Abstract
Objective: To observe changes in peripheral T lymphocytes of patients with lumbar disc herniation, and investigate the relationship between the type of herniation, signs and T lymphocyte subsets.
Methods: Blood samples from 20 healthy blood donors (control group), and 49 patients (27 male and 22 female) with single‐level lumbar intervertebral disc herniation were collected, the latter preoperatively. T lymphocytes subsets were detected by flow cytometer. According to the position of the intervertebral disc observed during surgery, the patients were divided into ruptured disc herniation (RDH) and degenerative disc herniation (DDH) groups. Straight leg raising (SLR) was assessed preoperatively.
Results: Percentages of CD3+, CD4+, and ratio of CD4+/CD8+ in the RDH group were significantly higher, and of percentage of CD8+ significantly lower, than were those in the control group. Percentages of CD4+ and ratio of CD4+/CD8+ were significantly higher, and percentage of CD8+ significantly lower, in the positive SLR test group than were those in the negative SLR test group. The positive rate of SLR testing was significantly higher in the RDH than in the DDH group.
Conclusion: Our results suggest that changes in T lymphocyte subsets in peripheral blood take place after herniation of the lumbar intervertebral disc. T lymphocyte mediated immune responses may play an important role in the occurrence and development of signs in patients with herniated lumbar intervertebral discs. The SLR test may help to confirm that disc herniation has caused nerve root impairment by mechanical loading or inflammatory stimulus and provide guidance on the choice of treatment.
Keywords: Intervertebral disc displacement, Signs and symptoms, T‐lymphocyte subsets
Introduction
Lumbar disc herniation (LDH) is a major cause of low back and unilateral leg pain. Mechanical compression or deformity of nerve roots due to displacement and effacement of neural tissue by disk herniation as a cause of pain or nerve dysfunction is a classic concept which dates back to the observations of Mixter and Barr 1 . A variety of morphological changes occur in the nerve root with compression, including venous stasis, edema, and ultimately intraneural and perineural fibrosis. Compression‐induced impairment of both arterial supply and venous drainage is one mechanism for nerve root dysfunction. Intraneural edema can occur even at low compression pressure levels 2 . However, the concept of neural compression by itself is inadequate to explain part or all of many symptom complexes. Additionally, some scholars have demonstrated that pain may be caused by the inflammatory and immune changes that can occur with disc herniation 3 , 4 . The type of LDH cannot be classified without surgery, not even with magnetic resonance imaging (MRI) or discography.
In this study, we performed physical examinations and surgery in patients with LDH, and assessed changes in the T lymphocyte subsets in patients with different pathological types of LDH as observed during surgical treatment, in order to determine the associations between the different pathological types of LDH, some immune parameters, and results of the straight leg raising (SLR) test.
Materials and methods
Study population
From January 2008 to October 2008, 49 patients with single segment LDH (46.9% female, mean age ± standard deviation [SD], 44.55 ± 13.9; mean body mass index [BMI]± SD, 23.45 ± 3.1) were recruited. The mean duration of illness was 2.2 ± 1.9 years (ranges, 8 months to 7 years). Analgesics and glucocorticoids were discontinued for a week before the blood samples were taken. Before surgical treatment, all patients with LDH were divided into two groups according to the results of their SLR tests, A1 being the positive and A2 the negative group. The patients were classified into a ruptured disc herniation (RDH) or degenerative disc herniation (DDH) group according to the position of intervertebral disc as observed during surgery. The diagnosis of LDH required the following two criteria: (i) diagnosis of LDH by MRI (sagittal and axial images obtained with a 1.5‐T imaging system); (ii) a history of unilateral pain radiating from the back along the femoral or sciatic nerve to the corresponding dermatome of the nerve root for more than 3 months. Primary exclusion criteria included synovial cyst, spinal tumor, spondylolisthesis, spondylosis, and trauma. The patients all had moderate socioeconomic status. All subjects provided informed consent after having been given a complete explanation of the nature and methods of the study. Twenty health blood donor volunteers (40.0% female; mean age ± SD, 39.15 ± 10.9; mean BMI ± SD, 23.64 ± 3.5) comprised the control group. There were no obvious differences in general characteristics between the two groups of test subjects.
Sample collection
After pain drawing, 2 ml of peripheral venous blood were collected from every subject, then added to a test tube containing disodium ethylene glycol tetraacetic acid (EDTA‐2Na), mixed and put into a refrigerator at −80°C, prior to detecting the T lymphocyte subsets.
