Abstract
Objective
To evaluate the changes in lung morphology in subjects with adolescent idiopathic scoliosis (AIS) following posterior spinal fusion surgery.
Methods
From April 2009 to August 2013, 30 AIS patients (nine males and 21 females) were enrolled in this study. All scans were obtained with the patient in the supine position and the breath held in deep inspiration and performed both before and after surgery. Syngo software was used to manage the computed tomography scan imaging and to calculate the lung volume, lung height and pulmonary cross‐sectional area in the apical vertebral plane.
Results
Left lung, right lung and total lung volumes and convex to concave lung volume ratio did not change significantly after corrective surgery. There was a statistically significant improvement in left lung and right lung heights after posterior spinal fusion surgery. However, the pulmonary cross‐sectional area in the apical vertebrae plane was smaller postoperatively than preoperatively.
Conclusions
This study showed that lung height in AIS patients increased significantly immediately postoperatively whereas lung volume did not change significantly. Thoracic symmetry was improved postoperatively in these patients.
Keywords: Adolescent idiopathic scoliosis, Lung morphology, Pulmonary function, Thoracic symmetry, Three‐dimensional computed tomography
Introduction
Adolescent idiopathic scoliosis (AIS), the commonest form of idiopathic scoliosis, is a three‐dimensional spinal deformity that typically affects adolescent girls (10–16 years of age)1. It has been reported that spinal deformity limits the space for pulmonary growth and that if scoliosis is untreated or improperly treated, the subject may be at high risk of pulmonary impairment2. Restrictive pulmonary dysfunction is the commonest reported pulmonary impairment in AIS patients. Reduction in pulmonary function is reportedly correlated with bigger Cobb angle, hypokyphosis, larger vertebral rotation, greater number of vertebrae in the thoracic curve and more cephalad location of the major curve2, 3, 4, 5. Thus, the goal of corrective operation is to correct the above‐mentioned factors.
Before surgical correction, pulmonary function and lung volumes are poorer in AIS patients than in normal controls6, 7. Pulmonary function immediately following posterior spinal fusion (PSF) is reportedly worse than preoperatively, whereas on follow‐up pulmonary function shows minor improvements, no change, or minor decline depending on the duration of follow‐up8, 9, 10, 11, 12, 13, 14.
Chu et al. have used dynamic breathing MRI to investigate lung volume in AIS patients15. They found that lung volumes before PSF surgery and six months after it did not change significantly. Iwano et al. found that lung volume calculated using 3‐D CT volumetry correlated well with lung volume measured using spirometry16. thus, 3‐D‐CT volumetry can be used to evaluate pulmonary function. Moreover, Yu et al. evaluated the relationship between CT‐determined lung volume and pulmonary function tests in patients with AIS17. They concluded that total lung volume (TLV) correlated positively with results of the pulmonary function tests and that right lung volume (RLV), left lung volume (LLV) and TLV were all smaller in AIS patients than in age matched controls. To our knowledge, there has been little research on postoperative lung morphology in AIS patients.
The aims of the current study were: i) to use a 3‐D‐CT‐based lung reconstruction technique to calculate the lung volume, lung height, convex to concave lung volume ratio (CCLVR) and pulmonary cross‐sectional area (PCSA) in the apical vertebral plane in AIS patients before and after surgery; and ii) to assess changes in postoperative lung morphology after PSF surgery.
Materials and Methods
Subjects
This prospective study investigated AIS patients who underwent corrective surgery performed by the corresponding author in our hospital. Inclusion criteria were as follows: i) a diagnosis of AIS with a predominant right‐sided thoracic curve; ii) undergoing posterior segmental spinal fusion surgery with all pedicle screw instrumentation; iii) pre‐ and postoperative full spine X‐ray films in natural standing position; and iv) pre‐ and postoperative 3‐D‐CT scans including the full lung. Exclusion criteria were as follows: i) undergoing anterior spinal fusion, rib resection or thoracoplasty (because opening of the chest wall leads to immediate deterioration in pulmonary function with improvement to preoperative values after 6 months in patients who have undergone rib resection group and after 24 months in those who have undergone thoracoplasty)18; ii) preoperative brace treatment (because preoperative brace treatment can reduce pulmonary function)19; iii) lack of imaging data; and iv) comorbidities that affect lung morphology, such as chronic obstructive pulmonary disease, pulmonary heart disease, asthma and so on.
