly fewer monthly migraine days with monthly dosing (–1.5 days) and with a single higher dose at baseline (–1.3 days) over 12 weeks.
Meaning
Fremanezumab as a preventive treatment for episodic migraine reduced the mean number of monthly migraine days over a 12-week period compared with placebo. Further research is needed to assess effectiveness against other preventive medications and in patients in whom multiple preventive drug classes have failed and to determine long-term safety and efficacy.
Abstract
Importance
Fremanezumab, a fully humanized monoclonal antibody that targets calcitonin gene-related peptide, may be effective for treating episodic migraine.
Objective
To assess the efficacy of fremanezumab compared with placebo for prevention of episodic migraine with a monthly dosing regimen or a single higher dose.
Design and Setting
Randomized, double-blind, placebo-controlled, parallel-group trial conducted at 123 sites in 9 countries from March 23, 2016 (first patient randomized), to April 10, 2017, consisting of a screening visit, 28-day pretreatment period, 12-week treatment period, and final evaluation at week 12.
Participants
Study participants were aged 18 to 70 years with episodic migraine (6-14 headache days, with at least 4 migraine days, during 28-day pretreatment period). Patients who had previous treatment failure with 2 classes of migraine-preventive medication were excluded.
Interventions
Patients were randomized 1:1:1 to receive subcutaneous monthly dosing of fremanezumab (n = 290; 225 mg at baseline, week 4, and week 8); a single higher dose of fremanezumab, as intended to support a quarterly dose regimen (n = 291; 675 mg of fremanezumab at baseline; placebo at weeks 4 and 8); or placebo (n = 294; at baseline, week 4, and week 8).
Main Outcomes and Measures
The primary end point was mean change in mean number of monthly migraine days during the 12-week period after the first dose.
Results
Among 875 patients who were randomized (mean age, 41.8 [SD, 12.1] years; 742 women [85%]), 791 (90.4%) completed the trial. From baseline to 12 weeks, mean migraine days per month decreased from 8.9 days to 4.9 days in the fremanezumab monthly dosing group, from 9.2 days to 5.3 days in the fremanezumab single-higher-dose group, and from 9.1 days to 6.5 days in the placebo group. This resulted in a difference with monthly dosing vs placebo of –1.5 days (95% CI, –2.01 to –0.93 days; P < .001) and with single higher dosing vs placebo of –1.3 days (95% CI, –1.79 to –0.72 days; P < .001). The most common adverse events that led to discontinuation were injection site erythema (n = 3), injection site induration (n = 2), diarrhea (n = 2), anxiety (n = 2), and depression (n = 2).
Conclusions and Relevance
Among patients with episodic migraine in whom multiple medication classes had not previously failed, subcutaneous fremanezumab, compared with placebo, resulted in a statistically significant 1.3- to 1.5-day reduction in the mean number of monthly migraine days over a 12-week period. Further research is needed to assess effectiveness against other preventive medications and in patients in whom multiple preventive drug classes have failed and to determine long-term safety and efficacy.
Trial Registration
clinicaltrials.gov Identifier: NCT02629861
This randomized clinical trial compares the effect of a monthly vs a single dose of fremanezumab, a humanized monoclonal antibody targeting calcitonin gene-related peptide, on monthly migraine days in patients with episodic migraine.
Introduction
Migraine is a prevalent disease characterized by headaches that are often severe and throbbing and accompanied by associated symptoms, such as photophobia, phonophobia, nausea, vomiting, vertigo, cutaneous allodynia, and cognitive dysfunction.1,2,3,4,5 Migraine is a leading cause of neurological disability worldwide and has a substantial effect on society.6,7,8
Episodic migraine, with headache occurring on fewer than 15 days per month, is the most common form of migraine.6 Although in many instances episodic migraine occurs at low frequency, nearly 30% of people with migraine experience headaches more than once per week, and about 8% of people with episodic migraine experience a high frequency of attacks (at least 10-14 headache days per month) and are at risk of transition to chronic migraine (at least 15 headache days per month).6,8,9 Accordingly, US guidelines recommend initiating preventive treatment in people who have at least 4 headache days per month.8 Among individuals with episodic migraine who should be considered for preventive treatment, less than 15% use preventive therapies.8,10 There is a need for effective preventive therapy that targets the pathophysiology of migraine and is safe and well tolerated.
Calcitonin gene-related peptide is a 37–amino acid neuropeptide involved in central and peripheral pathophysiological events in migraine.11,12 Fremanezumab (TEV-48125) is a fully humanized monoclonal antibody (immunoglobulin G isotype 2a) that potently and selectively binds to both isoforms of the calcitonin gene-related peptide ligand (not the receptor), has a flexible dosing regimen, and is administered by subcutaneous injection.13,14 In a previous placebo-controlled clinical trial of preventive treatment of episodic migraine, fremanezumab demonstrated efficacy and had a favorable tolerability profile, with no serious treatment-related adverse events.13
The purpose of this phase 3 trial was to evaluate the efficacy, adverse events, and immunogenicity of 2 dosing regimens of fremanezumab for the preventive treatment of episodic migraine.
Methods
Study Oversight
The protocol was approved by relevant ethics committees and institutional review boards. Written informed consent was obtained from each patient before any study procedures or assessments were done. This trial was conducted in accordance with the protocol (available in Supplement 1; statistical analysis plan available in Supplement 2) and with the International Conference for Harmonisation Guidelines for Good Clinical Practice, the Declaration of Helsinki,15 and relevant national and local regulations.
