Key Points
Question
Does sustained vitamin D supplementation until 6 months of age affect recurrent wheezing in black infants born preterm?
Findings
In this randomized clinical trial of 300 infants, sustained supplementation with 400 IU/d of vitamin D until 6 months of age, compared with a diet-limited approach, resulted in a likelihood of recurrent wheezing at 12 months of 31.1% vs 41.8%, respectively, a statistically significant difference.
Meaning
Among black infants born preterm, sustained supplementation with vitamin D, compared with diet-limited supplementation, resulted in a reduced risk of recurrent wheeze by 12 months’ adjusted age.
Abstract
Importance
Black infants born preterm face high rates of recurrent wheezing throughout infancy. Vitamin D supplementation has the potential to positively or negatively affect wheezing through modulation of the pulmonary and immune systems.
Objective
To assess the effectiveness of 2 vitamin D dosing strategies in preventing recurrent wheezing.
Design, Setting, and Participants
A randomized clinical trial enrolled 300 black infants born at 28 to 36 weeks’ gestation between January 2013 and January 2016 at 4 sites in the United States, and followed them up through March 2017. Randomization was stratified by site and maternal milk exposure.
Interventions
Patients were enrolled prior to discharge from the neonatal intensive care unit or newborn nursery and received open-label multivitamin until they were consuming 200 IU/d of cholecalciferol from formula or fortifier added to human milk, after which they received either 400 IU/d of cholecalciferol until 6 months of age adjusted for prematurity (sustained supplementation) or placebo (diet-limited supplementation). One-hundred fifty three infants were randomized to the sustained group, and 147 were randomized to the diet-limited group.
Main Outcomes and Measures
Recurrent wheezing by 12 months’ adjusted age was the primary outcome.
Results
Among 300 patients who were randomized (mean gestational age, 33 weeks; median birth weight, 1.9 kg), 277 (92.3%) completed the trial. Recurrent wheezing was experienced by 31.1% of infants in the sustained supplementation group and 41.8% of infants in the diet-limited supplementation group (difference, −10.7% [95% CI, −27.4% to −2.9%]; relative risk, 0.66 [95% CI, 0.47 to 0.94]). Upper and lower respiratory tract infections were among the most commonly reported adverse events. Upper respiratory infections were experienced by 84 of 153 infants (54.9%) in the sustained group and 83 of 147 infants (56.5%) in the diet-limited group (difference, −1.6% [95% CI, −17.1% to 7.0%]). Lower respiratory infections were experienced by 33 of 153 infants (21.6%) in the sustained group and 37 of 147 infants (25.2%) in the diet-limited group (difference, −3.6% [95% CI, −16.4% to 4.4%]).
Conclusions and Relevance
Among black infants born preterm, sustained supplementation with vitamin D, compared with diet-limited supplementation, resulted in a reduced risk of recurrent wheezing by 12 months’ adjusted age. Future research is needed to better understand the mechanisms and longer-term effects of vitamin D supplementation on wheezing in children born preterm.
Trial Registration
ClinicalTrials.gov Identifier: NCT01601847
This randomized clinical trial compares the effects of 2 vitamin D dosing strategies on recurrent wheezing in black infants at 12months of age who were born preterm.
Introduction
Wheezing is a common complication of prematurity. Early environmental and iatrogenic exposures may perturb the development of the lung, airway, or immune system and lead to recurrent wheezing. Substantial evidence suggests vitamin D is important in multiple pathways relevant to the development of wheezing in young children, including regulating inflammation, response to pathogens, lung and airway development, and propensity to allergic disease.1,2,3,4,5,6,7,8,9 Preterm infants, with developmentally immature pulmonary and immune systems perturbed by preterm birth, may be particularly vulnerable to any positive and negative effects of vitamin D. Black infants experience higher rates of both prematurity and prematurity-associated wheezing, and previous epidemiological work suggests that race may affect the relationship between vitamin D exposure and wheezing, leading to the design of the present study focusing exclusively on black infants.10,11
The Wheezing in Black Preterm Infants: Impact of Vitamin D Supplementation Strategy (D-Wheeze) Study assessed the effectiveness of 2 vitamin D supplementation strategies in black infants born preterm: (1) sustained supplementation with 400 IU/d of cholecalciferol until 6 months of age adjusted for prematurity, or (2) cessation of supplementation when the infant was taking 200 IU/d or more from diet. This trial focused on clinical outcomes as well as surrogate markers of pulmonary health, allergy, and bone health.
Methods
This study was approved by the institutional review board at each participating institution. The protocol is available in Supplement 1. Written informed consent was obtained from the infants’ parents prior to drawing any eligibility laboratories that were not obtained as part of clinical care and prior to randomization of eligible infants.
Study Design and Participants
We conducted a masked placebo-controlled randomized clinical trial of the effectiveness of 2 vitamin D supplementation strategies in black infants born at 280/7 to 366/7 weeks’ gestational age (GA). Patients were enrolled between January 2013 and January 2016; the follow-up window closed March 2017. Infants were recruited from University Hospitals Cleveland Medical Center (Cleveland, Ohio), MetroHealth Medical Center (Cleveland, Ohio), the Medical University of South Carolina (Charleston), and Montefiore Medical Center (Bronx, New York).
Infants were randomized to receive 400 IU of cholecalciferol daily until 6 months’ adjusted age (sustained group) or until they were taking at least 200 IU/d of vitamin D from formula or human milk fortifier (diet-limited group). Patients received open-label multivitamin until they were consuming 200 IU/d of cholecalciferol from formula or human milk fortifier, after which they received masked study drug (liquid cholecalciferol or a placebo, dispensed in an amber bottle). Parents, clinical caregivers, and study staff with patient contact were masked. Exclusively breastfed infants received open-label multivitamin as long as they were exclusively breastfed, up to 12 months’ adjusted age. The study protocol did not dictate what infants were fed.