Experimental materials and equipment
Immune reagent used included mouse anti‐human CD4/CD8/CD3,FITC/PE/PE‐Cy5 labeled antibody(Beijing Jingmei Jiyingu Technology, Beijing, China) and red blood cell (RBC) lysis solution (Becton Dickinson, Sparks, MD, USA).
The T cell subsets were assessed with FACSCalibur (Becton Dickinson).
T cell subset detection procedure
FACSCalibur (Becton Dickinson) was used to detect T lymphocyte subsets as follows: 20 µl anti‐human CD4/CD8/CD3, FITC/PE/PE‐Cy5 labeled antibody was placed in the bottom of a test tube, then 100 µl of peripheral blood with EDTA anticoagulant was added to the bottom of test tube, and gently shaken until the antibody was fully mixed. This was then incubated in the dark at room temperature for 30 min, then 2 ml of RBC lysis solution was added to the bottom of the test tube and buffer blended in a dark place for 10 min to lyse the red blood cells. The test tube was then centrifuged at 1000 rev/min for 5 min; the supernatant fluid discarded, 2 ml phosphate buffered saline (PBS) solution added to resuspend the cells, centrifuged at 1000 rev/min for 5 min, the supernatant again discarded, 0.5 ml (PBS) lotions added to resuspend the cells, then the samples were subject to flow cytometry, and the resultant data recorded.
Statistical analysis
Statistical analysis was implemented using SPSS 16.0 software package (SPSS, Chicago, IL, USA). Comparisons between the two groups were performed by conducting independent samples t‐test. Quantitative data were compared by one‐way analysis of variance (anova), and group comparisons were performed using the Scheffe method. For T cell count data the χ2 test was used. A P‐value < 0.05 was considered statistically significant.
Results
Results of assessing T lymphocyte subsets in patients with lumbar disc herniation
Percentages of CD3+ T lymphocytes in patients in the RDH and DDH groups were significantly higher than for subjects in the control group. Percentages of CD4+ T lymphocytes in patients in the RDH group were significantly higher than in the DDH and control groups. Percentages of CD8+T lymphocytes in patients in the RDH group were significantly lower than in the control group. The ratio of CD4+/CD8+ T lymphocytes in patients in the RDH group was significantly higher than in the control group (Fig. 1).
Figure 1.
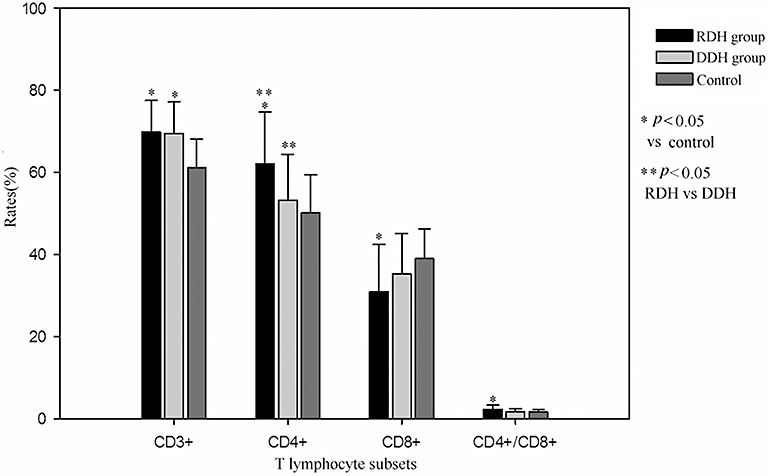
Comparison between LDH group and control groups of T lymphocyte subsets.
Comparison of T lymphocyte subsets between the positive and negative SLR test groups
There were no significant differences between these two groups in the percentages of CD3+ T lymphocytes. Percentages of CD4+ T lymphocytes in patients in the positive SLR test group were significantly higher than in the negative SLR test group. Percentages of CD8+ T lymphocytes in patients in the positive SLR test group were significantly lower than in the negative SLR test group. The ratio of CD4+/CD8+ T lymphocytes in patients in the positive SLR test group was significantly higher than in the negative SLR test group (Fig. 2).
Figure 2.
