Key Points
Question
Is there a significant difference in subsequent colorectal cancer (CRC) incidence between individuals with either advanced or nonadvanced adenomas compared with those with no adenomas?
Findings
In this prospective cohort study that included 15 935 participants undergoing colonoscopy following an abnormal flexible sigmoidoscopy screening with a median follow-up of 13 years, the CRC incidence per 10 000 person-years was 20.0 for baseline advanced adenoma, 9.1 for nonadvanced adenoma, and 7.5 for no adenoma. The difference compared with no adenoma was statistically significant for advanced adenoma but not for nonadvanced adenoma.
Meaning
Identification of advanced adenoma was associated with increased risk for subsequent CRC incidence; nonadvanced adenoma may not be associated with increased risk.
Abstract
Importance
Individuals with adenomatous polyps are advised to undergo repeated colonoscopy surveillance to prevent subsequent colorectal cancer (CRC), but the relationship between adenomas at colonoscopy and long-term CRC incidence is unclear.
Objective
To compare long-term CRC incidence by colonoscopy adenoma findings.
Design, Setting, and Participants
Multicenter, prospective cohort study of participants in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer randomized clinical trial of flexible sigmoidoscopy (FSG) beginning in 1993 with follow-up for CRC incidence to 2013 across the United States. Participants included 154 900 men and women aged 55 to 74 years enrolled in PLCO of whom 15 935 underwent colonoscopy following their first positive FSG screening result. The final day of follow-up was December 31, 2013.
Exposures
Enrolled participants had been randomized to FSG or usual care. Participants who underwent FSG and had abnormal findings were referred for follow-up. Subsequent colonoscopy findings were categorized as advanced adenoma (≥1 cm, high-grade dysplasia, or tubulovillous or villous histology), nonadvanced adenoma (<1 cm without advanced histology), or no adenoma.
Main Outcomes and Measures
The primary outcome was CRC incidence within 15 years of the baseline colonoscopy. The secondary outcome was CRC mortality.
Results
There were 15 935 participants who underwent colonoscopy (men, 59.7%; white, 90.7%; median age, 64 y [IQR, 61-68]). On initial colonoscopy, 2882 participants (18.1%) had an advanced adenoma, 5068 participants (31.8%) had a nonadvanced adenoma, and 7985 participants (50.1%) had no adenoma; median follow-up for CRC incidence was 12.9 years. CRC incidence rates per 10 000 person-years of observation were 20.0 (95% CI, 15.3-24.7; n = 70) for advanced adenoma, 9.1 (95% CI, 6.7-11.5; n = 55) for nonadvanced adenoma, and 7.5 (95% CI, 5.8-9.7; n = 71) for no adenoma. Participants with advanced adenoma were significantly more likely to develop CRC compared with participants with no adenoma (rate ratio [RR], 2.7 [95% CI, 1.9-3.7]; P < .001). There was no significant difference in CRC risk between participants with nonadvanced adenoma compared with no adenoma (RR, 1.2 [95% CI, 0.8-1.7]; P = .30). Compared with participants with no adenoma, those with advanced adenoma were at significantly increased risk of CRC death (RR, 2.6 [95% CI, 1.2-5.7], P = .01), but mortality risk in participants with nonadvanced adenoma was not significantly different (RR, 1.2 [95% CI, 0.5-2.7], P = .68).
Conclusions and Relevance
Over a median of 13 years of follow-up, participants with an advanced adenoma at diagnostic colonoscopy prompted by a positive flexible sigmoidoscopy result were at significantly increased risk of developing colorectal cancer compared with those with no adenoma. Identification of nonadvanced adenoma may not be associated with increased colorectal cancer risk.
Trial Registration
clinicaltrials.gov Identifier: NCT00002540
This cohort study of participants in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer randomized clinical trial compares long-term CRC incidence between individuals with either advanced or nonadvanced adenomas compared with those with no adenomas.
Introduction
Observational studies and randomized trials of endoscopic screening for colorectal cancer (CRC) with colonoscopy or flexible sigmoidoscopy demonstrate a significant reduction in CRC incidence and mortality.1,2,3,4,5,6,7 The reduction in incidence is due to the removal of the precursor lesion of colorectal cancer, the adenomatous polyp.1,2,3,5,6,8
Adenomatous polyps occur in about one-third of patients undergoing screening colonoscopy.9 To prevent subsequent cancer after the removal of adenomatous polyps, individuals are advised to undergo periodic colonoscopy surveillance with the timing determined by the number of adenomas and whether they were advanced by size or histology. International guidelines for the timing and frequency of colonoscopy surveillance vary and have weak supporting evidence.10 Individuals with advanced adenomas (≥1 cm, high-grade dysplasia, or villous or tubulovillous histology) are advised to return in 3 years.11 In the United States, individuals with 1 to 2 nonadvanced adenomas (<1 cm and no high-grade dysplasia or villous or tubulovillous histology), the most common preneoplastic finding, are advised to return in 5 to 10 years, but evidence to inform guidelines for who should return at 5 as opposed to 10 years is lacking. The timing for surveillance colonoscopy should be based on the ensuing risk of developing CRC, but few studies have examined CRC incidence after adenoma removal.12,13,14,15
The relationship between adenoma findings on diagnostic colonoscopy prompted by a positive flexible sigmoidoscopy (FSG) screening result and long-term risk of CRC incidence was examined in participants in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial.
Methods
Study Population and Design
This study was a prospective cohort study of participants enrolled in the multicenter, randomized PLCO Cancer Screening Trial of FSG. The trial enrolled 154 900 men and women aged 55 to 74 years between 1993 and 2001. Individuals were randomized to receive FSG at baseline and again at year 3 (for those randomized before April 1995) or year 5 vs usual care. Participants who had a positive FSG result (findings of a polyp or mass) did not have the polyps removed but were referred to their primary care physicians for decisions regarding diagnostic follow-up, about 75% of whom underwent colonoscopy.16 Details of the trial have been previously published.2,17 Each participating center's institutional review board approved the protocol and all study participants provided written informed consent. The PLCO trial protocol and statistical analysis plan are available in Supplement 1.