From April 2009 to August 2013, 30 of the 325 AIS patients (nine males and 21 females) who underwent PSF and instrumentation by the corresponding author and met all inclusion and exclusion criteria were eligible for enrollment in our study. Their ages ranged from 11.3 to 16.0 years (mean 14.1). The preoperative Cobb angle of the predominant thoracic curve was 58.8° ± 13.8° (41.3°–95.0°). The preoperative thoracic kyphosis angle from T5 to T12 was 28.9° ± 11.0° (17.9°–40.0°). Nineteen of the patients had Lenke type 1 curves, four type 2 curves and seven type 3 curves. Eleven of the subjects had Risser III signs, seven Risser IV, and 12 Risser V.
All patients provided written informed consent according to the guidelines of the institutional review board.
3‐D‐CT Examination
Preoperative 3‐D‐CT scans of the full spine were routinely performed preoperatively in AIS patients to assess the structure of the vertebrae and vertebral pedicles and to ensure accuracy of pedicle screw placement. In addition, patients with suspected pedicle screw malposition on postoperative X‐ray films underwent postoperative 3‐D‐CT scans of the spine. All pre‐ and postoperative 3‐D‐CT examinations were performed using a sliding gantry 40 slice CT scanner (Samatom Sensation Open; Siemens Medical Solutions, Forchheim, Germany). The slices were 1.5 mm, which guarantees accuracy and decreases spatial resolution. The voltage of the X‐ray tube was adjusted to 80 kV to minimize radiation. The ALARA (as low as reasonably accurate) protocols popularized by the American College of Radiology were also used to limit the average radiation dose to less than 2 mGy. All CT scans were performed by a professional radiologist with the patient a supine position and the breath held in deep inspiration.
3‐D‐CT Volumetric Lung Reconstruction
All thin‐section CT data for each patient were transferred to a computer workstation, from which 3‐D models were reconstructed using 3‐D‐CT volumetric lung reconstruction software (Syngo). Threshold limits of window level and window width were −198 to −992 Hounsfield units, as reported by Gollogly et al. in 200420. Soft tissue surrounding the lungs and large vessels within the lung were excluded. The 3‐D models were viewed as volume‐rendering displays at multiple angles to ensure that the models were valid. The trachea, main‐stem bronchi and lobar to segmental bronchi were semi‐automatically and selectively removed from the 3‐D models (Fig. 1).
Figure 1.
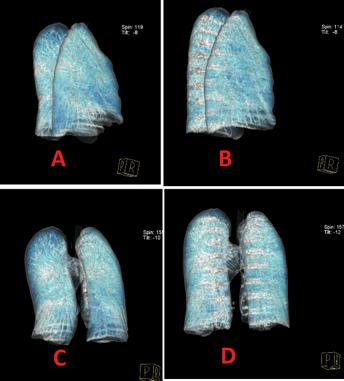
Preoperative and postoperative lung morphology changes in a 3‐D reconstruction of the lung. (A) Lateral view of preoperative reconstructed lung. (B) Lateral view of postoperative reconstructed lung. (C) Posteroanterior view of preoperative reconstructed lung. (D) Posteroanterior view of postoperative reconstructed lung.
Measured Variables
All measurements were performed by the first author of this paper. The measured variables included: i) TLV, RLV, LLV and CCLVR; ii) right lung height (RLH) and left lung height (LLH); iii) PCSA; and iv) thoracic symmetry as reflected by the concave to convex pulmonary cross‐sectional area ratio in the apical vertebrae plane (CCPCSAR).