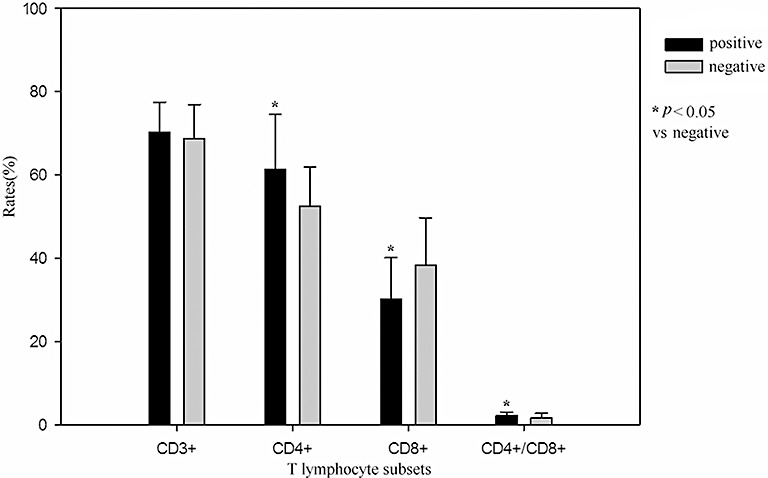
Comparison between positive and negative SLR test groups of T lymphocyte subsets.
Association between pathological type of lumbar disc herniation and straight leg raising test
Thirty‐four of 49 patients (69%) with lumbar disc herniation were positive on SLR test. Positive rates for the SLR test in the RDH and DDH group were 80% and 52.6%, respectively (P < 0.05, Table 1).
Table 1.
The association between pathological type and SLR test
| Type | SLR test | Total | |
|---|---|---|---|
| Positive | Negative | ||
| RDH | 24 (80.0%) | 6 (20.0%) | 30 |
| DDH | 10 (52.6%) | 9 (47.4%) | 19 |
| Total | 34 | 15 | 49 |
χ2 test: χ2= 4.102; P < 0.05.
Discussion
LDH is the commonest cause of sciatica, a condition which severely affects activities of daily living. Although there is a high incidence of LDH in our society, controversy still surrounds its pathogenesis and treatment. For example, from the basic science point of view, growing evidence indicates that the progressive development of symptomatic radiculopathy is caused not only by mechanical effects but also by biochemical factors, but further studies are needed to elucidate these factors. In recent years, many authors have been interested in a possible immunologic mechanism for nerve injury in LDH. Herniated disc material acting as a biochemical or immunologic irritant may contribute to a patient's clinical signs and symptoms.
In this study all LDH patients were divided into RDH and DDH groups based on whether the nucleus pulposus had broken through the posterior longitudinal ligament. In the RDH type, the herniated nucleus pulposus is in direct contact with epidural tissue, while in the DDH type, the outer layer of the annulus fibrosus is intact and the herniated nucleus pulposus is not in direct contact with epidural tissue 5 . The two types constitute two anatomically different types of LDH 5 . This study shows that the percentage of of CD4+ and the ratio of CD4+/CD8+ T lymphocytes in the peripheral blood is higher in patients with RDH than that in an age‐and sex‐matched control group. This indicates that some changes take place in the immune system after disc herniation, support an immune theory of LDH and conforming to the findings of other scholars. CD4+ and CD8+T lymphocytes are functional T lymphocytes forming two different subgroups which respectively exert positive and negative regulatory roles in the immune response. The body's relative immune balance is maintained mainly by the interaction between CD4+ and CD8+T lymphocytes, imbalance in the proportion of these two T lymphocyte subsets results in immune dysfunction, therefore the CD4+/CD8+ ratio represents the overall immune balance 6 .
Ma et al. collected specimens of lumbar intervertebral discs from 19 patients to study the histopathologic features; immunohistochemical staining showed that there were more T lymphocytes and macrophages in herniated lumbar disc specimens than in protruding specimens 7 . Concentrations of the immunoglobulins IgM and IgG in peripheral blood were higher in patients with herniated type than in patients with protruding type LDH 7 . Tian et al. found that IgM and IgG were deposited around new blood vessels and nucleus pulposus cells in herniated lumbar intervertebral discs, and concluded that an IgM‐ and IgG‐mediated immune response plays an important role in LDH 8 . In an experimental study on pigs, Geiss et al. discovered that, after autologous nucleus pulposus was placed subcutaneously in a perforated titanium chamber, the proportion of activated T cells (CD4 and CD8) was significantly higher in the exudate of the nucleus pulposus filled chamber than in that from the empty chambers 4 . The proportion of activated B cells expressing immunoglobulin kappa was also significantly increased in the exudate of the nucleus pulposus chambers 4 . Windsor et al. observed that lymphocytic pleocytosis is most common in dogs with chronic progression or acute‐on‐chronic intervertebral disc herniation, and that lymphocytic inflammation in the CSF of some dogs might suggest an immune‐mediated response to chronically herniated disc material 9 . Geiss et al. showed that after being exposed to the immune system, for example, in association with disc herniation, the nucleus pulposus may prime T (H) cells to develop into interleukin‐4‐producing T(H)2 cells 10 .