Definition of Exposure and Study Cohort
For this study, participants who had a positive FSG result at the baseline (time zero [T0]) or the year 3 or 5 (time 3 y or time 5 y [T3 or T5]) examination and had a diagnostic colonoscopy within 1 year of the FSG were included. Medical records related to follow-up were collected and abstracted in a standardized fashion including polyp size, number, and histology. Pathologic findings were abstracted from local pathologic reports; specimens were not reviewed centrally. The number, size, and histologic characteristics of lesions detected at the colonoscopy were ascertained and categorized hierarchically in accordance with current US guidelines as (1) advanced adenoma (any adenoma ≥1 cm, high-grade dysplasia, or with tubulovillous or villous histology); (2) nonadvanced adenoma (adenomas <1 cm without advanced histology); or (3) no adenoma. Within the nonadvanced adenoma category, participants were subcategorized as 1 to 2 adenomas or 3 adenomas or more.11
Lesions in the rectum through the splenic flexure were defined as distal and those in the transverse colon to the cecum were defined as proximal. Colonoscopy preparation and cecal intubation were abstracted on the report forms. If the colonoscopy preparation was designated as inadequate or if the cecum was not visualized, the examination was classified as inadequate. If the preparation quality was not reported but the cecum was visualized, the colonoscopy examination was considered adequate. Baseline participant demographics including age, body mass index, and smoking status at enrollment, sex, participant-identified fixed-category race, family history of cancer(s), and aspirin or ibuprofen use were recorded as these factors are known to influence colonic neoplastic risk.18,19,20,21,22,23,24
The primary exclusion criteria were a history of prostate, lung, colorectal, or ovarian cancer or ongoing treatment for any type of cancer except basal or squamous-cell skin cancer. Beginning in 1995, participants with a colonoscopy, FSG, or barium enema within the prior 3 years were ineligible. Participants who underwent a colonoscopy within a year of the first positive FSG result and were diagnosed with colorectal cancer within 2 months of the colonoscopy were excluded.
Definition of Outcomes
The primary outcome was colorectal cancer incidence and the secondary outcome was colorectal cancer mortality. Incident cancers and deaths were ascertained primarily by an active, trial-led process that involved mailing annual study update questionnaires to participants, obtaining and abstracting medical records pertaining to cancer using certified tumor registrars, and obtaining death certificates to confirm mortality. Starting in 2011, the trial switched to a centralized follow-up system that utilized passive linkages to state cancer registries to assess cancer incidence and linkages to the National Death Index to assess mortality. Some participants declined to be reconsented and thus opted out of extended follow-up. Participants who consented for extended follow-up (85.0% of those alive in 2011) were followed up for CRC incidence through the end of 2013 and for mortality through the end of 2012; those who declined were followed for incidence until December 31, 2009, and for mortality until their date of opting out (generally in 2011). The final date of follow-up was December 31, 2013. Therefore, for those who declined, deaths and incident CRC cases were ascertained solely by the active process, and for consenters, deaths and cases were ascertained by the active process through the end of 2009 and through passive linkages after that (with some overlap during 2010-2011). Cause of death was reviewed blinded to study group and categorized as due to colorectal cancer in a formal adjudication process until 2009 and by death certificate or National Death Index-linked records after 2009.25,26
Statistical Methods
Categorical variables were compared using χ2 or Fisher exact test when appropriate. Continuous variables were compared using Wilcoxon rank sum testing for pairwise comparisons or Kruskal-Wallis for multiple comparisons.
Follow-up for CRC incidence and mortality began 2 months from the date of participants’ colonoscopy. CRC incidence and mortality rates were computed for the period within 15 years of the start of follow-up as incident CRC cases or deaths divided by person-years of observation; rates were similarly computed for other observation periods (eg, within 10 years, within 5 years). Cumulative CRC incidence through 15 years was computed using Kaplan-Meier curves. Participants without CRC were censored at the end of follow-up.
Colorectal incidence and mortality for participants with advanced adenoma, nonadvanced adenoma, and no adenoma were compared using rate ratios with corresponding 95% CIs. The referent was the no adenoma group unless otherwise stated. Additionally, Poisson regression models for colorectal cancer incidence were constructed using baseline adenoma status and variables previously shown to be associated with outcomes including age, sex, regular aspirin or ibuprofen use, baseline vs postbaseline status of the initial colonoscopy, as well as the timing of the initial diagnostic colonoscopy. Response rate to baseline questionnaire items (race, family history, body mass index, aspirin or ibuprofen use, smoking status, prior lower endoscopy) ranged from 98.3% to 99.3%. A dummy variable was used for missing data on aspirin or ibuprofen use. Model goodness of fit was assessed using χ2 tests on model deviance. All tests were 2-sided and significance was considered at an α of .05, unless otherwise stated. All analyses were performed using SAS software (SAS Institute), version 9.3.
Subgroup Analyses
Participants With a Negative Screening Result
To be included in the no adenoma group, participants had to have an initial positive FSG result but no findings or nonadenomatous findings at subsequent colonoscopy. To evaluate the representativeness of the no adenoma group, distal CRC incidence in the no adenoma group was compared with participants with a negative, adequate, initial FSG result. The analysis was restricted to the distal colon because participants with a negative baseline screening result could have had undetected proximal disease. To account for time from initial FSG screen to subsequent colonoscopy and ensure comparability between negative and positive FSG result groups, CRC follow-up began at 133 days from the negative FSG result, where 133 days represented the median interval from FSG screen to colonoscopy for participants in the no adenoma group. CRC incidence rates were computed similarly as for the primary analysis.
Analysis of Subsequent Colonoscopy
In participants with adenomas, repeat colonoscopy after initial colonoscopy could have affected the subsequent incidence of CRC. The trial did not routinely collect information on utilization or yield of surveillance colonoscopy. However, an ancillary study of colonoscopy utilization conducted in 2005-200727,28 collected such information in a randomly selected subset of participants (n = 3492, 21.9% of current study cohort). In the study of colonoscopy utilization, participants whose first positive screening result was in T3 or T5 were excluded. Otherwise, the eligibility criteria were having a positive initial FSG result and a follow-up colonoscopy, similar to the current study. In the study of colonoscopy utilization, participants with advanced adenomas were oversampled. Participants were interviewed by phone about colonoscopy use, and confirmatory medical records of colonoscopy examinations including the indication and pathology reports were obtained and abstracted. The proportion of participants with advanced adenoma, nonadvanced adenoma, and no adenoma who underwent subsequent colonoscopy and the removal of adenoma for each of these groups was determined.
Results
Study Cohort
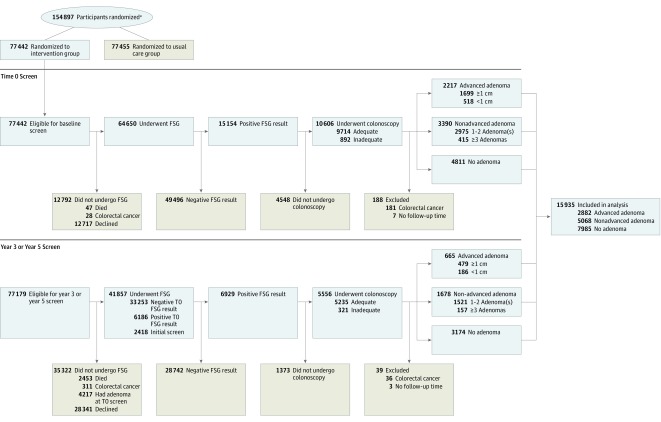
Of 77 442 participants randomized to FSG screening, 64 650 (83.5%) underwent an initial (T0) FSG examination, of whom 15 154 (19.6%) had a positive examination result and 10 606 (70.0%) had follow-up colonoscopy within 1 year. Of 35 671 participants who either had a negative initial FSG result and underwent repeat screening at T3 or T5 or whose initial screen was at T3 or T5, 6929 (19.4%) had a positive result and 5556 (80.2%) underwent colonoscopy within 1 year. In total, 16 162 participants had follow-up with diagnostic colonoscopy prompted by FSG screening. There were 217 cases of CRC diagnosed with colonoscopy and these participants were excluded. Ten participants had no follow-up time and were excluded, resulting in 15 935 included (Figure 1).