Study visits occurred at 3, 6, 9, and 12 months of age, adjusted for degree of prematurity, in the infant’s home and in the clinic. A monthly diet questionnaire was completed until 6 months’ adjusted age (or longer if exclusively breastfed) to assess 25-hydroxyvitamin D (25[OH]D) intake.
Infants were eligible for enrollment if they were 280/7 to 366/7 weeks’ GA at birth, the family identified the child as black or African American, they received 28 days or less of supplemental oxygen, were admitted to a participating nursery as a neonate, were 406/7 weeks’ adjusted GA or younger at enrollment, and lived within a predefined geographic area at each site. Infant race was considered “black” or “African American” if the parents affirmed that they believed one of those descriptors applied to their infant.
Infants were not eligible if they were diagnosed as having bronchopulmonary dysplasia; had a preexisting diagnosis of moderate to severe osteopenia of prematurity and/or alkaline phosphatase level greater than 700 U/L (to convert to μkat/L, multiply by 0.0167); a history of fracture; a history of gastrointestinal surgery including for necrotizing enterocolitis, known gastrointestinal malabsorption, a major congenital anomaly, a congenital pulmonary or airway disorder, documented wheezing, or stridor prior to enrollment; previous vitamin D supplementation with more than 400 IU/d; or the family planned to move out of the region. Infants were also ineligible if their serum phosphorus concentration was outside of the range of 4.0 to 9.5 mg/dL (to convert to mmol/L, multiply by 0.323) or serum calcium was outside of the range of 8.5 to 10.7 mg/dL (to convert to mmol/L, multiply by 0.25). A 25(OH)D concentration less than 10 ng/mL or greater than 80 ng/mL also made infants ineligible (to convert to nmol/L, multiply by 2.496). Eligible ranges for laboratory values were modified in the first year of enrollment (eMethods in Supplement 2). Serum bone markers of health and 25(OH)D levels were processed at each site’s clinical chemistry laboratory.
Adherence was estimated by weighing bottles of either open-label multivitamins or masked study drug before they were dispensed and after they were returned. Ideal adherence was defined as a weight difference averaging approximately 1 g/d.
Adverse Event Monitoring
The trial was monitored by a National Institutes of Health–convened data and safety monitoring board. A medical monitor reviewed certain categories of adverse events and laboratory values out of a predetermined range (eMethods in Supplement 2). She had independent authorization to either remove a child from the study or refer for further medical care. She was authorized to view group allocation as needed for safety, but did not access this information.
Randomization, Stratification, and Blinding
Infants were randomized with randomly permuted blocks, sizes 2 to 6, using computer-generated random numbers. Randomization was stratified by site and whether the infant had received any of their own mother’s milk within 24 hours of randomization. Twins and triplets were randomized together. Families, clinical caregivers, and study staff were blinded to assignment and block size.
Outcomes
The primary outcome was parent-reported recurrent wheezing (≥2 episodes of wheezing with or without an infection) by 12 months’ adjusted age, using a modified International Study of Asthma and Allergies in Childhood questionnaire.12 These questions have been shown to perform well in infants born preterm.13 Recurrent wheezing was defined as 1 report of more than 1 wheezing episode since the last interview or reports of single wheezing episodes at multiple visits. The ascertainment of recurrent wheezing was standardized with other concurrent trials.14,15
Secondary outcomes included parental report of upper and lower respiratory tract infections, pulmonary medications, hospitalization and emergency department visits, allergies, and eczema. These were assessed by questionnaire at each follow-up point. The modified Asthma Predictive Index was calculated, which yields a positive or negative prediction.16 Eosinophil counts were obtained at 12 months. Scoring Atopic Dermatitis skin examinations for eczema were completed at each follow-up point; Scoring Atopic Dermatitis scores range from 0 to 103; and a value of 25 or more indicates moderate to severe eczema.17 Circulating 25(OH)D3 concentrations were measured at the 3-, 6-, and 12-month visits, using the immunoassays that were standard of care at each site. Clinical recommendations for infants generally target levels 20 ng/mL or greater.18,19
Serum markers of bone health (alkaline phosphatase, calcium, phosphorus) were also measured at the clinical chemistry laboratory at each site. A speed-of-sound measure of tibial bone density assessment was done at 12 months. Published norms have described the mean and 2 SDs below the mean for 1-year-old girls to be 3139 m/s and 2919 m/s, respectively, and for boys to be 3189 m/s and 2871 m/s, respectively.20 Values less than 2 SDs below the mean were considered abnormal. A panel of allergen-specific IgE antibodies (ImmunoCAP Phadiatop Infant; Phadia AB) was obtained at 12 months.21 The panel included common food and inhalant allergies (egg, cow’s milk, peanut, shrimp, cat, dog, house dust mite, birch, timothy, ragweed, and wall pellitory). Results range from 0 to 100 kA/L; a level of 0.35 kA/L or greater is considered positive.
Statistical Analysis
Power and Sample Size
Based on a baseline rate of recurrent wheezing of 30%, a 2-sided significance level of .05, and a power of 80% to detect a relative risk of 1.6, a sample size of 115 infants per group was estimated. Allowing for 15% loss to follow-up and 20% of enrolled infants to be part of a twin pair, a sample size of 300 infants was planned. In the absence of a generally accepted minimal clinically important difference for studies of recurrent wheezing in infancy, a relative risk of 1.6 was chosen to be sufficiently large to be meaningful at face value and to be plausible based on prior studies.10
Baseline Characteristics
Standard summary statistics were used to describe baseline characteristics. For characteristics measured at the family level, such as maternal age, Pearson χ2 tests or 2-sample t tests were used. For comparisons of patient-level characteristics, such as birth weight, we used generalized estimating equations (GEEs) with exchangeable working covariance to reflect within-family covariance. All statistical analyses were conducted using SPSS version 25 (IBM Corp) or SAS for Windows 9.4 (SAS Institute Inc).