Lung Volume Measurement
The number of voxels on the 3‐D lung model was calculated as lung volume. When the total lung in the axial plane was selected, the Syngo software calculated the TLV (Fig. 2). Right or LLV was measured when the corresponding lung of the 3‐D model was selected in the axial plane (Fig. 3).
Figure 2.
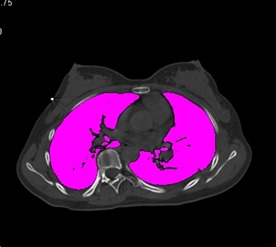
The whole lung has been selected in the axial plane and total lung volume calculated.
Figure 3.
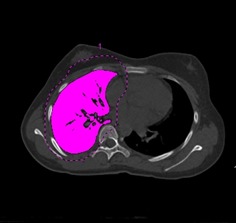
The right lung has been selected in the axial plane and the right lung volume calculated.
Lung Height Measurement
The “lung height” in this article is not the actual lung height but rather CT layer thickness calculated from the pulmonary apex to its base in the sagittal plane (Fig. 4).
Figure 4.
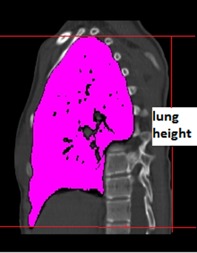
The lung height was defined as CT layer thickness calculated from the pulmonary apex to base in the sagittal plane and is not the actual lung height.
Pulmonary Cross‐sectional Area in the Apical Vertebrae Plane
The apical vertebrae plane of the 3‐D model in axial plane was selected: the PCSA in the apical vertebrae plane was the number of voxels calculated by the Syngo software in this plane (Fig. 5). From this, the concave and convex pulmonary cross‐sectional areas could be calculated (Fig. 6).
Figure 5.
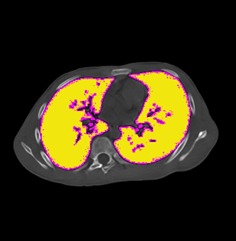
The pulmonary cross‐sectional area in the apical vertebral plane was selected in the axial plane and the number of voxels in this region calculated as the pulmonary cross‐sectional area in the apical vertebral plane.
Figure 6.
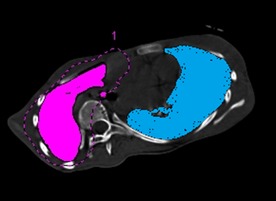
The concave (blue region) and convex (pink region) pulmonary cross‐sectional areas in the apical vertebral plane were calculated in the axial plane.
Statistical Analysis
Statistical analyses were performed with SPSS 16.0 for Windows. Descriptive data are presented as mean ± standard deviation (SD). Comparisons between preoperative and postoperative lung volume, CCLVR, lung height, PCSA and CCPCSAR were made using Wilcoxon signed‐rank tests. Potential relationships between sex, age, Lenke classification, Risser sign, correction of predominant thoracic curve, correction of kyphosis angle from T5 to T12 and changes in lung volume, CCLVR, lung height, PCSA and CCPCSAR were determined using Spearman rank correlation and Pearson's coefficient analysis. A P value less than 0.05 was considered statistically significant.
Results
Analysis of Results of Surgery
All subjects achieved good corrective outcomes and hypokyphosis was not aggravated. The preoperative main thoracic Cobb angle decreased from 58.8° ± 13.8° to 11.6° ± 7.8° postoperatively (P < 0.05). The average postoperative thoracic kyphosis angle from T5 to T12 was 25.6° ± 8.6° whereas the preoperative thoracic kyphosis angle from T5 to T12 was 28.9° ± 5.8° (P > 0.05).
Lung Volume and CCLVR
Preoperative LLV was 966.4 ± 301.1 cm3 and postoperative LLV 953.2 ± 274.3 cm3 (P > 0.05). Preoperative RLV was 1099.5 ± 294.3 cm3 and postoperative RLV 1070.1 ± 210.1 cm3 (P > 0.05). Preoperative TLV was 2089.0 ± 556.1 cm3 and postoperative TLV 2045.8 ± 455.6 cm3 (P > 0.05). Preoperative CCLVR was 1.167 ± 0.178 and postoperative CCLVR 1.172 ± 0.247 (P > 0.05). That is, both lung volume and CCLVR has not changed significantly after surgery (Table 1).