Figure 1. Flow of Included Participants Through the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
FSG indicates flexible sigmoidoscopy.
aData for number screened for eligibility and reasons for exclusion were not available.
Index colonoscopy findings identified 2882 participants (18.1%) with advanced adenoma, 5068 (31.8%) with nonadvanced adenoma, and 7985 (50.1%) with no adenoma (Table 1, Figure 1). Within the advanced adenoma group, 2178 participants (75.6%) had a lesion of 1 cm or larger and 704 participants (24.4%) had advanced histology (114 [16.2%] with high-grade dysplasia and 590 participants [83.8%] had villous or tubulovillous pathology). In the nonadvanced adenoma group, 572 participants (11.3%) had 3 or more nonadvanced adenomas and 4496 participants (88.7%) had 1 to 2 nonadvanced adenoma(s). Those with any adenomas were more likely to be men (Table 1). Aspirin or ibuprofen use, family history of CRC, smoking status, body mass index, and race did not differ by adenoma status. Overall median follow-up for CRC incidence was 12.9 years with 13.6, 13.1, and 12.5 years for advanced adenoma, nonadvanced adenoma, and no adenoma, respectively. The proportion of each group who declined extended follow-up ranged from 9.5% to 9.8% with no significant difference between adenoma groups (eTable 1 in Supplement 2). Those who declined extended follow-up were significantly younger, women, and white and had an overall median follow-up of 11.1 years compared with 13.2 years for those who agreed to extended follow-up.
Table 1. Study Population Baseline Demographics and Characteristics by Index Colonoscopy Findings Among Participants Aged 55 to 74 Years Enrolled in the Prostate, Lung, Colorectal, and Ovarian Cancer Randomized Clinical Trial.
| Advanced Adenoma, No. (%) | Nonadvanced Adenoma, No. (%) | No Adenoma, No. (%) | Total Population, No. (%) (N = 15 935) |
|||||
|---|---|---|---|---|---|---|---|---|
| All (n = 2882) |
≥1 cm (n = 2178) |
<1 cm (n = 704)a |
All (n = 5068) |
≥3 (n = 572) |
1-2 (n = 4496) |
All (n = 7985) |
||
| Age, median (IQR), yb | 65 (61-69) | 65 (61-69) | 65 (61-69) | 64 (61-68) | 64 (61-69) | 64 (61-68) | 64 (61-68) | 64 (61-68) |
| Men | 1937 (67.2) | 1465 (67.3) | 472 (67.0) | 3307 (65.3) | 432 (75.5) | 2875 (63.9) | 4263 (53.4) | 9507 (59.7) |
| Race/ethnicity | ||||||||
| White | 2608 (90.5) | 1955 (89.8) | 653 (92.8) | 4551 (89.8) | 518 (90.6) | 4033 (89.7) | 7289 (91.3) | 14 448 (90.7) |
| Black | 140 (4.9) | 115 (5.3) | 25 (3.6) | 202 (4.0) | 19 (3.3) | 183 (4.1) | 415 (5.2) | 757 (4.8) |
| Hispanic | 37 (1.3) | 27 (1.2) | 10 (1.4) | 89 (1.8) | 2 (0.3) | 87 (1.9) | 122 (1.5) | 248 (1.6) |
| Asian | 58 (2.0) | 51 (2.3) | 7 (1.0) | 156 (3.1) | 21 (3.7) | 135 (3.0) | 78 (1.0) | 292 (1.8) |
| Other or unknown | 39 (1.4) | 30 (1.4) | 9 (1.3) | 70 (1.4) | 12 (2.1) | 58 (1.3) | 81 (1.0) | 190 (1.2) |
| First-degree family history of colorectal cancer | 355 (12.3) | 274 (12.6) | 81 (11.5) | 557 (11.0) | 70 (12.2) | 487 (10.8) | 858 (10.7) | 1770 (11.1) |
| Smoking status | ||||||||
| Never | 1043 (36.2) | 775 (35.6) | 268 (38.1) | 1921 (37.9) | 183 (32.0) | 1738 (38.7) | 3061 (38.3) | 6025 (37.8) |
| Current | 459 (15.9) | 372 (17.1) | 87 (12.4) | 749 (14.8) | 94 (16.4) | 655 (14.6) | 1156 (14.5) | 2364 (14.8) |
| Former | 1362 (47.3) | 1018 (46.7) | 344 (48.9) | 2359 (46.5) | 286 (50.0) | 2073 (46.1) | 3717 (46.5) | 7438 (46.7) |
| Unknown | 18 (0.6) | 13 (0.6) | 5 (0.7) | 39 (0.8) | 9 (1.6) | 30 (0.7) | 51 (0.6) | 108 (0.7) |
| BMI, median (IQR) | 27.2 (24.5-30.3) |
27.2 (24.5-30.3) |
27.2 (24.5-30.5) |
27.3 (24.5-30.3) |
27.5 (25.1-30.7) |
27.3 (24.5-30.2) |
27.2 (24.5-30.4) |
27.3 (24.5-30.3) |
| Aspirin or ibuprofen in past 12 mo (≥3 times/wk) | 1136 (39.4) | 853 (39.2) | 283 (40.2) | 2170 (42.8) | 243 (42.5) | 1927 (42.9) | 3518 (44.1) | 6824 (42.8) |
| Prior lower GI endoscopyc | 249 (8.6) | 180 (8.3) | 69 (9.8) | 599 (11.8) | 72 (12.6) | 527 (11.7) | 964 (12.1) | 1812 (11.4) |
| First positive FSG at baseline | 2217 (76.9) | 1699 (78.0) | 518 (73.6) | 3390 (66.9) | 415 (72.6) | 2975 (66.2) | 4811 (60.3) | 10 418 (65.4) |
| Adequate colonoscopy following first positive FSGd | 2627 (91.2) | 2010 (92.3) | 617 (87.6) | 4711 (93.0) | 531 (92.8) | 4180 (93.0) | 7333 (91.8) | 14 671 (92.1) |
| Year of initial colonoscopy | ||||||||
| 1993-1995 | 372 (12.9) | 293 (13.5) | 79 (11.2) | 667 (13.2) | 94 (16.4) | 573 (12.7) | 871 (10.9) | 1910 (12.0) |
| 1996-1997 | 818 (28.4) | 620 (28.5) | 198 (28.1) | 1162 (22.9) | 153 (26.7) | 1009 (22.4) | 1544 (19.3) | 3524 (22.2) |
| 1998-1999 | 786 (27.3) | 576 (26.4) | 210 (29.8) | 1216 (24.0) | 132 (23.1) | 1084 (24.1) | 1904 (23.8) | 3906 (24.5) |
| 2000-2002 | 581 (20.2) | 448 (20.6) | 133 (18.9) | 1159 (22.9) | 98 (17.1) | 1061 (23.6) | 1863 (23.3) | 3603 (22.6) |
| 2003-2007 | 325 (11.3) | 241 (11.1) | 84 (11.9) | 864 (17.0) | 95 (16.6) | 769 (17.1) | 1803 (22.6) | 2992 (18.8) |
| Length of follow-up, median (IQR), y | 13.6 (10.3-15) |
13.6 (10.3-15) |
13.4 (10.3-15) |
13.1 (9.9-15) |
13.1 (9.7-15) |
13.1 (9.9-15) |
12.5 (9.7-15) |
12.9 (9.8-15) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FSG, flexible sigmoidoscopy; GI, gastrointestinal; IQR, interquartile range.