Primary Outcome
Analyses were based on a modified intent-to-treat approach. Because the primary outcome was a composite of longitudinal points, and most participants who withdrew did so before 3 months (Figure 1), withdrawn participants were not included. The 4 participants who were lost to all follow-up were not included in the primary analysis. Type I error was assumed to be .05, except for the primary outcome. One interim analysis was performed. The significance level used at the interim analysis was .003, while the significance level used at the final analysis was .047.
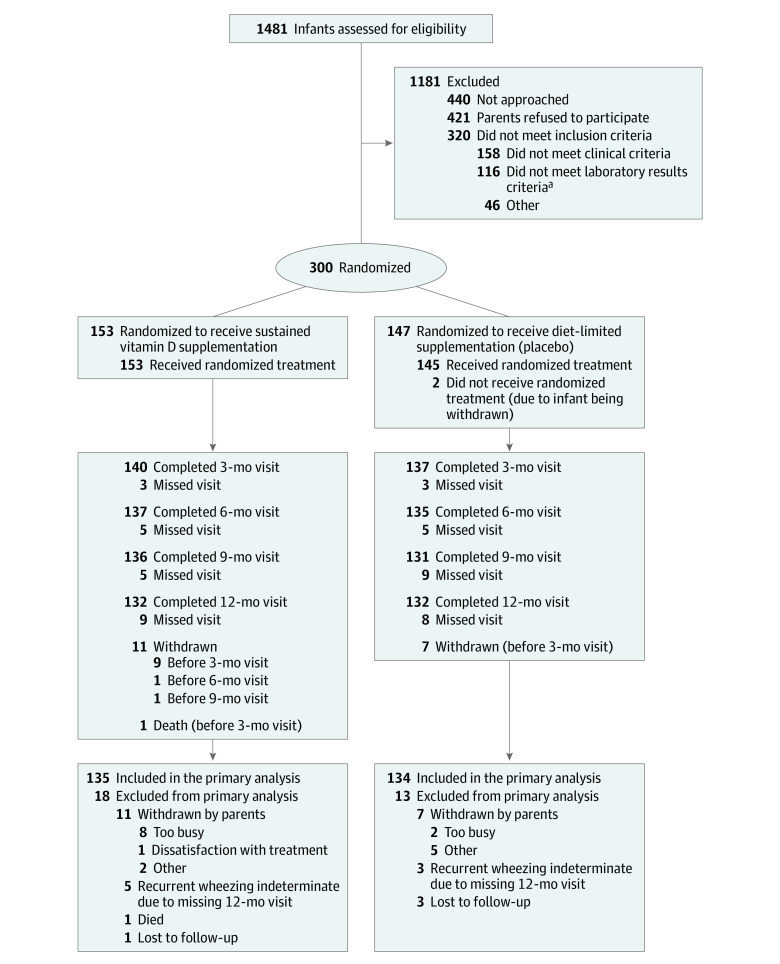
Figure 1. Flow of Patients in the D-Wheeze Study.
If all of the eligibility laboratory tests had not been obtained as part of clinical care, infants were consented prior to obtaining study eligibility laboratory tests. If they continued to be eligible, they were then randomized to the sustained or diet-limited group. In both groups, infants received an open-label multivitamin, which provided 400 IU/d of vitamin D until they were receiving 200 IU/d or more from formula or human milk fortifiers. When they were ingesting 200 IU/d or more of vitamin D, they received blinded study drug, which provided 400 IU/d of cholecalciferol (sustained group) or placebo (diet-limited group). Study drug was continued until 6 months’ adjusted gestational age, unless the infant continued to be exclusively fed human milk. In both groups, infants were provided open-label multivitamin if they were exclusively fed maternal milk, up to 12 months’ adjusted gestational age. Two-hundred eighteen infants were enrolled at University Hospitals Cleveland Medical Center (Cleveland, Ohio), 39 infants were enrolled at Medical University of South Carolina (Charleston), 38 infants were enrolled at Montefiore Medical Center (Bronx, New York), and 5 infants were enrolled at MetroHealth Medical Center (Cleveland, Ohio).
aLaboratory tests included calcium, phosphorus, alkaline phosphatase, and 25-hydroxyvitamin D.
For the primary outcome of recurrent wheezing, we used GEEs to account for the nonindependence of twins and triplets and to estimate the risk ratio and risk difference.22,23 Two Poisson regression models, adjusted and unadjusted, were planned a priori, with the unadjusted model considered primary. Covariates in the secondary adjusted model included randomization strata (site and maternal milk at enrollment), GA, time in study, and baseline covariates associated with recurrent wheezing within a significance level of .10. Adjustment for unbalanced baseline characteristics not associated with recurrent wheezing was not planned because, if a covariate is not strongly related to an outcome but is imbalanced between groups, adjustment for an imbalanced covariate may dilute the effect of treatment.24
A pattern mixture modeling approach was used to assess the sensitivity of the primary finding to missing data. This included 8 participants whose recurrent wheezing outcome could not be determined due to a missing 12-month visit and not having yet met the definition of recurrent wheezing. We considered a range of scenarios where the outcome was no longer independent of whether or not the data were missing (ie, not missing at random). This missing data approach is detailed in the eMethods in Supplement 2.