Study Participants
Patients were recruited from 123 investigative sites in 9 countries (eTable 1 in Supplement 3) between February 2016 (first patient was screened and signed consent form on February 22, 2016; first patient was randomized on March 23, 2016) and January 2017. Study participants included women and men aged 18 to 70 years with a history of migraine based on International Classification of Headache Disorders 3 beta version2 (ICHD-3 beta) diagnostic criteria for at least 12 months prior to screening and with onset prior to age 50 years. Patients were required to have episodic migraine based on information collected during a 28-day pretreatment baseline period and defined as a headache occurring on 6 to 14 days, with at least 4 days fulfilling ICHD-3 beta criteria for migraine with aura (code 1.2; B and C) or without aura (code 1.1; C and D), probable migraine, or use of triptans or ergot derivatives.
Patients were excluded if they used onabotulinumtoxinA during the 4 months before screening, used opioids or barbiturates on more than 4 days during the pretreatment baseline period, or had previous failure of 2 or more of the following medication clusters after at least 3 months of treatment for episodic or chronic migraine: divalproex sodium and sodium valproate; flunarizine and pizotifen; amitriptyline, nortriptyline, venlafaxine, and duloxetine; and atenolol, nadolol, metoprolol, propranolol, and timolol. Patients who received an intervention or used a device (eg, scheduled nerve blocks, transcranial magnetic stimulation) for migraine during the 2 months prior to screening were excluded. A subset of patients was allowed to use 1 concomitant preventive migraine medication if the dosing was stable for at least 2 months prior to the beginning of the pretreatment period and without any change in dose during the study. Acute headache medications were permitted.
Study Design and Procedures
This randomized, double-blind, placebo-controlled, parallel-group study consisted of a screening visit, 28-day pretreatment period, 12-week treatment period, and a final evaluation at week 12. At the screening visit, patients signed 2 consent forms, one for the current study for individuals with episodic migraine and one for a concurrent study for chronic migraine. Based on information from the screening visit and daily headache information captured during the pretreatment period, individuals were randomized into the appropriate study or were excluded if they did not meet eligibility criteria for either study.
Patients with episodic migraine were randomized 1:1:1 (stratified by sex, country, and baseline preventive migraine medication use) to receive (1) fremanezumab monthly, (2) a single higher dose of fremanezumab intended to support a quarterly dose regimen, or (3) placebo. Block size was fixed at 3 within each stratum. Monthly dosing consisted of 225 mg of fremanezumab at baseline (one 225-mg/1.5-mL injection and 2 placebo 1.5-mL injections) and at weeks 4 and 8 (one 225-mg/1.5-mL injection). Single higher dosing consisted of 675 mg of fremanezumab at baseline (three 225-mg/1.5-mL injections) and placebo (one 1.5-mL injection) at weeks 4 and 8. Placebo dosing consisted of placebo injections at baseline (three 1.5-mL injections) and at weeks 4 and 8 (one 1.5-mL injection). Randomization was performed using electronic interactive response technology. Patients, investigators, the sponsor, and designated personnel were blinded to treatment assignments.
Patients were seen at 5 scheduled site visits for protocol-specified evaluations: screening, baseline (dose 1), week 4 (dose 2), week 8 (dose 3), and at the end of treatment (week 12) or early withdrawal. Patients who withdrew from the study before the end of the 12-week evaluation period had final visit procedures and assessments performed as soon as possible after withdrawal. Headache data (eg, occurrence, duration, and severity of headache; occurrence of photophobia, phonophobia, nausea, or vomiting; and any migraine medication use) were captured daily during the study period via an electronic headache diary device.
Outcomes
The primary end point was the mean change from baseline (28-day pretreatment period) in the mean number of monthly migraine days during the 12-week period after the first injection. A migraine day was defined as a calendar day with either at least 2 consecutive hours of a headache meeting criteria for migraine (with or without aura); probable migraine (only 1 migraine criterion absent); or a day, regardless of duration, when acute migraine-specific medication (triptans or ergots) was used to treat a headache.
Secondary efficacy end points included the proportion of patients achieving at least a 50% reduction in the mean number of monthly migraine days from baseline to week 12, the mean change from baseline to week 12 in the monthly mean number of monthly days with use of any acute headache medications, the mean change from baseline to week 4 in the number of migraine days, the mean change from baseline to week 12 in mean number of monthly migraine days in patients not receiving concomitant migraine preventive medication, and the mean change in the Migraine Disability Assessment (MIDAS) score. The MIDAS questionnaire assesses headache-related disability based on lost days of activity over the previous 3 months; possible scores range from 0 to 270, with 0 to 5 indicating little or no disability; 6 to 10, mild disability; 11 to 20, moderate disability; and 21 or higher, severe disability.16,17
Minimal clinically important differences for the primary and secondary outcomes have not been established for patients with episodic migraine.
Adverse events and tolerability were assessed by evaluating reported adverse events, vital signs (systolic and diastolic blood pressure, pulse, temperature, and respiratory rate), 12-lead electrocardiogram, clinical laboratory tests (serum chemistry, hematology, coagulation, and urinalysis), physical examinations, and concomitant medication use. Suicidal ideation and behavior were assessed by the electronic Columbia-Suicide Severity Rating Scale. Systematic assessment of injection sites included examination for pain, erythema, induration, and ecchymosis immediately and 1 hour after dosing. Serum antidrug antibodies were assessed from blood samples using a validated method.
Statistical Analysis
Estimations based on the phase 2b fremanezumab study in patients with episodic migraine13 predicted that a sample of 675 evaluable patients completing the study (225 per treatment group) would provide 90% power to detect a 1.6-day (SD, 5.2 days) difference in migraine days between an active group and a placebo group at α = .05. Therefore, 768 patients were planned for randomization in this study, with an anticipated 12% dropout rate.
Efficacy analyses were conducted in the full analysis set, which included all randomized patients (intention-to-treat population) who received at least 1 dose of study drug and had at least 10 days of postbaseline efficacy assessments for the primary end point. Analyses of adverse events were performed in all randomized patients who received at least 1 dose of study drug.