The current study shows that the percentage of the CD4+T lymphocyte subset and the ratio of CD4+/CD8+ in patients with a positive SLR test group is significantly higher than in those with a negative SLR test, which indicates that T lymphocytes are associated with sciatica. The SLR test was positive significantly more often in the RDH than in the DDH group. Inflammation and an immune response to herniated nucleus pulposus may widely injure the nerve root, and even spread to the contralateral nerve root, which may explain the occurrence of sciatica in the absence of obvious nerve root compression, and also explain how unilateral nerve root compression can induce contralateral sciatica. A recent study indicated that tumor necrosis factor‐alpha (TNF‐α) is associated with sciatica 11 ; moreover TNF‐α was found to be present in nucleus pulposus cells 12 . Liu et al. have confirmed the role of circulating monocytes/macrophages in the development of neuropathic hyperalgesia and Wallerian degeneration due to partial nerve injury 13 . Macrophage depletion immediately after nerve injury could have some clinical potential in the prevention of neuropathic pain 13 . Kawakami et al. have shown that neuropathic pain produced by placing the nucleus pulposus on the nerve root may be related to inflammatory cell infiltration induced by relocation of the nucleus pulposus, rather than the nucleus pulposus itself 14 . TNF‐α, a proinflammatory cytokine affecting matrix metalloproteinase expression and increasing prostaglandin E2, is overexpressed in degenerated disks. Weiler et al. have demonstrated TNF‐α in cross‐sections of human disks, synthesis of TNF‐α in the annular and disk regions, increased TNF‐α with symptomatic disk disease, and TNF‐α expression associated with increasing disk degeneration 15 . Virri et al. studied 205 patients and showed some statistically significant associations between inflammatory cell infiltrates in herniated lumbar disc specimens and SLR, most clearly when comparing bilaterally positive and negative SLR 16 . But Rothoerl et al. studied 179 patients and found no statistically significant correlation between macrophage infiltrates in herniated lumbar disc specimens and clinical data 17 . The results of the study by Grönblad et al. also do not support a clinically relevant role for inflammatory cells with disc herniation in sciatica 18 .
The current study showed that positive rates for the SLR test in the RDH and DDH groups were 80% and 52.6%, respectively. This accords with the study of Roussel et al., which demonstrated that the SLR test had high test‐retest reliability in the diagnosis of lumbar disc herniation 19 . But Majlesi et al. showed that the Slump test might be a more sensitive physical examination tool in patients with symptoms of LDH 20 . In contrast, owing to its higher specificity, the SLR test may especially help to identify patients who have herniations with root compression requiring surgery. In the current study the SLR test was negative in 31% of all LDH patients, which shows that not all patients with LDH have typical symptoms and signs. Any study looking at the natural history of degenerative disk disease, the prognostic value of imaging, or its effect on therapeutic decision making will be confounded by the high prevalence of morphologic change in the asymptomatic population 21 , 22 . Twenty to twenty‐eight per cent of asymptomatic patients demonstrate disk herniations, and the majority have evidence of additional degenerative disk disease 21 , 22 .
In a word, the current results provide further proof for the theory of autoimmunity in LDH. The SLR test can confirm that the nerve root had been damaged by inflammatory stimulation or mechanical compression by a herniated disc, which can support the choice of surgical treatment. Continuing research is needed to find more relatively specific indicators so as to determine the differences between the various pathological types of LDH. There are many differences between the different pathological types of LDH in nerve conduction pathway neurotransmitter and receptor mechanisms, so it is necessary to judge comprehensively in combination with individual differences in patients, which factors are of crucial significance in assessing treatment options and prognosis of LDH.
Acknowledgments
This work was supported by Scientific Research Funds of Tianjin Medical University (2008ky22) and the Tianjin Municipal Science and Technology Commission (09JCZDJC19600).