Secondary Outcomes
For secondary binary outcomes that were planned a priori, we fit unadjusted Poisson GEE regression models. For continuous outcomes, we used linear GEE models. Secondary outcomes were also analyzed by follow-up point. Corresponding 95% CIs of risk and mean difference were generated from these models.
Post Hoc Analyses
Post hoc analyses assessed the role of baseline maternal milk status and sex on treatment outcomes through modeling of interaction with randomization group. In addition, a post hoc analysis of the primary outcome was conducted, which added baseline unbalanced covariates (P < .05) to the adjusted model.
Results
Characteristics of the Trial Population
Of the 300 infants enrolled in the study, 18 withdrew from the study and 1 died while cosleeping (Figure 1). Follow-up rates of surviving nonwithdrawn infants at the 3-, 6-, 9-, and 12-month visits were 97.9%, 96.5%, 95.0%, and 94.0%, respectively. Due to missing 12-month visits in infants who had not yet met criteria for recurrent wheezing, we were unable to determine recurrent wheezing status for 8 children, and these cases were considered as missing data in the primary analysis. Overall, the population had multiple risk factors for wheezing, including high rates of asthma in the family, smokers in the home, and other children younger than 5 years of age in the home (Table 1).
Table 1. Baseline Characteristics.
| Characteristic | No./Total No. (%) | |
|---|---|---|
| Sustained Vitamin D Supplementation | Diet-Limited Supplementation | |
| Infant | n = 153 | n = 147 |
| Gestational age at birth, median (IQR), wk | 33.0 (31.4-35.3) | 33.5 (32.0-35.2) |
| 28-33 | 99/153 (64.7) | 84/147 (57.1) |
| 34-36 | 54/153 (35.3) | 63/147 (42.9) |
| Adjusted gestational age at randomization, median (IQR), wk | 35.3 (34.3-36.4) | 35.4 (34.6-36.6) |
| Age at randomization, median (IQR), d | 11 (6-21) | 13 (6-20) |
| Birth weight, median (IQR), kg | 1.84 (1.53-2.25) | 1.96 (1.60-2.38) |
| Multiple birtha | 58/153 (37.9) | 38/146 (26.0) |
| Male | 77/153 (50.3) | 89/146 (61.0) |
| Antenatal steroids | 82/153 (53.6) | 78/146 (53.4) |
| Received surfactant | 25/153 (16.3) | 29/146 (19.9) |
| History of ventilator support | 27/153 (17.6) | 26/146 (17.8) |
| Time on ventilator, median (IQR), d | 0 (0-0) | 0 (0-0) |
| History of any oxygen treatment | 66/153 (43.1) | 53/146 (36.3) |
| Time on oxygen, median (IQR), d | 0 (0-1) | 0 (0-1) |
| Referred for outpatient palivizumabb | 24/153 (15.7) | 19/145 (13.1) |
| Receiving maternal breast milk at randomization | 107/153 (69.9) | 104/147 (70.7) |
| Discharge season, October-March | 69/153 (45.1) | 67/146 (45.9) |
| Supplemented with vitamin D prior to enrollmentc | 82/152 (53.9) | 78/146 (53.4) |
| Laboratory values, median (IQR) | ||
| Calcium, mg/dL | 9.5 (9.0-9.9) | 9.5 (8.7-10.0) |
| Phosphorus, mg/dL | 7.0 (6.5-7.7) | 6.9 (6.3-7.5) |
| Alkaline phosphatase, U/L | 268 (211-343) | 257 (210-309) |
| Total circulating 25(OH)D, ng/mL | 19.1 (15.7-28.0) | 21.0 (17.0-25.0) |
| Family | n = 126 | n = 126 |
| Maternal age, median (IQR), y | 26 (22-30) | 25 (22-30) |
| Black race | ||
| Maternal | 124/125 (99.2) | 118/124 (95.2) |
| Paternal | 123/125 (98.4) | 123/126 (97.6) |
| Hispanic ethnicity | ||
| Maternal | 9/126 (7.1) | 10/125 (8.0) |
| Paternal | 12/126 (9.5) | 7/125 (5.6) |
| Maternal education | ||
| Did not graduate high school | 25/126 (19.8) | 29/126 (23.0) |
| High school graduate | 33/126 (26.2) | 39/126 (31.0) |
| Any college | 45/126 (35.7) | 41/126 (32.5) |
| Trade school | 8/126 (6.3) | 5/126 (4.0) |
| Associates degree | 6/126 (4.8) | 3/126 (2.4) |
| College graduate | 5/126 (4.0) | 6/126 (4.8) |
| Graduate degree | 4/126 (3.2) | 3/126 (2.4) |
| Paternal education | ||
| Did not graduate high school | 22/126 (17.5) | 18/126 (14.3) |
| High school graduate | 53/126 (42.1) | 58/126 (46.0) |
| Any college | 30/126 (23.8) | 27/126 (21.4) |
| Trade school | 3/126 (2.4) | 2/126 (1.6) |
| Associates degree | 6/126 (4.8) | 1/126 (0.8) |
| College graduate | 3/126 (2.4) | 9/126 (7.1) |
| Graduate degree | 0/126 (0.0) | 1/126 (0.8) |
| Unknown | 9/126 (7.1) | 10/126 (7.9) |
| Family history of food allergy | 49/124 (39.5) | 42/124 (33.9) |
| First-degree relative with food allergy | 33/124 (26.6) | 28/124 (22.6) |
| Family history of hay fever or pollen allergy | 87/123 (70.7) | 74/126 (58.7) |
| First-degree relative with hay fever | 65/123 (52.8) | 55/126 (43.7) |
| Family history of eczema | 87/124 (70.2) | 79/126 (62.7) |
| First-degree relative with eczema | 60/124 (48.4) | 62/126 (49.2) |
| Family history of asthma | 86/123 (69.9) | 73/126 (57.9) |
| First-degree relative with asthma | 56/123 (45.5) | 46/126 (36.5) |
| Children aged <5 y in home | 70/126 (55.6) | 62/126 (49.2) |
| Pets in home | 36/126 (28.6) | 34/126 (27.0) |
| Daycare planned | 33/126 (26.2) | 37/126 (29.4) |
| Smokers in home | 48/126 (38.1) | 47/126 (37.3) |
| WIC enrollmentd | 118/122 (96.7) | 116/122 (95.1) |
| Housing | ||
| Owns home | 10/126 (7.9) | 9/126 (7.1) |
| Rents home | 78/126 (61.9) | 82/126 (65.1) |
| Lives with family and friends | 36/126 (28.6) | 34/126 (27.0) |
| Lives in a shelter | 2/126 (1.6) | 1/126 (0.8) |
| Insurance | ||
| Private | 18/126 (14.3) | 11/126 (8.7) |
| Public | 107/126 (84.9) | 113/126 (89.7) |
| No Insurance | 1/126 (0.8) | 2/126 (1.6) |
Abbreviations: IQR, interquartile range; 25(OH)D, 25-hydroxyvitamin D; WIC, Women Infants and Children.