Table 1.
Comparison of measured variables pre‐ and post‐operatively (mean ± SD)
| Variable | Preoperative | Postoperative | P value |
|---|---|---|---|
| LLV (cm3) | 966.4 ± 301.1 | 953.2 ± 274.3 | 0.7136 |
| RLV (cm3) | 1099.5 ± 294.3 | 1070.1 ± 210.1 | 0.2660 |
| TLV (cm3) | 2089.0 ± 556.1 | 2045.8 ± 455.6 | 0.4993 |
| CCLVR | 1.167 ± 0.178 | 1.172 ± 0.247 | 0.9051 |
| LLH (cm) | 21.4 ± 2.6 | 22.6 ± 2.5 | 0.0008 |
| RLH (cm) | 21.9 ± 2.1 | 22.7 ± 2.7 | 0.0152 |
| PCSA (cm2) | 232.9 ± 43.6 | 223.1 ± 38.4 | 0.0147 |
| CCPCSAR | 1.378 ± 0.180 | 1.218 ± 0.122 | 0.0000 |
The subjects were divided into the following three groups according to their main thoracic Cobb angles: group A (n = 18), main thoracic Cobb angle >60°; group B (n = 9), main thoracic Cobb angle >60° and <80°; and group C (n = 3), main thoracic Cobb angle >80° (Table 2). LLV, RLV, and TLV did not change significantly after surgery in groups A and B (P > 0.05). However, in group C TLV increased from 2067.5 ± 783.5 cm3 to 2392.6 ± 848.2 cm3 (P < 0.05). Because Group C comprised only three patients, a larger study is required to confirm this finding.
Table 2.
Comparison of variables pre‐ and postoperatively in groups A and B (mean ± SD)
| Parameters | Group A (Cobb < 60°, n = 18) | Group B (60°–80°, n = 9) | Group C (Cobb > 80°, n = 3) |
|---|---|---|---|
| LLV (cm3) | |||
| Preoperative | 970.9 ± 277.4 | 922.5 ± 327.6 | 1071.0 ± 454.3 |
| Postoperative | 924.9 ± 209.6 | 921.8 ± 279.9 | 1217.3 ± 536.2 |
| P value | 0.2762 | 0.9928 | 0.3357 |
| RLV (cm3) | |||
| Preoperative | 1137.1 ± 280.6 | 1102.2 ± 284.3 | 969.8 ± 433.1 |
| Postoperative | 1098.9 ± 196.9 | 1086.3 ± 216.5 | 1026.1 ± 263.4 |
| P value | 0.2506 | 0.7691 | 0.6472 |
| TLV(cm3) | |||
| Preoperative | 2119.4 ± 516.7 | 2038.7 ± 624.6 | 2067.5 ± 783.5 |
| Postoperative | 1999.1 ± 367.6 | 2018.3 ± 475.1 | 2392.6 ± 848.2 |
| P value | 0.1300 | 0.8758 | 0.0363 |
Lung Height
Both LLH and RLH were increased significantly after surgery in these AIS patients (Table 1). Preoperative LLH was 21.4 ± 2.6 cm whereas postoperative LLH was 22.6 ± 2.5 cm (P < 0.05). RLH also increased, being 21.9 ± 2.1 cm preoperatively and 22.7 ± 2.7 cm postoperatively (P < 0.05).
PCSA and CCPCSAR
Pulmonary cross‐sectional area decreased from 232.9 ± 43.6 cm2 preoperatively to 223.1 ± 38.4 cm2 postoperatively (P < 0.05). Preoperative CCPCSAR was 1.378 ± 0.180 and declined to 1.218 ± 0.122 after surgery (P < 0.05). Thus, these AIS patients’ thoracic symmetry was improved postoperatively (Table 2).