Advanced adenomas <1 cm were defined as villous or tubulovillous or high-grade dysplasia on histology.
At start of colorectal cancer follow-up.
Within 3 y of study enrollment. After 1995, participants with a colonoscopy, FSG, or barium enema within the prior 3 y were excluded.
If the colonoscopy preparation was designated as inadequate or if the cecum was not visualized, the examination was classified as inadequate. If the preparation quality was not reported but the cecum was visualized, the colonoscopy examination was considered adequate.
CRC Incidence by Adenoma Group
Through the end of follow-up, there were a total of 196 CRC cases: 70 in the advanced adenoma group; 55 in the nonadvanced adenoma group; and 71 in the no adenoma group. Incidence rates per 10 000 person-years were 20.0 (95% CI, 15.3 to 24.7) in the advanced adenoma group, 9.1 (95% CI, 6.7 to 11.5) in the nonadvanced adenoma group, and 7.5 (95% CI, 5.8 to 9.7) in the no adenoma group (Table 2).
Table 2. Colorectal Cancer Incidence Rates per 10 000 Person-Years by Adenoma Category at Index Colonoscopy Among Participants Aged 55 to 74 Years Enrolled in the Prostate, Lung, Colorectal, and Ovarian Cancer Randomized Clinical Trial.
| Advanced Adenoma | Nonadvanced Adenoma | No Adenoma All (n = 7985) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 2882) | ≥1 cm (n = 2178) | <1 cm (n = 704)a | All (n = 5068) | ≥3 (n = 572) | 1-2 (n = 4496) | |||||||||
| No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | |
| No. of person-years at 15 y | 34 993 | 26 494 | 8499 | 60 650 | 6838 | 53 812 | 94 248 | |||||||
| Colorectal Cancer Incidence | ||||||||||||||
| Within 3 y | 16 | 18.9 | 14 | 21.7 | 2 | 9.6 | 2 | 1.3 | 0 | 0 | 2 | 1.5 | 8 | 3.4 |
| Within 5 y | 27 | 19.2 | 22 | 20.7 | 5 | 14.6 | 11 | 4.4 | 1 | 3.6 | 10 | 4.6 | 22 | 5.6 |
| >5 to 10 y | 29 | 23.1 | 20 | 21.1 | 9 | 29.3 | 26 | 11.8 | 3 | 12.1 | 23 | 11.7 | 26 | 7.5 |
| Within 10 y | 56 | 21.1 | 42 | 20.9 | 14 | 21.5 | 37 | 7.9 | 4 | 7.6 | 33 | 7.9 | 48 | 6.5 |
| >10 to 15 y | 14 | 16.7 | 9 | 14.1 | 5 | 25.0 | 18 | 13.1 | 3 | 19.4 | 15 | 12.3 | 23 | 11.4 |
| Within 15 y (cumulative) | 70 | 20.0 | 51 | 19.2 | 19 | 22.4 | 55 | 9.1 | 7 | 10.2 | 48 | 8.9 | 71 | 7.5 |
| RD vs No Adenoma |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
|
| At 10-y | 14.6 (8.8 to 20.4) |
<.001 | 14.4 (7.8 to 21.0) |
<.001 | 15.0 (3.6 to 26.4) |
.01 | 1.4 (−1.7 to 4.5) |
.38 | 1.1 (−6.6 to 8.8) |
.78 | 1.4 (−1.9 to 4.7) |
.40 | 0 [Reference] | |
| At 15-y | 12.5 (7.5 to 17.5) |
<.001 | 11.7 (6.1 to 17.3) |
<.001 | 14.9 (4.7 to 25.1) |
.004 | 1.6 (−1.4 to 4.6) |
.29 | 2.7 (−5.1 to 10.5) |
.49 | 1.4 (−1.5 to 4.5) |
.37 | 0 [Reference] | |
| RR vs No Adenoma |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
|
| At 10-y | 3.3 (2.2 to 4.8) |
<.001 | 3.2 (2.1 to 4.9) |
<.001 | 3.3 (1.8 to 6.0) |
<.001 | 1.2 (0.8 to 1.9) |
.37 | 1.2 (0.4 to 3.2) |
.77 | 1.2 (0.8 to 1.9) |
.37 | 1 [Reference] | |
| At 15-y | ||||||||||||||
| Unadjusted | 2.7 (1.9 to 3.7) |
<.001 | 2.6 (1.8 to 3.7) |
<.001 | 3.0 (1.8 to 4.9) |
<.001 | 1.2 (0.8 to 1.7) |
.30 | 1.4 (0.6 to 3.0) |
.44 | 1.2 (0.8 to 1.7) |
.37 | 1 [Reference] | |
| ARR (model)b | 2.6 (1.9 to 3.7) |
<.001 | 2.5 (1.8 to 3.7) |
<.001 | 2.8 (1.7 to 4.7) |
<.001 | 1.2 (0.8 to 1.7) |
.32 | 1.2 (0.5 to 2.7) |
.71 | 1.2 (0.8 to 1.7) |
.33 | 1 [Reference] | |
| ARR (model excluding participants with inadequate colonoscopy [N = 1213])b,c | 3.0 (2.1 to 4.3) |
<.001 | 2.9 (2.0 to 4.3) |
<.001 | 3.2 (1.8 to 5.5) |
<.001 | 1.3 (0.9 to 1.9) |
.20 | 1.3 (0.9 to 1.9) |
.73 | 1.2 (0.5 to 2.9) |
.19 | 1 [Reference] | |
Abbreviations: ARR, adjusted rate ratio; RD, rate difference; RR, rate ratio.
Advanced adenomas <1 cm were defined as villous or tubulovillous or high-grade dysplasia on histology.
Adjusted risk-ratio from Poisson regression (controlling for age, sex, aspirin or ibuprofen use, and time of initial colonoscopy [baseline vs postbaseline] for 15-y data).