SI conversion factors: To convert alkaline phosphatase to μkat/L, multiply by 0.0167; calcium to mmol/L, multiply by 0.25; 25(OH)D to nmol/L, multiply by 2.496; and phosphorus to mmol/L, multiply by 0.323.
Infants of multiple births were randomized together and therefore received the same study drug allocation.
Infants who qualify for outpatient palivizumab prophylaxis for respiratory syncytial virus are routinely referred to a prophylaxis program prior to discharge.
Some infants were supplemented with 200 to 400 IU/d of vitamin D, per clinical practice, prior to study enrollment.
WIC is a program that offers supplemental food, health care referrals, and nutrition education for low-income women and children.
At the first diet screen, 1 month after enrollment, 61.4% of those receiving maternal milk at randomization and 94.1% of those not receiving maternal milk were above the 200 IU/d threshold to transition to blinded study drug. At the 3-month adjusted age point, 90.2% of the infants receiving maternal milk at randomization and 100% of those not receiving maternal milk had been transitioned to blinded study drug. Feeding patterns are described in eTable 1 in Supplement 2.
Adherence was estimated for participants who received their final bottle of study drug and returned all bottles. Among participants who received their final bottle of study drug, rates of return of all bottles were 75 of 141 (53.2%) in the sustained group and 60 of 133 (45.1%) in the diet-limited group. Average estimated daily intake was 0.94 g in the sustained group and 0.91 g in the diet-limited group.
Primary Outcome
Recurrent wheezing was experienced by 42 of 135 (31.1%) in the sustained supplementation group compared with 56 of 134 (41.8%) in the diet-limited supplementation group in the primary unadjusted analysis (difference, −10.7% [95% CI, −27.4% to −2.9%]; relative risk, 0.66 [95% CI, 0.47 to 0.94]; P = .02) (Table 2). Missing data sensitivity analysis supports the primary finding, in that associated averaged P values across a range of imputation scenarios were consistently less than .047 (eMethods in Supplement 2).
Table 2. Primary and Secondary Outcomes by Randomization Group.
| Outcome | No./Total No. (%) | Risk Difference, % (95% CI)a | |
|---|---|---|---|
| Sustained Vitamin D Supplementation | Diet-Limited Supplementation | ||
| Primary | |||
| Recurrent wheezing | 42/135 (31.1) | 56/134 (41.8) | −10.7 (−27.4 to −2.9) |
| Secondary | |||
| Diagnosis of asthmab | 13/141 (9.2) | 15/137 (10.9) | −1.7 (−10.8 to 3.8) |
| Diagnosis of eczemab | 53/140 (37.9) | 63/137 (46.0) | −8.1 (−21.1 to 3.3) |
| Diagnosis of food allergyb | 9/141 (6.4) | 7/137 (5.1) | 1.3 (−4.8 to 6.7) |
| Pollen or dust allergyb | 3/141 (2.1) | 3/137 (2.2) | −0.1 (−3.5 to 3.4) |
| Positive panel of IgE antibodies (≥0.35 kA/L) at 12 mo | 7/116 (6.0) | 3/112 (2.7) | 3.4 (−1.8 to 8.6) |
| Positive modified Asthma Predictive Index | 15/140 (10.7) | 19/137 (13.9) | −3.2 (−12.4 to 4.6) |
| Any pediatrician sick visit | 74/141 (52.5) | 71/137 (51.8) | 0.7 (−13.6 to 11.3) |
| Respiratory sick visit | 52/141 (36.9) | 45/137 (32.9) | 4.0 (−12.2 to 12.0) |
| Any emergency department visit | 95/141 (67.4) | 103/137 (75.2) | −7.8 (−19.4 to 3.0) |
| Respiratory emergency department visit | 75/141 (53.2) | 69/137 (50.4) | 2.8 (−13.2 to 11.9) |
| Respiratory non-ICU hospitalizationb,c | 14/141 (9.9) | 20/137 (14.6) | −4.7 (−13.2 to 2.9) |
| Respiratory ICU hospitalizationb,c | 1/141 (0.7) | 6/137 (4.4) | −3.7 (−7.4 to 0.02) |
| Any adverse eventsd | 128/153 (83.7) | 127/147 (86.4) | −2.7 (−12.2 to 4.7) |
| Asthma or wheezing | 41/153 (26.8) | 42/147 (28.6) | −1.8 (−14.2 to 7.5) |
| Upper respiratory | 84/153 (54.9) | 83/147 (56.5) | −1.6 (−17.1 to 7.0) |
| Lower respiratory | 33/153 (21.6) | 37/147 (25.2) | −3.6 (−16.4 to 4.4) |
| Other infections | 56/153 (36.6) | 66/147 (44.9) | −8.3 (−20.6 to 2.7) |
| Rash | 38/153 (24.8) | 43/147 (29.3) | −4.4 (−14.8 to 6.3) |
| Any serious adverse eventd | 36/153 (23.5) | 38/147 (25.9) | −2.3 (−13.2 to 7.4) |
| Eosinophils >4% at 12 mo | 26/114 (22.8) | 24/112 (21.4) | 1.4 (−10.8 to 12.1) |
| Calcium >10.7 mg/dL | |||
| 3 mo | 17/135 (12.6) | 20/134 (14.9) | −2.3 (−10.2 to 7.4) |
| 6 mo | 9/134 (6.7) | 16/132 (12.1) | −5.4 (−13.0 to 1.9) |
| Alkaline phosphatase >500 U/L | |||
| 3 mo | 10/135 (7.4) | 4/134 (3.0) | 4.4 (−1.7 to 9.2) |
| 6 mo | 3/134 (2.2) | 8/131 (6.1) | −3.9 (−8.6 to 1.0) |
| Tibial speed of sound at 12 mo, median (interquartile range)e | 3155 (3081-3209) (n = 105) |
3149 (3095-3225) (n = 107) |
|
Abbreviation: ICU, intensive care unit.