Correlation Analysis of Postoperative Changes in Measured Variables
The change in CCPCSAR correlated positively with changes in correction of predominant thoracic curve (r = 0.8636; P < 0.01) and correction of kyphosis angle from T5 to T12 (r = 0.6482; P < 0.05). However, the changes in LLH, RLH and PCSA did not correlate significantly with patients’ sex, age, Lenke classification, Risser sign, correction of predominant thoracic curve, or correction of kyphosis angle from T5 to T12. With better coronal and sagittal balance, thoracic symmetry did improve (Table 3).
Table 3.
Correlation analyses of postoperative changes in variables (values of correlation coefficient)
| Variable | Sex | Age | Lenke classification | Risser sign | Main thoracic Cobb's angle | T5–T12 kyphosis angle |
|---|---|---|---|---|---|---|
| LLH | 0.1826 | 0.2634 | 0.2720 | 0.2334 | 0.3592 | 0.0894 |
| RLH | 0.1054 | 0.0469 | 0.3389 | 0.1844 | 0.3737 | 0.2322 |
| PCSA | −0.0100 | 0.0244 | 0.1897 | −0.1161 | −0.2914 | −0.2861 |
| CCPCSAR | −0.1548 | 0.2569 | 0.0576 | −0.2154 | 0.8636* | 0.6482† |
*, P < 0.05; †, P < 0.01.
Discussion
Spine surgeons have always paid close attention to pre‐ and post‐operative pulmonary function and lung volume in AIS patients21. The purpose of corrective surgery is to prevent the deterioration in pulmonary function caused by progressive spinal deformity. Previous studies have evaluated pulmonary function after spinal fusion surgery and most of them have shown minor improvements, no change, or minor declines after surgery11, 12, 13, 14. In our study, 3‐D CT scans and 3‐D reconstruction software were used to evaluate changes in lung morphology in AIS patients after PSF surgery.
Convex to Concave Lung Volume Ratio
Adam et al. used 3D‐CT volumetric reconstruction to assess 28 patients with idiopathic scoliosis22. They found statistically significant differences in both lung volumes and lung volume ratios compared with normal controls. Chun et al. studied 99 cases (77 asymptomatic AIS patients and 22 normal controls) and reported that concave side lung volume was relatively more decreased than convex side lung volume. In our study, the value for CCLVR was 1.167 ± 0.178, which does not differ significantly from that reported by Chun (1.1823 ± 0.1158) (P > 0.05)23, showing that both convex and concave lung volumes were reduced, the impact on the concave lung being greater.
Reported Pulmonary Function and Lung Volume
Previous studies have reported that pulmonary function of AIS patients treated with anterior release and posterior fusion surgery clearly decreases 3–6 months after surgery and recovers to preoperative levels 12–24 months postoperatively8, 24. Lonner et al. reported that pulmonary function of AIS patients at 2‐year follow‐up declined slightly after thoracotomy and thoracoscopic surgery, the decline being significantly greater than in subjects who underwent open thoracotomy10. These findings indicate that pulmonary impairment occurs more frequently in AIS patients who have undergone thoracoplasty than in those who have not. Thus, opening of the chest wall leads to postoperative deterioration in pulmonary function.
According to several studies, posterior spinal surgery stabilizes or improves pulmonary function postoperatively7, 8, 9, 25, 26, 27. Vedantam et al. reported that in 47 patients who had undergone PSF without thoracoplasty, the absolute forced vital capacity and forced expiratory volume in one second had improved significantly by final follow‐up. However, the percent predicted values for pulmonary function did not differ significantly between preoperatively and final follow‐up25. Kim et al. studied 139 AIS patients following posterior segmental spinal fusion and instrumentation with iliac crest bone graft and demonstrated statistically significant improvement in absolute and percent predicted pulmonary function tests 2 years postoperatively26. More recently, Demura et al. examined 154 cases following PSF without thoracoplasty and showed significant increases in the absolute values for forced vital capacity, forced expiratory volume in one second and total lung capacity27. However, in contrast with the report of Kim et al.26, the changes in percent predicted values were not statistically significant.