Excludes participants with inadequate colonoscopy (n = 1213). If the colonoscopy preparation was designated as inadequate or if the cecum was not visualized, the examination was classified as inadequate. If the preparation quality was not reported but the cecum was visualized, the colonoscopy examination was considered adequate.
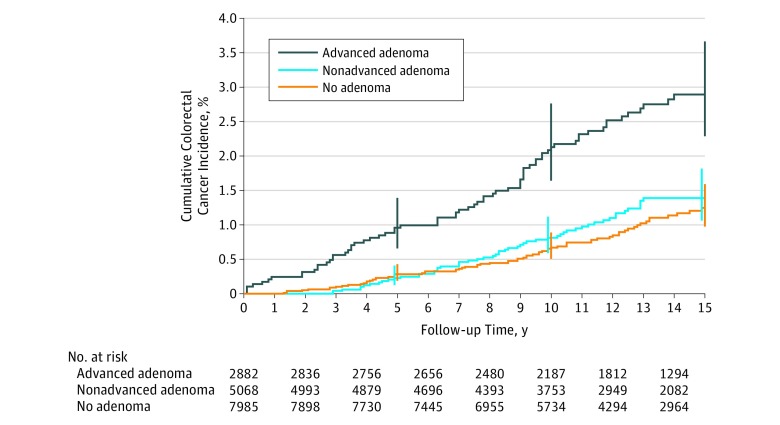
Cumulative incidence over 15 years was 2.9% (95% CI, 2.3% to 3.7%) in the advanced adenoma group, 1.4% (95% CI, 1.1% to 1.8%) in the nonadvanced adenoma group, and 1.2% (95% CI, 1.0% to 1.6%) in the no adenoma group (Figure 2). Absolute differences were 1.7% (95% CI, 0.9% to 2.4%; P < .001) for advanced adenoma vs no adenoma, 0.2% (95% CI, −0.3% to 0.6%; P = .69) for nonadvanced adenoma vs no adenoma, and 1.5% (95% CI, 0.7% to 2.3%; P < .001) for advanced adenoma vs nonadvanced adenoma.
Figure 2. Cumulative Colorectal Cancer Incidence by Adenoma Status Among Participants Aged 55 to 74 Years Enrolled in the Prostate, Lung, Colorectal, and Ovarian Cancer Randomized Clinical Trial.
Error bars indicate 95% CIs at the given time point. Median time of follow-up was 13.6 years (interquartile range [IQR], 10.3-15.0) for advanced adenoma, 13.1 years (IQR, 9.9-15.0) for nonadvanced adenoma, and 12.5 years (IQR, 9.7-15.0) for no adenoma. P values for pairwise comparisons (log-rank test) were P < .001 for advanced adenoma vs no adenoma, P < .001 for advanced adenoma vs nonadvanced adenoma, and P = .32 for nonadvanced adenoma vs no adenoma.
Within the advanced adenoma category, there was no significant difference in incidence rate per 10 000 person-years between those with adenomas of 1 cm or larger vs those with adenomas less than 1 cm but with advanced histology (19.2 [95% CI, 13.9 to 24.5] for ≥ 1 cm vs 22.4 [95% CI, 12.3 to 32.5] for <1 cm , P = .58; rate difference, 3.2 [95% CI, −8.3 to 14.4]) (Table 2). Within advanced adenoma less than 1 cm, there was no significant difference in CRC incidence per 10 000 person-years between high-grade dysplasia and villous or tubulovillous histology (28.6 [95% CI, 0.6 to 56.6] for high-grade dysplasia vs 21.1 [95% CI, 10.4 to 31.8] for villous or tubulovillous histology, P = .59; rate difference, 7.5 [95% CI, −22.5 to 37.5]).
In the advanced adenoma group, there was no significant difference in CRC risk between years 5 to 10 compared with years 0 to 5 (rate ratio [RR], 1.2 [95% CI, 0.7 to 2.0]; P = .49; rate difference, 3.9 [95% CI, −7.2 to 15.0]). There was no significant difference between participants with 3 or more nonadvanced adenomas and those with no adenomas (RR, 1.4 [95% CI, 0.6 to 3.0], P = .44; rate difference, 2.7 [95% CI, −5.1 to 10.5]).
Multiple Poisson regression analysis demonstrated a significantly increased risk of CRC in advanced adenoma compared with no adenoma participants (RR, 2.6 [95% CI, 1.9 to 3.7]; P < .001) (Table 3). There was no significant difference between nonadvanced adenoma and no adenoma (RR, 1.2 [95% CI, 0.8 to 1.7], P = .32).
Table 3. Mortality Rates per 10 000 Person-Years by Adenoma Category at Index Colonoscopy Among Participants Aged 55 to 74 Years Enrolled in the Prostate, Lung, Colorectal, and Ovarian Cancer Randomized Clinical Trial.
| Advanced Adenoma | Nonadvanced Adenoma | No Adenoma All (n = 7985) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 2882) | ≥1 cm (n = 2178) | <1 cm (n = 704)a | All (n = 5068) | ≥3 (n = 572) | 1-2 (n = 4496) | |||||||||
| No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | No. of Cases | Rate | |
| No. of person-years at 15 y | 34 708 | 26 294 | 8414 | 59 418 | 6741 | 52 677 | 91 906 | |||||||
| Colorectal Cancer Mortality | ||||||||||||||
| Within 15 y | 13 | 3.8 | 9 | 3.4 | 4 | 4.8 | 10 | 1.7 | 1 | 1.5 | 9 | 1.7 | 13 | 1.4 |
| RD vs No Adenoma |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
P Value |
RD (95% CI) |
|
| At 15 y | 2.4 (0.2 to 4.6) |
.03 | 2.0 (−0.3 to 4.3) |
.09 | 3.4 (−1.4 to 8.2) |
.16 | 0.3 (−1.0 to 1.6) |
.65 | 0.1 (−2.9 to 3.1) |
.95 | 0.3 (−1.0 to 1.6) |
.66 | 0 [Reference] | |
| RR vs No Adenoma |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
P Value |
RR (95% CI) |
|
| At 15 y | 2.6 (1.2 to 5.7) |
.01 | 2.4 (1.0 to 5.6) |
.04 | 3.4 (1.1 to 10.2) |
.03 | 1.2 (0.5 to 2.7) |
.68 | 1.05 (0.1 to 8.1) |
.96 | 1.2 (0.5 to 2.8) |
.66 | 1 [Reference] | |
Abbreviations: RD, rate difference; RR, rate ratio.
Advanced adenomas <1 cm were defined as villous or tubulovillous or high-grade dysplasia on histology.
Within the nonadvanced adenoma group, participants with 3 or more nonadvanced adenomas were not at significantly higher CRC risk compared with those with 1 to 2 nonadvanced adenoma(s) (RR, 1.01 [95% CI, 0.4 to 2.4]; P = .98). Limiting the analysis to 14 722 (92.4%) participants with an adequate colonoscopy did not significantly alter the results (Table 3). All Poisson regression models showed adequate goodness of fit.