The primary end point was analyzed using an analysis of covariance method. Treatment, sex, region (US vs non-US), and baseline preventive medication use were used as fixed effects, and baseline number of migraine days and years since onset of migraine were covariates. Ninety-five percent confidence intervals were constructed for the least-squares mean (LSM) differences between each fremanezumab group and the placebo group. The Wilcoxon rank-sum test was performed as the primary analysis if there was deviation from normality assumption as assessed by the Shapiro-Wilk test. The same analyses were used for relevant secondary end points. A mixed-effects repeated-measures analysis model was implemented as a sensitivity analysis to estimate the mean change from baseline in the end points for the overall 3-month treatment period and for each month to support the primary analysis; it included baseline value, treatment, sex, region (US vs non-US), baseline preventive migraine medication use, years since onset of migraines, month and treatment × month interaction as fixed effects, and patient in the repeated statement as a random effect. An additional post hoc sensitivity analysis using a mixed-effects model that included country instead of region as a random effect was also performed.
For withdrawals or patients with missing e-diary days and 10 or more days of data for a month, the monthly number of days of efficacy variables was prorated to 28 days for that month. A multiple imputation method was also conducted as a sensitivity analysis using the following steps. For patients with missing days and fewer than 10 days of e-diary data for 1 month, the monthly number of days of efficacy variables was considered missing before the multiple imputation procedure. Patients in active treatment groups who discontinued because of adverse events or lack of efficacy were assigned to the placebo group. The statistics were based on 10 sets of imputed data from SAS PROC MI, where the mean is the average of the means from the 10 data sets and the standard error of the mean is adjusted based on the within-imputation variance estimates and the between-imputation variance. The mean number of monthly migraine days during the 12-week period after the first dose of study drug was a mean of month 1, 2, and 3 values.
For the proportion of responders defined as having at least a 50% reduction in mean number of monthly migraine days, the Cochran-Mantel-Haenszel test was used with baseline preventive medication as a stratification variable. In the primary overall analysis, patients who discontinued early were considered nonresponders.
To control the type I statistical error rate at .05, a hierarchical testing procedure with a preplanned sequence of comparisons was applied. Each comparison was interpreted inferentially at α = .05 only if the preceding comparison had a 2-sided P ≤ .05. All data are presented in the order of hierarchical testing (eTable 2 in Supplement 3).
SAS software version 9.2 (SAS Institute Inc) was used to generate all data listings, summaries, and statistical analyses.
Results
Study Participants
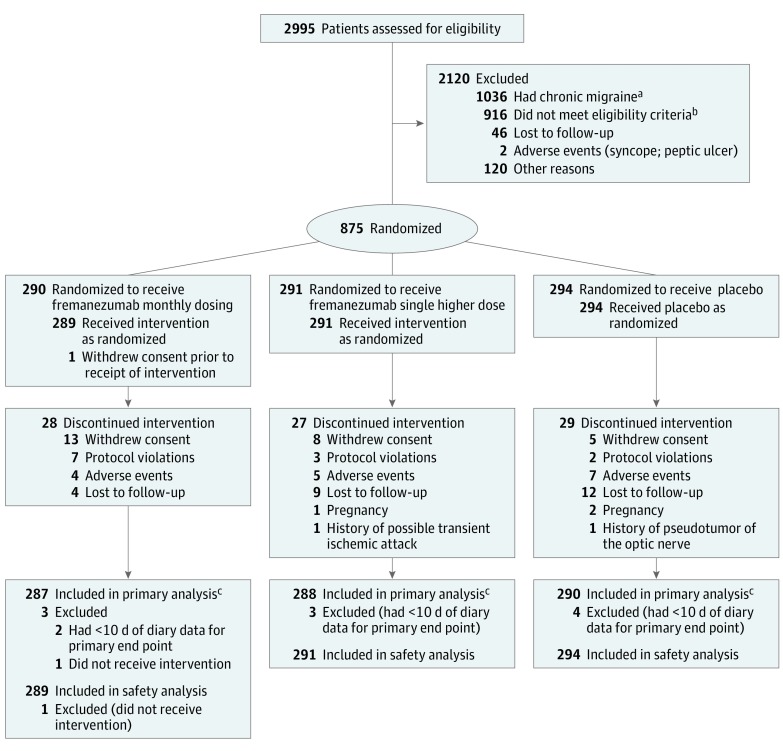
A total of 875 patients were enrolled and randomized at 123 sites (number of patients per site: mean, 7.1; median, 6; range, 1-22) to 1 of 3 treatment groups (fremanezumab monthly, n = 290; fremanezumab single higher dose, n = 291; placebo, n = 294) (Figure 1). Baseline demographics and clinical characteristics were similar among all treatment groups; patients with episodic migraine had an overall mean 9.1 (SD, 2.6) migraine days per month and had severe disability based on the MIDAS score (mean score, 39 points) (Table 1). Twenty-one percent of patients were allowed to continue treatment with 1 concomitant preventive medication (Table 1). A total of 791 patients (262 receiving fremanezumab monthly, 264 receiving fremanezumab single higher dose, and 265 receiving placebo) completed the study. A total of 10 (1%) of 875 patients in the intention-to-treat population had missing data on the primary end point.