Improvement in Diaphragmatic Motion and Thoracic Symmetry
Dynamic movement of the thoracic cage and diaphragm have been studied by ultrasonography28, infrared rays29, CT30 and MRI6, 31. Kotani et al. used dynamic breathing MRI to compare movement of the thoracic cage in healthy subjects and patients with scoliosis and found no significant differences in movement of the diaphragm and lateral expansion of the thoracic cage; however, they found that lower chest wall movement in the anteroposterior direction was significantly restricted in patients with scoliosis31. Chu et al. used dynamic MR to study the lung volume, chest wall and diaphragmatic motion between inspiration and expiration in AIS patients before and after surgery15. They found that lateral chest wall and diaphragmatic motions were improved six months after PSF. This shows that chest wall and diaphragmatic movements can be further increased by corrective surgery. The increased lateral chest wall movement could be related to the correction of the abnormal chest wall after surgery, whereas the improvement in diaphragmatic motion may be explained by the reconstructed chest wall decreasing the compressive effect by both the scoliotic spine and the rotated mediastinum.
In the current study, 3‐D‐CT imaging and lung volume and height measurement were used to evaluate changes in pre‐ and post‐operative lung morphology in AIS patients after PSF surgery. We found that the corrective surgery only corrected spinal deformity and increased lung height (P < 0.05), whereas the PCSA in the apical vertebral plane was decreased postoperatively (P < 0.05). Additionally, the concave to convex pulmonary cross‐sectional area ratio in the apical vertebrae plane declined after surgery (P < 0.05). In other words, thoracic symmetry in AIS patients was improved whereas lung volume did not change postoperatively.
Although the lung volume does not change postoperatively in AIS patients, lung morphology and thoracic symmetry improves after surgery. Improvement in thoracic symmetry denotes reestablishment of symmetry in the chest cage and may lead to improvements in deep breathing, which involves larger movements of the chest cage and diaphragm. Improving the symmetry of the chest cavity and correcting spinal deformity can make the diaphragm and ribs move more naturally15, which may contribute to an improvement in pulmonary function.
Limitations
In our study, all CT scans were obtained in the supine position with the breath held in deep inspiration, which eliminated the effects of breathing and position on lung volume. This prospective study had a few limitations. The first was the radiation exposure. However, the 3‐D‐CT scans performed on AIS patients pre‐ and postoperatively were also to assess the vertebral structure and ensure the accuracy of pedicle screw placement rather than for purposes of research alone. It should be noted that we do not advocate the routine use of 3‐D‐CT scan as a means of assessing lung function. Also, all scans were obtained using ALARA protocols. Another weakness of this study was the lack of follow‐up postoperative pulmonary function tests. Future studies are needed to examine correlations between postoperative pulmonary function tests and lung volume in AIS patients.
In conclusion, the current study demonstrated changes in lung morphology of AIS patients after PSF surgery, showing that lung height was increased significantly postoperatively whereas lung volume did not. Moreover, thoracic symmetry in these AIS patients was improved postoperatively, which may contribute to postoperative improvement in pulmonary function.
Disclosure: This manuscript does not contain information about medical device(s)/drug(s). No funds were received in support of this work. No financial activities related to the submitted work.