References
- 1. Mixter W, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med, 1934, 211: 210–215. [Google Scholar]
- 2. Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine, 1989, 14: 569–573. [PubMed] [Google Scholar]
- 3. McLain RF, Kapural L, Mekhail NA. Epidural steroids for back and leg pain: mechanism of action and efficacy. Cleve Clin J Med, 2004, 71: 961–970. [DOI] [PubMed] [Google Scholar]
- 4. Geiss A, Larsson K, Rydevik B, et al. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine, 2007, 32: 168–173. [DOI] [PubMed] [Google Scholar]
- 5. Delauche‐Cavallier MC, Budet C, Laredo JD, et al. Lumbar disc herniation. Computed tomographic scan changes after conservative treatment of nerve root compression. Spine, 1992, 17: 927–933. [PubMed] [Google Scholar]
- 6. Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: functional differentiation and homeostasis. Immunol Rev, 2006, 211: 39–48. [DOI] [PubMed] [Google Scholar]
- 7. Ma XL, Xu YQ, Zhang YX, et al. The study on autoimmune factors of lumbar disc herniation (Chin). Zhongguo Xian Dai Shen Jing Ji Bing, 2004, 4: 291–296. [Google Scholar]
- 8. Tian W, Cui GY, Zhao DH, et al. Clinical signs and symptoms relate to IgM and IgG in herniated lumbar intervertebral disc (Chin). Zhonghua Gu Ke Za Zhi, 2008, 28: 288–291. [Google Scholar]
- 9. Windsor RC, Vernau KM, Sturges BK, et al. Lumbar cerebrospinal fluid in dogs with type I intervertebral disc herniation. Vet Intern Med, 2008, 22: 954–960. [DOI] [PubMed] [Google Scholar]
- 10. Geiss A, Larsson K, Junevik K, et al. Autologous nucleus pulposus primes T cells to develop into interleukin‐4‐producing effector cells: an experimental study on the autoimmune properties of nucleus pulposus. J Orthop Res, 2009, 27: 97–103. [DOI] [PubMed] [Google Scholar]
- 11. Igarashi T, Kikuchi S, Shubayev V, et al. Exogenous tumor necrosis factor‐alpha mimics nucleus pulposus‐induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine, 2000, 25: 2975–2980. [DOI] [PubMed] [Google Scholar]
- 12. Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus‐pulposus‐induced nerve root injury. Spine, 1998, 23: 2538–2544. [DOI] [PubMed] [Google Scholar]
- 13. Liu T, Van Rooijen N, Tracey DJ. Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain, 2000, 86: 25–32. [DOI] [PubMed] [Google Scholar]
- 14. Kawakami M, Tamaki T, Matsumoto T, et al. Role of leukocytes in radicular pain secondary to herniated nucleus pulposus. Clin Orthop Relat Res, 2000, 376: 268–277. [DOI] [PubMed] [Google Scholar]
- 15. Weiler C, Nerlich AG, Bachmeier BE, et al. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine, 2005, 30: 44–53. [DOI] [PubMed] [Google Scholar]
- 16. Virri J, Gronblad M, Seitsalo S, et al. Comparison of the prevalence of inflammatory cells in subtypes of disc herniations and associations with straight leg raising. Spine, 2001, 26: 2311–2315. 11679814 [Google Scholar]
- 17. Rothoerl R, Woertgen C, Holzschuh M, et al. Macrophage tissue infiltration,clinical symptoms and signs in patients with lumbar disc herniations: a clinicopathological study on 179 patients. Acta Neurochir, 1998, 140: 1245–1248. [DOI] [PubMed] [Google Scholar]
- 18. Grönblad M, Virri J, Seitsalo S, et al. Inflammatory cells, motor weakness, and straight leg raising in transligamentous disc herniations. Spine, 2000, 25: 2803–2807. [DOI] [PubMed] [Google Scholar]
- 19. Roussel NA, Nijs J, Truijen S, et al. Low back pain: clinimetric properties of the Trendelenburg test, active straight leg raise test, and breathing pattern during active straight leg raising. J Manipulative Physiol Ther, 2007, 30: 270–278. [DOI] [PubMed] [Google Scholar]
- 20. Majlesi J, Togay H, Unalan H, et al. The sensitivity and specificity of the Slump and the Straight Leg Raising tests in patients with lumbar disc herniation. J Clin Rheumatol, 2008, 14: 87–91. [DOI] [PubMed] [Google Scholar]
- 21. Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic‐resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am, 1990, 72: 403–408. [PubMed] [Google Scholar]
- 22. Jensen MC, Brant‐Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med, 1994, 331: 69–73. [DOI] [PubMed] [Google Scholar]