SI conversion factors: To convert alkaline phosphatase to μkat/L, multiply by 0.0167; and calcium to mmol/L, multiply by 0.25.
Risk difference was calculated as the difference in percentage between sustained and diet-limited. The confidence interval for risk difference was obtained through Poisson generalized estimating equation regression with identity link.
Assessed by parental report at each follow-up point.
This represents the number of patients who experienced each type of hospitalization. Patients may have experienced more than 1 hospitalization. In the sustained group, there were 1 ICU hospitalization and 16 non-ICU hospitalizations. In the diet-limited group, there were 7 ICU hospitalizations and 34 non-ICU hospitalizations.
Adverse events by organ system are shown in eTable 4 in Supplement 2.
Tibial speed of sound is a quantitative ultrasound method that allows for radiation-free assessments of bone density. No participants had values lower than 2 SDs below the mean of previously published norms.21
A planned model of recurrent wheezing adjusting for randomization strata, time in study, GA, and the variables associated with recurrent wheezing in bivariate analyses was also run. In this model, recurrent wheezing was significantly decreased in the sustained supplementation group (relative risk, 0.62 [95% CI, 0.44 to 0.87]; P = .005) (eTable 2 in Supplement 2).
Secondary Outcomes
For secondary outcomes, values were determined for each infant included, so there were no missing outcomes in the analyses. No significant differences between groups were seen in total medically attended illnesses or markers of allergy or bone health (Table 2). The Asthma Predictive Index was positive for 15 of 140 (10.7%) and 19 of 137 (13.9%) infants in the sustained and diet-limited groups, respectively (difference, −3.2% [95% CI, −12.4% to 4.6%]). Emergency department visits for a respiratory cause were experienced by 75 of 141 (53.2%) in the sustained group and 69 of 137 (50.4%) in the diet-limited group (difference, 2.8% [95% CI, −13.2% to 11.9%]). There was also not a significant difference in respiratory hospitalizations. No participants had a tibial speed of sound measurement more than 2 SDs below the mean, based on previously published norms, at 12 months.20 Cases of elevated calcium and alkaline phosphatase levels were generally transient and did not differ between treatment groups. In analyses by time point, a significant decrease was seen in the sustained group at some, but not all, points for wheezing, respiratory medication use, and hospitalization (eTable 3 in Supplement 2).
Adverse Events
There were no statistically significant differences between the groups in the number of infants experiencing any adverse events or serious adverse events (Table 2). Upper and lower respiratory tract infections were among the most commonly reported adverse events. Upper respiratory infections were experienced by 84 of 153 infants (54.9%) in the sustained group and 83 of 147 infants (56.5%) in the diet-limited group (difference, −1.6% [95% CI, −17.1% to 7.0%]). Lower respiratory infections were experienced by 33 of 153 infants (21.6%) in the sustained group and 37 of 147 infants (25.2%) in the diet-limited group (difference, −3.6% [95% CI, −16.4% to 4.4%]). No adverse events were attributed to vitamin D treatment or deficiency. No infants were diagnosed as having rickets. Adverse event counts by organ system are presented in eTable 4 in Supplement 2.
Post Hoc Analyses
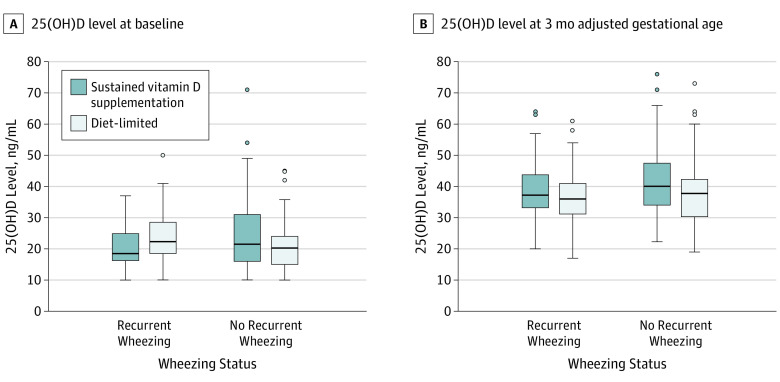
Median achieved circulating 25(OH)D concentrations were greater than 30 ng/mL at follow-up (Figure 2 and eTable 5 in Supplement 2). Separation between groups was primarily seen in the infants who were not receiving maternal milk at randomization. However, there was no significant interaction between maternal milk at randomization and group (P = .20). There was also no significant interaction between sex and randomization group (P = .32).