Of the 196 CRC cases, the majority (59%) were proximal, whereas 16 (8%) were in an unknown location. Compared with distal advanced adenoma, participants with a proximal advanced adenoma at the index colonoscopy were at significantly greater overall risk for subsequent CRC (RR, 2.6 [95% CI, 1.6 to 4.3]; P < .001; rate difference, 23.9 [95% CI, 7.9 to 39.9]) (eTable 2 in Supplement 2). Advanced adenoma location was not significantly associated with subsequent CRC location; proximal CRC comprised 54% (19 of 35) of cases in those with distal advanced adenomas vs 78% (18 of 23) of cases in those with proximal advanced adenomas (P = .06). Among those with 1 to 2 nonadvanced adenomas, there was no significant association between initial nonadvanced adenoma location and risk for CRC (RR, 1.5 [95% CI, 0.7 to 2.8]; P = .24; rate difference, 3.8 [95% CI, −3.1 to 10.7]) for proximal only vs distal only adenoma location. There was also no significant association between nonadvanced adenoma location and subsequent CRC location, with proximal CRC comprising 57% (16 of 28) and 62% (8 of 13) of CRC cases in those with distal adenomas and proximal adenomas, respectively (P = .79) (eTable 2 in Supplement 2).
The majority (56.6%) of CRC detected was stage I or II, whereas 32.7% were stage III or IV and 10.7% were unknown (eTable 3 in Supplement 2). There was no significant difference in stage I or II CRC by adenoma group (advanced adenoma, 60% [42 of 70 cases]; nonadvanced adenoma, 54.5% [30 of 55 cases]; no adenoma, 54.9% [39 of 71 cases]; P = .78) or in stage III or IV CRC (advanced adenoma, 31.4% [22 of 70 cases]; nonadvanced adenoma, 32.7% [18 of 55 cases], no adenoma, 33.8% [24 of 71 cases]; P = .96).
Within the no adenoma group, there were 4410 (55.2%) participants with hyperplastic polyp(s) or other nonadenomatous lesion(s) and 3575 (44.8%) participants with no polyp findings on follow-up colonoscopy. There were 42 CRC cases (8.1 per 10 000 person-years [95% CI, 5.7 to 10.6]) within the hyperplastic or nonadenomatous polyp group and 29 cases (6.8 per 10 000 person-years [95% CI, 4.3 to 9.3]) in participants with no polyp findings, with no significant difference between them (P = .47; RR, 1.2 [95% CI, 0.7 to 1.9]; rate difference, 1.3 [95% CI, −2.2 to 4.8]).
Participants With a Negative Screening Result
Distal CRC incidence was compared in 42 348 participants with a negative T0 FSG result with the 4811 participants with a positive T0 FSG result but no adenomatous findings on subsequent colonoscopy. Distal incidence rates per 10 000 person-years were not significantly different (3.0 [95% CI, 1.7 to 4.4] for the no adenoma group vs 2.3 [95% CI, 1.9 to 2.7] for the negative FSG result group; RR, 1.3 [95% CI, 0.8 to 2.0], P = .32; rate difference, 0.7 [95% CI, −0.7 to 2.1]). For overall CRC, rates were 8.7 (95% CI, 6.4 to 11.0) for the no adenoma group vs 8.3 (95% CI, 7.6 to 9.1) for the negative FSG result group (RR, 1.05 [95% CI, 0.8 to 1.4]; P = .74; rate difference, 0.4 [95% CI, −2.0 to 2.8]).
Subsequent Colonoscopy Utilization and Findings
Of 3561 participants in the study of colonoscopy utilization, 3492 (98.1%) were included in the current study, with a median length of follow-up after index colonoscopy of 9.0 years. Subsequent colonoscopy utilization was significantly higher in the advanced adenoma compared with both nonadvanced adenoma and no adenoma (82.5% for advanced adenoma vs 78.7% for nonadvanced adenoma [P = .008] vs 69.9% for no adenoma [P < .001]), although the 3 or more nonadvanced adenomas group had the highest proportion of subsequent colonoscopy (83.0%) during the 9-year period (Table 4). Only a small percentage of subsequent colonoscopies were for symptoms (advanced adenoma, 10.6%; nonadvanced adenoma, 14.0%; no adenoma, 19.4%) during the 9-year follow-up period. Advanced adenoma participants had a significantly higher rate of subsequent adenoma removal than nonadvanced adenoma and no adenoma participants (9-year rate: 40.4% for advanced adenoma vs 33.2% for nonadvanced adenoma [P < .001] vs 20.3% for no adenoma [P < .001]), as well as a significantly higher rate of subsequent advanced adenoma removal (13.0% for advanced adenoma vs 7.6% for nonadvanced adenoma [P < .001] vs 4.8% for no adenoma [P < .001]). Compared with the no adenoma group, participants with 1 to 2 nonadvanced adenoma(s) had an 8.2% higher frequency of subsequent colonoscopy (78.1% for the nonadvanced adenoma group vs 69.9% for the no adenoma group, P = .001), a 10.8% higher adenoma removal frequency (31.1% for the nonadvanced adenoma group vs 20.3% for the no adenoma group, P < .001), and a 2.3% higher advanced adenoma removal rate (7.1% for the nonadvanced adenoma group vs 4.8% for the no adenoma group, P < .001).
Table 4. Subsequent Colonoscopy Usage and Yield by Baseline Colonoscopy Findings in Participants Enrolled in the Study of Colonoscopy Utilizationa.