References
- 1. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet, 2008, 371: 1527–1537. [DOI] [PubMed] [Google Scholar]
- 2. Newton PO, Faro FD, Gollogly S, Betz RR, Lenke LG, Lowe TG. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis. A study of six hundred and thirty‐one patients. J Bone Joint Surg Am, 2005, 87: 1937–1946. [DOI] [PubMed] [Google Scholar]
- 3. Kearon C, Viviani GR, Kirkley A, Killian KJ. Factors determining pulmonary function in adolescent idiopathic thoracic scoliosis. Am Rev Respir Dis, 1993, 148: 288–294. [DOI] [PubMed] [Google Scholar]
- 4. Upadhyay SS, Mullaji AB, Luk KD, Leong JC. Relation of spinal and thoracic cage deformities and their flexibilities with altered pulmonary functions in adolescent idiopathic scoliosis. Spine (Phila Pa 1976), 1995, 20: 2415–2420. [DOI] [PubMed] [Google Scholar]
- 5. Lin MC, Liaw MY, Chen WJ, Cheng PT, Wong AM, Chiou WK. Pulmonary function and spinal characteristics: their relationships in persons with idiopathic and postpoliomyelitic scoliosis. Arch Phys Med Rehabil, 2001, 82: 335–341. [DOI] [PubMed] [Google Scholar]
- 6. Chu WC, Li AM, Ng BK, et al Dynamic magnetic resonance imaging in assessing lung volumes, chest wall, and diaphragm motions in adolescent idiopathic scoliosis versus normal controls. Spine (Phila Pa 1976), 2006, 31: 2243–2249. [DOI] [PubMed] [Google Scholar]
- 7. Johnston CE, Richards BS, Sucato DJ, et al Correlation of preoperative deformity magnitude and pulmonary function tests in adolescent idiopathic scoliosis. Spine (Phila Pa 1976), 2011, 36: 1096–1102. [DOI] [PubMed] [Google Scholar]
- 8. Kim YJ, Lenke LG, Bridwell KH, Kim KL, Steger‐May K. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure. J Bone Joint Surg Am, 2005, 87: 1534–1541. [DOI] [PubMed] [Google Scholar]
- 9. Newton PO, Perry A, Bastrom TP, et al Predictors of change in postoperative pulmonary function in adolescent idiopathic scoliosis: a prospective study of 254 patients. Spine (Phila Pa 1976), 2007, 32: 1875–1882. [DOI] [PubMed] [Google Scholar]
- 10. Lonner BS, Auerbach JD, Estreicher MB, et al Pulmonary function changes after various anterior approaches in the treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech, 2009, 22: 551–558. [DOI] [PubMed] [Google Scholar]
- 11. Jackson RP, Simmons EH, Stripinis D. Coronal and sagittal plane spinal deformities correlating with back pain and pulmonary function in adult idiopathic scoliosis. Spine (Phila Pa 1976), 1989, 14: 1391–1397. [DOI] [PubMed] [Google Scholar]
- 12. Lenke LG, White DK, Kemp JS, Bridwell KH, Blanke KM, Engsberg JR. Evaluation of ventilator efficiency during exercise in patients with idiopathic scoliosis undergoing spinal fusion. Spine (Phila Pa 1976), 2002, 27: 2041–2045. [DOI] [PubMed] [Google Scholar]
- 13. Pehrsson K, Danielsson A, Nachemson A. Pulmonary function in adolescent idiopathic scoliosis: a 25 year follow up after surgery or start of brace treatment. Thorax, 2001, 56: 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wood KB, Schendel MJ, Dekutoski MB, Boachie‐Adjei O, Heithoff KH. Thoracic volume changes in scoliosis surgery. Spine (Phila Pa 1976), 1996, 21: 718–723. [DOI] [PubMed] [Google Scholar]
- 15. Chu WC, Ng BK, Li AM, Lam TP, Lam WW, Cheng JC. Dynamic magnetic resonance imaging in assessing lung function in adolescent idiopathic scoliosis: a pilot study of comparison before and after posterior spinal fusion. J Orthop Surg Res, 2007, 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iwano S, Okada T, Satake H, Naganawa S. 3D‐CT volumetry of the lung using multidetector row ct: comparison with pulmonary function tests. Acad Radiol, 2009, 16: 250–256. [DOI] [PubMed] [Google Scholar]