Figure 2. 25-Hydroxyvitamin D (25[OH]D) Levels at Baseline (A) and 3 Months’ Adjusted Age (B), By Recurrent Wheezing and Randomization Group.
Recurrent wheezing is defined as 2 or more episodes of wheezing during the study period. Box and whisker plots are shown. The extreme horizontal bar for the upper whisker represents the largest value in the respective group that is within 1.5 times the interquartile range or the first quartile, while for the lower whisker, it is the smaller value within 1.5 times the interquartile range of the third quartile. The middle horizontal line in the box represents the median. Values beyond the range of box and whiskers are denoted as points. The 25(OH)D levels at baseline ranged from 10.0 to 71.0 ng/mL in the sustained group and 10.0 to 50.0 ng/mL in the diet-limited group. At 3 months’ adjusted gestational age, the levels ranged from 20.0 to 76.0 ng/mL in the sustained group and 17.0 to 73.0 ng/mL in the diet-limited group. Infants were eligible for enrollment with 25(OH)D levels of 10.0 to 80.0 ng/mL. At 3 months, levels outside of the range of 15.0 to 80.0 ng/mL were treated as adverse events and would have triggered a medical monitor review. At baseline, the sample sizes were 42 and 56 in the sustained and diet-limited groups, respectively, for recurrent wheezing and 93 and 78 for no recurrent wheezing; at 3 months, sample sizes were 42 and 55 in the sustained and diet-limited groups, respectively, for recurrent wheezing and 88 and 78 for no recurrent wheezing.
Discussion
In this randomized clinical trial of black preterm infants, sustained supplementation with vitamin D compared with diet-limited supplementation resulted in a reduced risk of recurrent wheezing by 12 months’ adjusted age. Neither group demonstrated superiority in terms of bone health or allergic disease.
Both supplementation strategies targeted a total daily intake between 200 and 1000 IU of vitamin D, generally within the high and low ranges recommended by the American Academy of Pediatrics and Institute of Medicine18,19,25; infants consuming a high volume of formula designed for feeding preterm infants may have exceeded that range. Infant dietary vitamin D intake also tends to increase over time; therefore, total intake was not constant over the study period. Epidemiologic studies in a northern Finland cohort showed an association between infant supplementation and allergy and atopy later in life,26 and other cohort studies have found increased wheezing or asthma in black infants being supplemented with up to 400 IU/d.10,11 However, this study suggests a benefit of sustained supplementation with 400 IU/d with regard to recurrent wheezing, without an increase in allergy or eczema. The mechanisms of such an effect are unknown, but could potentially include developmental alterations in the airway or immune system, or acute alterations in inflammation or response to infection.
In pediatric and adult populations, several trials have failed to show a significant benefit of vitamin D supplementation on respiratory outcomes, including asthma or upper respiratory infections.14,27,28,29 In addition, in a study of extremely preterm infants, vitamin D did not affect duration of respiratory support.30 However, multiple observational studies have shown an association between respiratory disease and low vitamin D levels.31,32,33,34,35 In term infants, differences in cord 25(OH)D levels were associated with meaningful differences in the risk of lower respiratory tract infection with respiratory syncytial virus.32 A recent individual patient data meta-analysis found that supplementation decreases respiratory infections, particularly in vitamin D–deficient patients or those receiving daily dosing.36,37 However, results of studies in other populations should not be extrapolated to preterm infants who may be in a critical developmental window with regard to the effects of vitamin D on the pulmonary and immune systems.
This effectiveness study has several strengths, including targeting clinically relevant outcomes in a high-risk and under-studied population of preterm infants, with low rates of attrition during the study. Validated questions were used that have been shown to perform well in this population and were concurrently used in other trials.12,13,14,15,38 Stratification of randomization by receipt of maternal milk intake in the prior 24 hours aimed to balance maternal milk exposure between randomization groups, due to the immunologic and dietary differences between breastfed and exclusively formula fed infants, including the potential protective role of maternal milk with regard to wheezing and infection.
Limitations
This study has several limitations. First, despite the use of a validated questionnaire, there is potential for misclassification of wheezing by questionnaire. Second, the study was not powered to detect significant differences in secondary outcomes such as hospitalization or serious adverse events. Third, resource utilization, such as medically attended illnesses, are also highly driven by nonbiological social factors. Fourth, the significance level for a priori secondary outcomes was not adjusted for multiple comparisons. Therefore, statistically significant results in secondary outcomes, such as the analyses by follow-up point, should be interpreted as exploratory. Fifth, this effectiveness study tested 2 dosing regimens within the bounds of common supplementation recommendations in a population of black preterm infants born at 280/7 to 366/7 weeks’ GA with a paucity of major neonatal morbidities. The sustained strategy may or may not reflect the optimum dosing strategy in this population; the results cannot be generalized to support the efficacy or adverse event profiles of higher doses; nor can they be generalized to infants of other races, those born at less than 28 weeks’ GA, or infants with bronchopulmonary dysplasia.
Conclusions
Among black infants born preterm, sustained supplementation with vitamin D, compared with diet-limited supplementation, resulted in a reduced risk of recurrent wheeze by 12 months’ adjusted age. Future research is needed to better understand the mechanisms and longer-term effects of vitamin D supplementation on wheezing in children born preterm.