| Subsequent Procedure by Years of Follow-up | Advanced Adenoma (n = 1304) |
Nonadvanced Adenoma | No Adenoma (n = 1208) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 980) | ≥3 (n = 123) | 1-2 (n = 857) | ||||||||
| No. at Riskb | Cumulative, No. With Event (%)c | No. at Risk | Cumulative No. With Event (%)c | No. at Risk | Cumulative No. With Event (%)c | No. at Risk | Cumulative No. With Event (%)c | No.at Risk | Cumulative No. With Event (%)c | |
| Colonoscopyd | ||||||||||
| 3 | 834 | 443 (33.4) | 771 | 178 (20.5) | 92 | 29 (25.7) | 679 | 149 (19.8) | 1022 | 174 (11.4) |
| 5 | 452 | 842 (62.5) | 437 | 531 (53.9) | 50 | 73 (59.9) | 387 | 458 (53.0) | 661 | 531 (36.9) |
| 7 | 255 | 1011 (76.3) | 261 | 712 (72.2) | 29 | 94 (77.0) | 232 | 618 (71.5) | 399 | 768 (59.9) |
| 9 | 99 | 1070 (82.5) | 117 | 769 (78.7) | 12 | 102 (83.0) | 105 | 667 (78.1) | 146 | 843 (69.9) |
| Adenoma removal | ||||||||||
| 3 | 1116 | 188 (13.2) | 917 | 63 (6.1) | 109 | 14 (10.5) | 808 | 49 (5.5) | 1169 | 39 (2.7) |
| 5 | 954 | 350 (26.5) | 796 | 184 (18.2) | 84 | 39 (30.5) | 712 | 145 (16.4) | 1084 | 124 (8.8) |
| 7 | 720 | 442 (34.1) | 707 | 263 (26.6) | 70 | 53 (42.6) | 637 | 210 (24.3) | 925 | 185 (15.7) |
| 9 | 309 | 494 (40.4) | 344 | 318 (33.2) | 37 | 57 (47.4) | 307 | 261 (31.1) | 396 | 225 (20.3) |
| Advanced adenoma removal | ||||||||||
| 3 | 1254 | 50 (3.5) | 964 | 16 (1.5) | 120 | 3 (2.1) | 844 | 13 (1.4) | 1201 | 7 (0.4) |
| 5 | 1203 | 101 (7.7) | 939 | 41 (4.0) | 114 | 9 (6.9) | 825 | 32 (3.6) | 1181 | 27 (1.8) |
| 7 | 968 | 133 (10.4) | 911 | 56 (5.5) | 110 | 12 (9.1) | 801 | 44 (5.0) | 1058 | 43 (3.8) |
| 9 | 446 | 154 (13.0) | 475 | 73 (7.6) | 62 | 13 (10.7) | 413 | 60 (7.1) | 474 | 55 (4.8) |
Participants were considered at risk for colonoscopy until undergoing first subsequent colonoscopy. If there were no adenomas removed, the participant was still considered at risk for adenoma removal.
No. at risk indicates number at risk at the given time point.
Cumulative percentages were computed using weighted Kaplan-Meier analysis, in which weights were the inverse sampling weights from the study of colonoscopy utilization.
Median follow-up for subsequent colonoscopy was 9.0 y.
Colorectal Cancer Mortality
Through the end of the follow-up period there were 36 deaths from CRC (13 advanced adenoma, 10 nonadvanced adenoma, 13 no adenoma) (Table 3). Compared with participants with no adenoma, those with advanced adenoma were at a significantly increased risk of CRC death (RR, 2.6 [95% CI, 1.2 to 5.7], P = .01), but those with nonadvanced adenoma were not (RR, 1.2 [95% CI, 0.5 to 2.7], P = .68). The rate difference per 10 000 person-years for the advanced adenoma and nonadvanced adenoma groups compared with the no adenoma group was 2.4 (95% CI, 0.2 to 4.6) and 0.3 (95% CI, −1.0 to 1.6), respectively.
Discussion
This post hoc follow-up of a randomized clinical trial of flexible sigmoidoscopy for a median observation time of 13 years demonstrates that participants with an advanced adenoma detected at colonoscopy were at significantly increased risk of subsequent colorectal cancer compared with those with no adenoma. Participants with nonadvanced adenoma did not have a significantly different CRC risk compared with those with no adenoma.
The risk in participants with advanced adenoma was unlikely to be due to partial removal of the initial lesion, as the risk in the first 5 years was not significantly different from risk between years 5 through 10. These data are consistent with studies that demonstrate increased CRC risk in patients with a history of an advanced adenoma.12,29,30,31 However, these data show a higher risk than that observed in a Norwegian study13 of colorectal cancer mortality in patients with a high-risk adenoma (standardized mortality ratio, 1.16 [95% CI, 1.02 to 1.31]). Other studies have demonstrated that finding an advanced adenoma on colonoscopy significantly increases the risk of finding an advanced adenoma on subsequent colonoscopy compared with those with no adenoma.18,27,30,32,33,34,35,36 The current data provide long-term assessment of incident CRC risk in a large population in comparison with a control group of nonadvanced and no adenoma. By demonstrating that individuals diagnosed with an advanced adenoma are at increased long-term risk for subsequent incident CRC, these findings support periodic, ongoing surveillance colonoscopy in these patients. Data from the United Kingdom in participants with advanced adenomas demonstrated a cumulative CRC incidence of 1.8% (210 cases of 11944 participants) after median follow-up of 7.9 years, similar to the current observed incidence of 1.9% (56 cases of 2882 participants) through 10 years of follow-up. Furthermore, in the UK study,14 surveillance colonoscopy was associated with a 50% to 60% reduction in subsequent CRC incidence. In the current study, proximal advanced adenoma location was significantly associated with overall subsequent CRC risk compared with distal advanced adenoma location.
There was no significant difference in CRC incidence in participants with advanced adenoma, whether the adenoma was 1 cm or larger or smaller than 1 cm with advanced histology. European guidelines offer the option of returning patients with an adenoma smaller than 1 cm with advanced histology to a low-risk group with a standard 10-year surveillance interval.10 The current data suggest this group is not significantly different in risk compared with those with a large adenoma and therefore they should continue to be followed with more frequent surveillance.
Participants with 1 to 2 nonadvanced adenoma(s) were not at significantly increased risk for CRC incidence compared with those with no adenoma. However, the 95% CIs are wide and may include a potentially clinically important effect size (RR: 1.7 for incidence and 2.8 for mortality) because the study had limited power to detect a significant difference. Furthermore, increased surveillance colonoscopy use and additional adenoma and advanced adenoma removal among participants with 1 to 2 nonadvanced adenoma compared with those with no adenoma in the follow-up period could have contributed to a lower cancer incidence rate in participants with 1 to 2 nonadvanced adenomas. However, the difference in colonoscopy use (8.2%) and adenoma removal (10.8%) between the nonadvanced adenoma and the no adenoma groups was small.
Current US guidelines recommend a colonoscopy follow-up interval of 5 to 10 years for patients with 1 to 2 nonadvanced adenoma(s),11 but the decision between 5 and 10 years is often left to physician discretion and evidence-based recommendations are unavailable.10 Individuals with no adenomatous findings on colonoscopy are advised to return in 10 years. If appropriately powered prospective trials were to replicate these findings demonstrating no significant difference in cancer incidence between participants with 1 to 2 nonadvanced adenoma(s) and no adenomas, colonoscopy use could be reduced by a large extent, as a surveillance examination at 5 years would not be needed. Small nonadvanced adenomas are the most common neoplastic finding on colonoscopy—occurring in about 30% of patients.9,37 The European Polyp Surveillance randomized clinical trial (EPoS, NCT02319928) is randomizing participants with 1 to 2 nonadvanced adenoma(s) to surveillance at 5 and 10 years vs surveillance at 10 years to determine if surveillance at 5 years will reduce CRC incidence and a similar type trial, FORTE, is in the planning stages in the United States.38
Participants with 3 or more adenomas are considered higher risk than those with 1 to 2 nonadvanced adenoma(s), and guidelines advise repeat colonoscopy examination in 3 years. In the current study, there was no significant difference in cancer incidence when comparing those with 3 or more adenomas to those with 1 to 2 nonadvanced adenoma(s), but the number of patients with 3 or more adenomas was small resulting in estimates with wide CIs that did not provide adequate guidance. Furthermore, subsequent colonoscopy was highly utilized in patients with 3 or more adenomas.