Figure 1. Flow of Participants in a Randomized Clinical Trial of Fremanezumab vs Placebo for Prevention of Episodic Migraine.
aThe 1036 patients with chronic migraine were enrolled in a separate clinical trial of fremanezumab in patients with chronic migraine.
bIneligible patients included those who met the following exclusion criteria: onabotulinumtoxinA use within 4 months of screening (n=6); opioid or barbiturate use >4 d/mo (n=29); ≥2 medication clusters previously failed (n=14); intervention or device use within 2 months of screening (n=4); clinically significant other disease (at discretion of investigator) (n=31); evidence or history of significant psychiatric issues (n=46); history of significant cardiovascular disease (n=21); known infection or history of infectious disease (n=9); cancer (current or past 5 years) (n=4); pregnant or nursing (n=5); history of hypersensitivity to injected proteins (n=1); participation in clinical study within 2 months or 5 half-lives (n=3); prior exposure to monoclonal antibody targeting calcitonin gene-related peptide pathway (n=2); clinically significant 12-lead electrocardiogram finding (n=13); clinically significant laboratory abnormality (n=16); hepatic enzymes >1.5 times upper limit of normal range or Hy’s law (presence of 3 components: aspartate aminotransferase or alanine aminotransferase ≥3 times upper limit of normal; total bilirubin ≥2 times upper limit of normal; and no other reason to explain these increases (eg, viral hepatitis A, B, or C; liver disease; or drug capable of causing injury) (n=24); serum creatinine >1.5 times upper limit of normal, clinically significant proteinuria, or renal disease (n=11); alcohol or drug abuse in past 2 years or dependence in past 5 years (n=14); unable to participate or complete study (n=16); study center or sponsor employee or relative of such an employee (n=7). Ineligible patients also included those who did not meet the following inclusion criteria: male or female aged 18-70 years with migraine onset at ≤50 years (n=5); provide written informed consent (n=2); history of migraine for ≥12 months (n=32); confirmed episodic migraine during pretreatment period (n=279); not using preventive medications or one with stable dose for ≥2 months (n=38); body mass index 17.5-37.5 and total body weight 45-120 kg (n=37); nonchildbearing potential (n=22); women of childbearing potential with negative pregnancy test result (n=3); about 85% electronic headache diary adherence (n=80); in overall good health (n=36); willing and able to adhere to study restrictions, remain at clinic during study period, and return to clinic for follow-up evaluation (n=111).
cThe primary analysis included all patients who were randomized, received at least 1 dose of study drug, and had at least 10 days of postbaseline efficacy assessments for the primary end point.
Table 1. Baseline Participant Characteristics.
| Characteristics | Fremanezumab Monthly Dosing (n = 290) | Fremanezumab Single Higher Dose (n = 291) | Placebo (n = 294) |
|---|---|---|---|
| Age, mean (SD), y | 42.9 (12.7) | 41.1 (11.4) | 41.3 (12.0) |
| Female, No. (%) | 244 (84.1) | 251 (86.3) | 247 (84.0) |
| Body mass index, mean (SD)a | 26.2 (5.2) | 27.0 (5.1) | 27.2 (4.9) |
| Disease history | |||
| Time since initial migraine diagnosis, mean (SD), y | 20.7 (12.9) | 20.0 (12.1) | 19.9 (11.9) |
| Current preventive medication use, No. (%) | 62 (21.4) | 58 (19.9) | 62 (21.1) |
| Current acute headache medication use, No. (%) | 279 (96.2) | 281 (96.6) | 280 (95.2) |
| Prior topiramate use, No. (%)b | 64 (22.1) | 51 (17.5) | 53 (18.0) |
| Disease characteristics during 28-d pretreatment period, mean (SD) | |||
| Migraine daysc | 8.9 (2.6) | 9.3 (2.7) | 9.1 (2.7) |
| Headache days of at least moderate severityd | 6.8 (2.9) | 7.2 (3.1) | 6.9 (3.1) |
| Days with use of any acute headache medications | 7.7 (3.4) | 7.8 (3.7) | 7.7 (3.6) |
| Days with use of migraine-specific acute headache medications | 6.1 (3.1) | 6.6 (3.1) | 7.1 (3.0) |
| MIDAS score, mean (SD)e | 38.0 (33.2) | 41.7 (33.0) | 37.3 (27.6) |
Calculated as weight in kilograms divided by height in meters squared.
One of the US Food and Drug Administration–approved preventive medications for episodic migraine.
A migraine day was defined as a calendar day in which a patient reported either a headache that lasted at least 2 consecutive hours and met criteria for migraine (with or without aura) or probable migraine (subtype in which only 1 migraine criterion is absent), or a day when a headache of any duration was treated with migraine-specific medications (triptans or ergots).
A headache day of at least moderate severity was defined as a calendar day in which a patient reported either headache pain that lasted at least 4 hours with a peak of at least moderate severity or a day when an acute migraine-specific medication (triptans or ergots) was used to treat a headache of any severity or duration.
For the Migraine Disability Assessment (MIDAS), the score ranges from 0 to 270, with 0 to 5 indicating little or no disability; 6 to 10, mild disability; 11 to 20, moderate disability; and 21 or higher, severe disability.
Efficacy
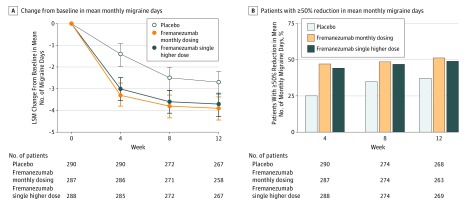
The baseline mean numbers of monthly migraine days were 8.9, 9.2, and 9.1 days in the monthly dosing, single-higher-dose, and placebo groups, respectively. During the 12-week period after the first dose, the mean numbers of migraine days per month were 4.9 days for the monthly fremanezumab dosing group (LSM change from baseline, –3.7 days) and 5.3 days for the fremanezumab single-higher-dose group (LSM change from baseline, –3.4 days) compared with 6.5 days for the placebo group (LSM change from baseline, –2.2 days). There was a statistically significant difference with monthly dosing vs placebo of –1.5 days (95% CI, –2.01 to –0.93 days; P < .001) and with the single higher dose vs placebo of –1.3 days (95% CI, –1.79 to –0.72 days; P < .001) (Table 2 and Figure 2A).