Trial Protocol
eMethods
eReference
eTable 1. Overview of Maternal Milk and Formula Feeding, by Randomization Group.
eTable 2. Full Estimates for Preplanned and Post Hoc Adjusted Models.
eTable 3. Outcomes by Reporting Time-Point.
eTable 4. Adverse Events by Organ System.
eTable 5. Post Hoc Analysis of Achieved 25(OH)D Levels by Maternal Breast Milk Status at Randomization.
References
- 1.Bossé Y, Maghni K, Hudson TJ. 1alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics. 2007;29(2):161-168. [DOI] [PubMed] [Google Scholar]
- 2.Edelson JD, Chan S, Jassal D, Post M, Tanswell AK. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim Biophys Acta. 1994;1221(2):159-166. [DOI] [PubMed] [Google Scholar]
- 3.Koroglu OA, Onay H, Cakmak B, et al. Association of vitamin D receptor gene polymorphisms and bronchopulmonary dysplasia. Pediatr Res. 2014;76(2):171-176. [DOI] [PubMed] [Google Scholar]
- 4.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112(3):585-592. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol. 1996;271(3, pt 1):L392-L399. [DOI] [PubMed] [Google Scholar]
- 6.Saadoon A, Ambalavanan N, Zinn K, et al. Effect of prenatal versus postnatal vitamin D deficiency on pulmonary structure and function in mice. Am J Respir Cell Mol Biol. 2017;56(3):383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foong RE, Bosco A, Jones AC, et al. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am J Respir Cell Mol Biol. 2015;53(5):664-675. [DOI] [PubMed] [Google Scholar]
- 8.Yurt M, Liu J, Sakurai R, et al. Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. Am J Physiol Lung Cell Mol Physiol. 2014;307(11):L859-L867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183(10):1336-1343. [DOI] [PubMed] [Google Scholar]
- 10.Hibbs AM, Babineau DC, Wang X, Redline S. Race differences in the association between multivitamin exposure and wheezing in preterm infants. J Perinatol. 2015;35(3):192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milner JD, Stein DM, McCarter R, Moon RY. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114(1):27-32. [DOI] [PubMed] [Google Scholar]
- 12.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483-491. [DOI] [PubMed] [Google Scholar]
- 13.Boggs E, Minich N, Hibbs AM. Performance of commonly used respiratory questionnaire items in a cohort of infants born preterm. Open J Pediatr. 2013;3(3):260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART Randomized Clinical Trial. JAMA. 2016;315(4):362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEvoy CT, Milner KF, Scherman AJ, et al. Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP): rationale, design, and methods of a randomized, controlled trial of vitamin C supplementation in pregnancy for the primary prevention of effects of in utero tobacco smoke exposure on infant lung function and respiratory health. Contemp Clin Trials. 2017;58:66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang TS, Lemanske RF Jr, Guilbert TW, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract. 2013;1(2):152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157(4):645-648. [DOI] [PubMed] [Google Scholar]
- 18.Abrams SA; Committee on Nutrition . Calcium and vitamin D requirements of enterally fed preterm infants. Pediatrics. 2013;131(5):e1676-e1683. [DOI] [PubMed] [Google Scholar]
- 19.Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition . Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142-1152. [DOI] [PubMed] [Google Scholar]
- 20.Zadik Z, Price D, Diamond G. Pediatric reference curves for multi-site quantitative ultrasound and its modulators. Osteoporos Int. 2003;14(10):857-862. [DOI] [PubMed] [Google Scholar]
- 21.Halvorsen R, Jenner A, Hagelin EM, Borres MP. Phadiatop infant in the diagnosis of atopy in children with allergy-like symptoms. Int J Pediatr. 2009;2009:460737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13-22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 23.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. [DOI] [PubMed] [Google Scholar]
- 24.Pocock SJAS, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21(19):2917-2930. [DOI] [PubMed] [Google Scholar]
- 25.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyppönen E, Sovio U, Wjst M, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84-95. [DOI] [PubMed] [Google Scholar]
- 27.Aglipay M, Birken CS, Parkin PC, et al. ; TARGet Kids! Collaboration . Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro M, King TS, Kunselman SJ, et al. ; National Heart, Lung, and Blood Institute’s AsthmaNet . Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311(20):2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379(9824):1419-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fort P, Salas AA, Nicola T, Craig CM, Carlo WA, Ambalavanan N. A comparison of 3 vitamin D dosing regimens in extremely preterm infants: a randomized controlled trial. J Pediatr. 2016;174:132-138.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alyasin S, Momen T, Kashef S, Alipour A, Amin R. The relationship between serum 25 hydroxy vitamin d levels and asthma in children. Allergy Asthma Immunol Res. 2011;3(4):251-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belderbos ME, Houben ML, Wilbrink B, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127(6):e1513-e1520. [DOI] [PubMed] [Google Scholar]
- 33.Brehm JM, Celedón JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freishtat RJ, Iqbal SF, Pillai DK, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156(6):948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maxwell CS, Carbone ET, Wood RJ. Better newborn vitamin D status lowers RSV-associated bronchiolitis in infants. Nutr Rev. 2012;70(9):548-552. [DOI] [PubMed] [Google Scholar]
- 36.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118(6, pt 2):1-120. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods
eReference
eTable 1. Overview of Maternal Milk and Formula Feeding, by Randomization Group.
eTable 2. Full Estimates for Preplanned and Post Hoc Adjusted Models.
eTable 3. Outcomes by Reporting Time-Point.
eTable 4. Adverse Events by Organ System.
eTable 5. Post Hoc Analysis of Achieved 25(OH)D Levels by Maternal Breast Milk Status at Randomization.