- 17. Yu W, Song K, Zhang Y, Zheng GQ, Dong T. Relationship between lung volume and pulmonary function in patients with adolescent idiopathic scoliosis: computed tomographic‐based three‐dimensional volumetric reconstruction of lung parenchyma. J Spinal Disord Tech, 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Bullmann V, Schulte TL, Schmidt C, Gosheger G, Osada N, Liljenqvist UR. Pulmonary function after anterior double thoracotomy approach versus posterior surgery with costectomies in idiopathic thoracic scoliosis. Eur Spine J, 2013, 22 (Suppl. 2): S164–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu B, Wang Y, Qiu G, Shen J, Zhang J, Lao L. The influence of preoperative brace treatment on the pulmonary function test in female adolescent idiopathic scoliosis. J Spinal Disord Tech, 2013, 26: E254–E258. [DOI] [PubMed] [Google Scholar]
- 20. Gollogly S, Smith JT, White SK, Firth S, White K. The volume of lung parenchyma as a function of age: a review of 1050 normal CT scans of the chest with three‐dimensional volumetric reconstruction of the pulmonary system. Spine (Phila Pa 1976), 2004, 29: 2061–2066. [DOI] [PubMed] [Google Scholar]
- 21. Lao L, Weng X, Qiu G, Shen J. The role of preoperative pulmonary function tests in the surgical treatment of extremely severe scoliosis. J Orthop Surg Res, 2013, 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adam CJ, Cargill SC, Askin GN. Computed tomographic‐based volumetric reconstruction of the pulmonary system in scoliosis: trends in lung volume and lung volume asymmetry with spinal curve severity. J Pediatr Orthop, 2007, 27: 677–681. [DOI] [PubMed] [Google Scholar]
- 23. Chun EM, Suh SW, Modi HN, Kang EY, Hong SJ, Song HR. The change in ratio of convex and concave lung volume in adolescent idiopathic scoliosis: a 3D CT scan based cross sectional study of effect of severity of curve on convex and concave lung volumes in 99 cases. Eur Spine J, 2008, 17: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou C, Liu L, Song Y, et al Pulmonary function changes after operation in patients with severe scoliosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2010, 24: 23–26. [PubMed] [Google Scholar]
- 25. Vedantam R, Lenke LG, Bridwell KH, Haas J, Linville DA. A prospective evaluation of pulmonary function in patients with adolescent idiopathic scoliosis relative to the surgical approach used for spinal arthrodesis. Spine (Phila Pa 1976), 2000, 25: 82–90. [DOI] [PubMed] [Google Scholar]
- 26. Kim YJ, Lenke LG, Bridwell KH, Cheh G, Whorton J, Sides B. Prospective pulmonary function comparison following posterior segmental spinal instrumentation and fusion of adolescent idiopathic scoliosis: is there a relationship between major thoracic curve correction and pulmonary function test improvement? Spine (Phila Pa 1976), 2007, 32: 2685–2693. [DOI] [PubMed] [Google Scholar]
- 27. Demura S, Bastrom TP, Schlechter J, Yaszay B, Newton PO, Harms Study Group . Should postoperative pulmonary function be a criterion that affects upper instrumented vertebra selection in adolescent idiopathic scoliosis surgery? Spine (Phila Pa 1976), 2013, 38: 1920–1926. [DOI] [PubMed] [Google Scholar]
- 28. Kotani T, Minami S, Takahashi K, et al Three dimensional analysis of chest wall motion during breathing in healthy individuals and patients with scoliosis using an ultrasonography‐based system In: Grivas TB, ed. Research into Spinal Deformities 4, 1st edn Amsterdam: IOS Press, 2002; 135–139. [PubMed] [Google Scholar]
- 29. Leong JC, Lu WW, Luk KD, Karlberg EM. Kinematics of the chest cage and spine during breathing in healthy individuals and in patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976), 1999, 24: 1310–1315. [DOI] [PubMed] [Google Scholar]
- 30. Kondo T, Arita H, Ohta Y, Yamabayashi H. Role of the mediastinum as a part of the chest wall: analyzed by computed tomography. Respiration, 1989, 56: 116–126. [DOI] [PubMed] [Google Scholar]
- 31. Kotani T, Minami S, Takahashi K, et al Respiratory motion analysis using dynamic breathing MRI in patients with idiopathic scoliosis. J Jpn Scoliosis Soc, 2003, 18: 31–35. (in Japanese). [Google Scholar]