Table 2. Summary of Primary and Secondary End Pointsa.
| Analysis of Covarianceb | Mixed-Effects Repeated Measuresc | |||||
|---|---|---|---|---|---|---|
| Fremanezumab Monthly Dosing (n = 287) | Fremanezumab Single Higher Dose (n = 288) | Placebo (n = 290) | Fremanezumab Monthly Dosing (n = 287) | Fremanezumab Single Higher Dose (n = 288) | Placebo (n = 290) | |
| Primary End Point | ||||||
| Mean monthly migraine days, baseline to wk 12 | ||||||
| Least-squares mean change (95% CI), d | −3.7 (−4.15 to −3.18) | −3.4 (−3.94 to −2.96) | −2.2 (−2.68 to −1.71) | −3.7 (−4.14 to −3.19) | −3.5 (−3.93 to −2.97) | −2.2 (−2.71 to −1.77) |
| Difference vs placebo (95% CI), d | −1.5 (−2.01 to −0.93) | −1.3 (−1.79 to −0.72) | −1.4 (−1.96 to −0.90) | −1.2 (−1.74 to −0.69) | ||
| P value | <.001 | <.001 | <.001 | <.001 | ||
| Secondary End Points | ||||||
| ≥50% Reduction in mean monthly migraine days, baseline to wk 12d | ||||||
| Patients achieving response, No. (%) | 137 (47.7) | 128 (44.4) | 81 (27.9) | |||
| Difference vs placebo (95% CI), % responders | 19.8 (12.0 to 27.6) | 16.5 (8.9 to 24.1) | ||||
| P value | <.001 | <.001 | ||||
| Mean monthly days with any acute headache medication, baseline to wk 12 | ||||||
| Least-squares mean change (95% CI), d | −3.0 (−3.41 to −2.56) | −2.9 (−3.34 to −2.48) | −1.6 (−2.04 to −1.20) | −3.0 (−3.43 to −2.58) | −2.9 (−3.35 to −2.49) | −1.7 (−2.09 to −1.24) |
| Difference vs placebo (95% CI), d | −1.4 (−1.84 to −0.89) | −1.3 (−1.76 to −0.82) | −1.3 (−1.81 to −0.86) | −1.3 (−1.73 to −0.78) | ||
| P value | <.001 | <.001 | <.001 | <.001 | ||
| Mean monthly migraine days, baseline to wk 4 | ||||||
| Least-squares mean change (95% CI), d | −3.5 (−4.05 to −2.93) | −3.3 (−3.85 to −2.71) | −1.7 (−2.24 to −1.13) | −3.3 (−3.80 to −2.74) | −3.0 (−3.55 to −2.48) | −1.4 (−1.97 to −0.92) |
| Difference vs placebo (95% CI), d | −1.8 (−2.43 to −1.18) | −1.6 (−2.22 to −0.97) | −1.8 (−2.45 to −1.20) | −1.6 (−2.20 to −0.95) | ||
| P value | <.001 | <.001 | <.001 | <.001 | ||
| Mean monthly migraine days in patients not receiving concomitant preventive migraine medications, baseline to wk 12 | n=225 | n=230 | n=230 | n=225 | n=230 | n=230 |
| Least-squares mean change (95% CI), d | −3.7 (−4.23 to −3.17) | −3.5 (−4.06 to −3.01) | −2.4 (−2.91 to −1.88) | −3.7 (−4.24 to −3.21) | −3.5 (−4.06 to −3.03) | −2.4 (−2.93 to −1.94) |
| Difference vs placebo (95% CI), d | −1.3 (−1.92 to −0.70) | −1.1 (−1.75 to −0.54) | −1.3 (−1.88 to −0.70) | −1.1 (−1.70 to −0.52) | ||
| P value | <.001 | <.001 | <.001 | <.001 | ||
| MIDAS scoree | ||||||
| Least-squares mean change (95% CI) | −24.6 (−27.68 to −21.45) | −23.0 (−26.10 to −19.82) | −17.5 (−20.62 to −14.47) | |||
| Difference vs placebo (95% CI) | −7.0 (−10.51 to −3.53) | −5.4 (−8.90 to −1.93) | ||||
| P value | <.001 | .002 | ||||
Efficacy analyses were conducted for all randomized patients who received ≥1 dose of study drug and had ≥10 days of postbaseline efficacy assessments for the primary end point. To control the type I statistical error rate at .05, a preplanned hierarchical testing procedure was applied, and end points are presented in the sequence in which they were evaluated. All P values are vs placebo.
Analysis of covariance was applied for the primary analysis of all end points unless otherwise noted. The model included treatment, sex, region (US vs non-US), and baseline preventive migraine medication use as fixed effects, with the baseline number of migraine days and years since onset of migraine as covariates.
A mixed-effects repeated-measures sensitivity analysis included treatment, sex, region (US vs non-US), baseline preventive medication use, month, and treatment × month as fixed effects, with baseline value of the end point and years since onset of migraine as covariates. Patient was a random effect, and an unconstructed covariance structure was used.
Analyzed via Cochran-Mantel-Haenszel test stratified by baseline preventive migraine medication use. Patients who discontinued early were considered nonresponders.
Mean change in Migraine Disability Assessment (MIDAS) score from baseline to 4 weeks after administration of the last (third) dose of the study drug. See Table 1 footnotes for description of score ranges.
Figure 2. Effect of Fremanezumab vs Placebo on Migraine Outcomes.