There are several strengths of the current study. It included participants cared for throughout the United States in a variety of practice settings and is thus representative of standard practice. Use of surveillance colonoscopy was tracked for 21.9% of the sample, so the effect of additional colonoscopy examinations could be estimated. The cohort was closely followed for CRC incidence with few lost to follow-up. Overall compliance with the annual study update was 93.8%.2
Limitations
This study has several limitations. First, the colonoscopy examination followed an abnormal or positive FSG result. As such, participants in the no adenoma group may not be representative of individuals with a negative initial colonoscopy result. However, distal and overall CRC incidence in participants with a negative FSG result were not statistically significantly different from those with a positive FSG result but no adenomatous findings on subsequent colonoscopy. Additionally, in the group with an abnormal sigmoidoscopy but no adenoma at follow-up colonoscopy, CRC incidence was not significantly different among those with hyperplastic polyps or nonadenomatous lesion(s) compared with those with no findings.
Second, although most participants (88.6%) had not had a lower gastrointestinal endoscopy in the 3 years prior to enrollment, information on previous examinations was not available. Thus, some participants may have had lower gastrointestinal endoscopy and removal of adenomas prior to enrollment in the trial that was not accounted for. However, the cohort was followed for up to 15 years, ample time for CRC risk to emerge. Third, colonoscopy follow-up was not standardized following the initial examination, was not uniform across adenoma groups, and data on subsequent colonoscopy use was only available for a minority of the study population. Fourth, limited data on the quality of colonoscopy was available; for example, endoscopists’ adenoma detection rates were not available, but restricting the analysis to participants with adequate colonoscopy preparation and confirmed cecal intubation did not affect the results. Fifth, pathology was not centrally reviewed, although this could be a strength because these assessments reflect real-world interpretations. Sixth, endoscopic procedures and pathologic assessments were performed from 1993 to 2006, and whether those findings are directly applicable to current techniques cannot be known with certainty. Seventh, this study was a post hoc analysis with limited statistical power.
Conclusions
Over a median of 13 years of follow-up, participants with an advanced adenoma at diagnostic colonoscopy prompted by a positive screening flexible sigmoidoscopy result were at significantly increased risk of developing colorectal cancer compared with those with no adenoma. Identification of nonadvanced adenoma may not be associated with increased colorectal cancer risk.
Trial Protocal and Statistical Analysis Plan
eTable 1. Demographics and Index Colonoscopy Findings by Extended Follow-up Status
eTable 2. Location of Adenoma on Initial Colonoscopy and Colorectal Cancer Incidence and Location
eTable 3. Colorectal Cancer Stage by Index Colonoscopy Adenoma Group
References
- 1.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE [published correction appears in J Natl Cancer Inst. 2011;103(24):1903]. J Natl Cancer Inst. 2011;103(17):1310-1322. [DOI] [PubMed] [Google Scholar]
- 2.Schoen RE, Pinsky PF, Weissfeld JL, et al. ; PLCO Project Team . Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med. 2013;158(5 Pt 1):312-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holme Ø, Løberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312(6):606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up. Lancet. 2017;389(10076):1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer. Gut. 2018;67(2):291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287-1297. [DOI] [PubMed] [Google Scholar]
- 10.Ladabaum U, Schoen RE. Post-polypectomy surveillance that would please goldilocks—not too much, not too little, but just right. Gastroenterology. 2016;150(4):791-796. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy. Gastroenterology. 2012;143(3):844-857. [DOI] [PubMed] [Google Scholar]
- 12.Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, Bonithon-Kopp C. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61(8):1180-1186. [DOI] [PubMed] [Google Scholar]
- 13.Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371(9):799-807. [DOI] [PubMed] [Google Scholar]
- 14.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence. Lancet Oncol. 2017;18(6):823-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy. Am J Gastroenterol. 2017;112(12):1790-1801. [DOI] [PubMed] [Google Scholar]
- 16.Weissfeld JL, Schoen RE, Pinsky PF, et al. Flexible sigmoidoscopy in the randomized Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. J Natl Cancer Inst. 2012;104(4):280-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6)(suppl):273S-309S. [DOI] [PubMed] [Google Scholar]
- 18.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136(3):832-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134(1):21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325(23):1593-1596. [DOI] [PubMed] [Google Scholar]
- 21.Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology. 2012;142(4):762-772. [DOI] [PubMed] [Google Scholar]
- 22.Stürmer T, Glynn RJ, Lee IM, Christen WG, Hennekens CH. Lifetime cigarette smoking and colorectal cancer incidence in the Physicians’ Health Study I. J Natl Cancer Inst. 2000;92(14):1178-1181. [DOI] [PubMed] [Google Scholar]
- 23.Schoen RE, Razzak A, Yu KJ, et al. Incidence and mortality of colorectal cancer in individuals with a family history of colorectal cancer. Gastroenterology. 2015;149(6):1438-1445.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the US. Cancer. 1999;85(8):1670-1676. [PubMed] [Google Scholar]
- 25.Miller AB, Yurgalevitch S, Weissfeld JL; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team . Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6)(suppl):400S-406S. [DOI] [PubMed] [Google Scholar]
- 26.Pinsky PF, Prorok PC, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer. 2017;123(4):592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol. 2009;7(1):86-92. [DOI] [PubMed] [Google Scholar]
- 28.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138(1):73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326(10):658-662. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. [DOI] [PubMed] [Google Scholar]
- 31.Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE. Ongoing colorectal cancer risk despite surveillance colonoscopy. Gastrointest Endosc. 2010;71(1):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification. Gut. 2011;60(11):1537-1543. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Li X, Wang Z, Su B. Five-year risk of colorectal neoplasia after normal baseline colonoscopy in asymptomatic Chinese Mongolian over 50 years of age. Int J Colorectal Dis. 2012;27(12):1651-1656. [DOI] [PubMed] [Google Scholar]
- 34.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines. Ann Intern Med. 2008;148(6):419-426. [DOI] [PubMed] [Google Scholar]
- 35.Laiyemo AO, Pinsky PF, Marcus PM, et al. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastroenterol Hepatol. 2009;7(5):562-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas. Gastrointest Endosc. 2006;64(4):614-626. [DOI] [PubMed] [Google Scholar]
- 37.Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7(12):1272-1278. [DOI] [PubMed] [Google Scholar]
- 38.Jover R, Bretthauer M, Dekker E, et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy. 2016;48(6):571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocal and Statistical Analysis Plan
eTable 1. Demographics and Index Colonoscopy Findings by Extended Follow-up Status
eTable 2. Location of Adenoma on Initial Colonoscopy and Colorectal Cancer Incidence and Location
eTable 3. Colorectal Cancer Stage by Index Colonoscopy Adenoma Group