Panel A shows the change in mean number of monthly migraine days from baseline to week 12, analyzed via mixed-effects repeated measures. Error bars represent 95% confidence intervals. LSM indicates least-squares mean. Differences from placebo were significant for both fremanezumab treatment groups at each time point. For the primary analysis (analysis of covariance) of mean migraine days per month from baseline to week 12, the difference vs placebo for the fremanezumab monthly dosing group was –1.5 days (95% CI, –2.01 to –0.93 days; P<.001) and for the fremanezumab single-higher-dose group was –1.3 days (95% CI, –1.79 to –0.72 days; P<.001). Panel B shows the percentage of patients with at least a 50% reduction in mean number of monthly migraine days during the 12 weeks following the first administration of the study drug. The overall difference vs placebo for the fremanezumab monthly dosing group was 19.8% (95% CI, 12.0%-27.6%; P<.001) and for the fremanezumab single-higher-dose group was 16.5% (95% CI, 8.9%-24.1%; P<.001).
The proportion of patients with response rates of at least a 50% reduction in mean number of monthly migraine days during the 12-week treatment period were 47.7% for the fremanezumab monthly dosing group (difference vs placebo, 19.8%; 95% CI, 12.0%-27.6%; P < .001) and 44.4% for the fremanezumab single-higher-dose group (difference vs placebo, 16.5%; 95% CI, 8.9%-24.1%; P < .001) compared with 27.9% for the placebo group (Table 2 and Figure 2B).
The baseline mean numbers of monthly days with any acute headache medication use were 7.7, 7.9, and 7.7 days in the fremanezumab monthly dosing, fremanezumab single-higher-dose, and placebo groups, respectively. The mean numbers of monthly days with any acute headache medication use during the 12-week treatment period were 4.4 days for the fremanezumab monthly dosing group (LSM change from baseline, –3.0 days; LSM difference from placebo, –1.4 days [95% CI, –1.84 to –0.89 days]; P < .001) and 4.6 days for the single-higher-dose group (LSM change from baseline, –2.9 days; LSM difference from placebo, –1.3 days [95% CI, –1.76 to –0.82 days]; P < .001) compared with 5.8 days for the placebo group (LSM change from baseline, –1.6 days) (Table 2).
During the 4-week period after the first dose, monthly migraine days were 5.3 days for the fremanezumab monthly dosing group (LSM change from baseline, –3.5 days; LSM difference from placebo, –1.8 days [95% CI, –2.43 to –1.18 days]; P < .001) and 5.7 days for the fremanezumab single-higher-dose group (LSM change from baseline, –3.3 days; LSM difference from placebo, –1.6 days [95% CI, –2.22 to –0.97 days]; P < .001) compared with 7.2 days for the placebo group (LSM change from baseline, –1.7 days).
Among patients not receiving concomitant preventive migraine medications, the baseline mean numbers of monthly migraine days were 8.9, 9.3, and 9.1 days in the fremanezumab monthly dosing, fremanezumab single-higher-dose, and placebo groups, respectively. Among these patients, the monthly mean numbers of migraine days were 4.8 days for the fremanezumab monthly dosing group (LSM change from baseline, –3.7 days; LSM difference from placebo, –1.3 days [95% CI, –1.92 to –0.70 days]; P < .001) and 5.3 days for the fremanezumab single-higher-dose group (LSM change from baseline, –3.5 days; LSM difference from placebo, –1.1 days [95% CI, –1.75 to –0.54 days]; P < .001) compared with 6.4 days for the placebo group (LSM change from baseline, –2.4 days) (Table 2).
Baseline mean MIDAS scores were 38.0, 41.7, and 37.3 points in the fremanezumab monthly dosing, fremanezumab single-higher-dose, and placebo groups, respectively. At 4 weeks after administration of the last (third) dose of the study drug, mean MIDAS scores were 12.6 points for the fremanezumab monthly dosing group (LSM change from baseline, –24.6 points; LSM difference from placebo, –7.0 points [95% CI, –10.51 to –3.53 points]; P < .001) and 14.6 points for the single-higher-dose group (LSM change from baseline, –23.0 points; LSM difference from placebo, –5.4 points [95% CI, –8.90 to –1.93 points]; P = .002) compared with 19.4 points for the placebo group (LSM change from baseline, –17.5 points) (Table 2).
Sensitivity and Additional Analyses
Prespecified mixed-effects repeated-measures and multiple imputation sensitivity analyses of the efficacy end points yielded results similar to the primary analyses (Table 2 and eTable 3 in Supplement 3). A post hoc mixed-effects repeated-measures sensitivity analysis using country as a random effect of the primary end point yielded results similar to those of the primary analysis (eTable 4 in Supplement 3). The distribution of monthly migraine days over the treatment period in the full analysis set is shown in the eFigure in Supplement 3.
Adverse Events and Tolerability
Adverse Events
A total of 192 patients (66%) who received fremanezumab monthly dosing and 193 patients (66%) who received a single higher dose of fremanezumab reported at least 1 adverse event, compared with 171 patients (58%) who received placebo (Table 3). Severe adverse events, serious adverse events, and adverse events leading to discontinuation were infrequent and had similar incidences (≤2%) across the treatment groups. Treatment-related adverse events were higher in the fremanezumab treatment groups (48% in the monthly group and 47% in the single-higher-dose group) compared with placebo (37%). The most common adverse events in patients treated with fremanezumab were injection site reactions: pain (fremanezumab monthly dosing: 87/290 [30.0%]; fremanezumab single higher dose: 86/291 [29.6%]), induration (fremanezumab monthly dosing: 71/290 [24.5%]; fremanezumab single higher dose: 57/291 [19.6%]), and erythema (fremanezumab monthly dosing: 52/290 [17.9%]; fremanezumab single higher dose: 55/291 [18.9%]). The proportion of patients with injection site pain, induration, and erythema was higher with fremanezumab than with placebo (76/293 [25.9%], 45/293 [15.4%], and 41/293 [14.0%], respectively). Injection site hemorrhage occurred infrequently and at similar rates among treatment groups (fremanezumab monthly dosing: 3/290 [1.0%]; fremanezumab single higher dose: 9/291 [3.1%]; placebo: 6/293 [2.0%]). One patient who withdrew consent (not study related) was subsequently reported as deceased by a family member. The event occurred 109 days after receiving a single higher dose of fremanezumab. The patient had withdrawn from the study 38 days earlier because of a family emergency. Cause of death per autopsy report was diphenhydramine overdose (suicide). Assessment by the investigator determined that the death was unrelated to treatment.
Table 3. Adverse Events in the Safety Populationa.
| Adverse Events | No. (%) of Patients | ||
|---|---|---|---|
| Fremanezumab Monthly Dosing (n = 290) | Fremanezumab Single Higher Dose (n = 291) | Placebo (n = 293) | |
| All events | |||
| ≥1 Adverse event | 192 (66.2) | 193 (66.3) | 171 (58.4) |
| ≥1 Treatment-related adverse event | 138 (47.6) | 137 (47.1) | 109 (37.2) |
| ≥1 Serious adverse event | 3 (1.0) | 3 (1.0) | 7 (2.4) |
| Any adverse event leading to study discontinuation | 5 (1.7) | 5 (1.7) | 5 (1.7) |
| Death | 0 | 1 (0.3) | 0 |
| Adverse event typeb | |||
| General disorders and administration site conditions | |||
| Injection site pain | 87 (30.0) | 86 (29.6) | 76 (25.9) |
| Injection site induration | 71 (24.5) | 57 (19.6) | 45 (15.4) |
| Injection site erythema | 52 (17.9) | 55 (18.9) | 41 (14.0) |
| Injection site hemorrhage | 3 (1.0) | 9 (3.1) | 6 (2.0) |
| Fatigue | 2 (0.7) | 6 (2.1) | 4 (1.4) |
| Infections and infestations | |||
| Upper respiratory tract infection | 16 (5.5) | 11 (3.8) | 15 (5.1) |
| Nasopharyngitis | 11 (3.8) | 11 (3.8) | 9 (3.1) |
| Urinary tract infection | 7 (2.4) | 10 (3.4) | 4 (1.4) |
| Bronchitis | 6 (2.1) | 4 (1.4) | 3 (1.0) |
| Sinusitis | 4 (1.4) | 2 (0.7) | 8 (2.7) |
| Gastrointestinal disorders | |||
| Nausea | 4 (1.4) | 7 (2.4) | 5 (1.7) |
| Protocol-defined adverse events of special interest | |||
| Hepatic enzyme increasedc | 2 (0.7) | 1 (0.3) | 0 |
| Blood bilirubin increasedd | 1 (0.3) | 0 | 1 (0.3) |
| Ophthalmic events of at least moderate intensity | 0 | 0 | 0 |
| Hy’s law eventse | 0 | 0 | 0 |
| Anaphylaxis | 0 | 0 | 0 |
| Severe hypersensitivity reactionsf | 0 | 0 | 1 (0.3) |
Adverse events were collected at each visit via inquiry and clinical laboratory tests. The safety population included all patients who were randomized and received at least 1 dose of study drug. If a patient had multiple types of adverse events, he/she was counted once for each type. One patient randomized to receive placebo inadvertently received 1 dose of fremanezumab, 225 mg.
Adverse events shown include those reported in more than 2% of patients in any group.
Aspartate aminotransferase or alanine aminotransferase at least 3 times the upper limit of normal.
Total bilirubin at least 2 times the upper limit of normal.
Presence of 3 components: aspartate aminotransferase or alanine aminotransferase at least 3 times the upper limit of normal; total bilirubin at least 2 times the upper limit of normal; and no other reason to explain these increases (eg, viral hepatitis A, B, or C; liver disease; or drug capable of causing injury).
One patient in the placebo group had a serious adverse event of drug hypersensitivity that was assessed as severe; the event was considered to be a generalized allergic reaction to ceftriaxone.
The proportion of patients who discontinued because of adverse events was similar in each treatment group (2%). The most common adverse events leading to discontinuation from the fremanezumab treatment groups included injection site erythema (n = 3), injection site induration (n = 2), diarrhea (n = 2), anxiety (n = 2), and depression (n = 2). No other adverse events leading to discontinuation from the study occurred in more than 1 patient.
Tolerability Measurements
No relevant changes in vital signs (blood pressure, pulse, temperature, and respiratory rate), physical examination measurements (including weight), or electrocardiogram findings were noted in patients in any of the treatment groups. There were no clinically significant changes in any laboratory parameter (serum chemistry, hematology, coagulation, and urinalysis), including liver function tests (Table 3). Four patients in the fremanezumab monthly dosing group developed antidrug antibodies against fremanezumab without any significant adverse events (eTable 5 in Supplement 3).
Discussion
Among patients with episodic migraine in whom multiple medication classes had not previously failed, subcutaneous fremanezumab, compared with placebo, significantly reduced the mean number of migraine days per month over the 12-week treatment period. In this phase 3 study, both monthly dosing and a single higher dose of fremanezumab intended to support a quarterly dosing regimen led to statistically significant improvements in the primary and secondary end points. A clinical response to fremanezumab, compared with placebo, was suggested by the greater proportion of patients who achieved a 50% or greater reduction in the mean number of migraine days per month.10
The low percentage of patients in this study who had prior use of topiramate (19%), one of the US Food and Drug Administration–approved preventive medications for episodic migraine, highlights the limited use of preventive medications for episodic migraine.8,10,18 The adverse event profile of fremanezumab in this trial is consistent with previous clinical trials, with no clinically significant pattern of adverse events or drug-related serious adverse events.13,19 As expected from prior studies, the most common adverse event reported was injection site pain, which occurred with greater incidence with fremanezumab than with placebo. Injection sites were systematically assessed for pain, erythema, induration, and ecchymosis, immediately and 1 hour after dosing, and these careful assessments may explain the higher proportion of reported injection site adverse events with both fremanezumab and placebo compared with the previous phase 2 studies.13,19
This study has several strengths, including the inclusion of a single higher subcutaneous dose. This study also allowed for inclusion of individuals taking monotherapy or those using preventive medications after proper stratification. To our knowledge, this program is the first to use this strategy in the development of drugs for episodic migraine.13
Limitations
This study has several limitations. First, the study was powered to detect a 1.6-day difference in the mean number of monthly migraine days between the fremanezumab and placebo groups, yet the observed effect sizes were a 1.5-day reduction for the fremanezumab monthly dosing group and a 1.3-day reduction for the fremanezumab single-higher-dosing group. However, no minimal clinically important difference has been established for this outcome measure in episodic or chronic migraine.
Second, although the trial included patients with a long history of disease (about 20 years) and those who were currently taking preventive medications or in whom preventive medications had previously failed, it did not include treatment-refractory patients with more than 2 failed preventive drug clusters or those who had continuous headache. Nonetheless, the trial was less restrictive in inclusion criteria than other trials and allowed patients currently taking other preventive medication.20,21,22,23 In addition, the results of a recently published chronic migraine clinical study support use of fremanezumab for the subset of patients who are more severely affected as well.24 However, further studies are needed to define the full spectrum of efficacy and tolerability of fremanezumab, including in patients who are treatment refractory and who have a range of coexistent diseases.
Third, this trial was limited to the evaluation of end points at a short-term follow-up of 3 months after randomization. An ongoing extension of this study is evaluating the long-term safety and efficacy of fremanezumab via blinded assessment over an additional 12 months. Fourth, the response of postrandomization attacks to acute treatment was also not assessed, although consumption of acute medication was significantly decreased. Fifth, the effect of fremanezumab on frequency of migraine aura was also not evaluated in this trial. Sixth, this study did not compare fremanezumab with other active treatments, although active comparators are usually not included in pivotal trials for migraine prevention. Seventh, this study did not include certain patient populations, such as those who are pregnant, who have acute coronary syndrome or ischemic stroke, or who may have a compromised blood-brain barrier. Further studies are needed to inform the use of fremanezumab in these populations.
Conclusions
Among patients with episodic migraine in whom multiple medication classes had not previously failed, subcutaneous fremanezumab, compared with placebo, resulted in a statistically significant 1.3- to 1.5-day reduction in the mean number of monthly migraine days over a 12-week period. Further research is needed to assess effectiveness against other preventive medications and in patients in whom multiple preventive drug classes have failed and to determine long-term safety and efficacy.
Trial Protocol
Statistical Analysis Plan
eTable 1. Number of Investigational Sites Per Country
eTable 2. Hierarchical Testing Sequence of Comparisons
eTable 3. Sensitivity Analysis of the Primary Efficacy Endpoint With Multiple Imputation
eTable 4. Sensitivity Analysis of the Primary Efficacy Endpoint Using Mixed-Effect Model With Country as a Random Effect
eFigure. Histograms of Migraine Days at Each Time Point
eTable 5. Other Safety Measures
References
- 1.Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(suppl 2):103-122. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646-657. [DOI] [PubMed] [Google Scholar]
- 5.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buse DC, Scher AI, Dodick DW, et al. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the CaMEO study [published online April 25, 2016]. Mayo Clin Proc. doi: 10.1016/j.mayocp.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 7.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF; AMPP Advisory Group . Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-349. [DOI] [PubMed] [Google Scholar]
- 9.Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71(11):848-855. [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644-655. [DOI] [PubMed] [Google Scholar]
- 11.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183-187. [DOI] [PubMed] [Google Scholar]
- 12.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6(10):573-582. [DOI] [PubMed] [Google Scholar]
- 13.Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015;14(11):1081-1090. [DOI] [PubMed] [Google Scholar]
- 14.Walter S, Bigal ME. TEV-48125: a review of a monoclonal CGRP antibody in development for the preventive treatment of migraine. Curr Pain Headache Rep. 2015;19(3):6. [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41(9):854-861. [PubMed] [Google Scholar]
- 17.Peng KP, Wang SJ. Migraine diagnosis: screening items, instruments, and scales. Acta Anaesthesiol Taiwan. 2012;50(2):69-73. [DOI] [PubMed] [Google Scholar]
- 18.Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015;14(11):1091-1100. [DOI] [PubMed] [Google Scholar]
- 20.Dodick DW, Goadsby PJ, Silberstein SD, et al. ; ALD403 Study Investigators . Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13(11):1100-1107. [DOI] [PubMed] [Google Scholar]
- 21.Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885-892. [DOI] [PubMed] [Google Scholar]
- 22.Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132. [DOI] [PubMed] [Google Scholar]
- 23.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382-390. [DOI] [PubMed] [Google Scholar]
- 24.Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113-2122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Number of Investigational Sites Per Country
eTable 2. Hierarchical Testing Sequence of Comparisons
eTable 3. Sensitivity Analysis of the Primary Efficacy Endpoint With Multiple Imputation
eTable 4. Sensitivity Analysis of the Primary Efficacy Endpoint Using Mixed-Effect Model With Country as a Random Effect
eFigure. Histograms of Migraine Days at Each Time Point
eTable 5. Other Safety Measures