Abstract
The study of bone tissue regeneration in orthopaedic diseases has stimulated great interest among bone tissue engineering specialists and orthopaedic surgeons. Combinations of biomaterials, growth factors and stem cells for repairing bone have been much studied and researched, yet remain a challenge for both scientists and clinicians pursuing regenerative medicine. The purpose of this review was to elucidate the role of sequential release of bone morphogenetic protein‐2 and vascular endothelial growth factor in producing better outcomes in the field of bone tissue regeneration.
Keywords: Bone morphogenetic protein‐2, Bone tissue regeneration, Vascular endothelial growth factor
Introduction
With the aging of populations worldwide and the associated increasing incidence of bone diseases, over the next few years repair of bone defects and fractures will be a major challenge for orthopaedic surgeons1, 2. Bone is a dynamic, highly vascularized tissue which has tendency to heal by itself; however, regeneration and growth of tissue are slow processes3. In addition, these processes can be affected by various physiological processes, biomaterials and growth factors; shortening healing time after bone repair is an important and popular clinical research focus in orthopaedics4, 5. Bone grafts are the gold standard for treating bone defects, autografts being the most commonly used; however, autografts have limitations in sources for bone sampling and complications can occur in both donor and recipient sites during and after such surgeries6, 7. Even allografts have their limitations and adverse consequences such as risk of infection, disease transmission and host immune responses8. These drawbacks have led to the development of new strategies for repairing bone defects, including the use of various factors. A combination of delivery of growth factors and stem cell support provides a controlled environment that can enhance bone healing by mimicking the bone environment9, 10. This review describes the individual and combined roles of bone morphogenetic protein‐2 (BMP‐2) and vascular endothelial growth factor (VEGF) in bone repair, including their sequential release and the importance of gene delivery in bone tissue regeneration.
Growth Factors for Bone Tissue Regeneration
Growth factors are defined as proteins secreted by cells that act on the appropriate target cell or cells to perform a specific function11, 12. They are part of a vast cellular communications network that influences such critical functions as cell division, matrix synthesis and tissue differentiation13. Many studies have established that growth factors play vital roles in healing of bone fractures14. The most studied growth factors are BMP‐2 and VEGF, whicha re involved in osteogenesis and angiogenesis, respectively15, 16.
BMP‐2 in Bone Tissue Regeneration
A number of key molecules that regulate the complex bone regenerative physiological process have been identified and are already in clinical use for enhancing bone repair. Of these molecules, BMP‐2 has been the most extensively studied regarding induction of new bone formation in ectopic and orthotopic sites, including where there are critical size defects (CSDs)17, 18. BMP‐2 has been found to be a promising alternative to autografts for non‐union of bone defects, open tibial fractures, spinal fusion and accelerated fracture healing19, 20. BMP‐2, a growth factor, belongs to the transforming growth factor‐β superfamily of protein; it acts as a disulfide‐linked homodimer and induces bone and cartilage formation21. It is a pleiotropic regulator that governs key steps in the bone induction cascade, such as chemotaxis, mitosis and differentiation of mesenchymal stem cells in the process of bone healing22, 23.
The use of BMP‐2 can be advantageous for bone regeneration or even for acceleration of normal bone healing to reduce the duration of fracture treatment24, 25. Its clinical use, either alone or combined with bone grafts, is constantly increasing26. However, there are several issues concerning its use, including safety (because of the supraphysiological concentrations of growth factors needed to obtain the desired osteoinductive effects), the high cost of treatment and, more importantly, the potential for ectopic bone formation27. Here we present findings of some among many studies reporting the effectiveness of BMP‐2 in bone tissue regeneration.
Li et al. reported a study on use of adipose tissue‐derived stem cells (ADSCs) and BMP‐2 for bone defects. Radiographic, histological and histomorphometry assessment at 16 weeks showed that ADSCs modified by BMP‐2 gene cause a significant increase in newly formed bone area. These authors concluded that ADSCs modified by the BMP‐2 gene can enhance the repair of CSDs in large animals28. The effect of brief incubation (15 min) with BMP‐2, which induces an osteogenic phenotype in adipose tissue‐derived mesenchymal stem cells (AT‐MSCs), was studied by Knippenberg et al. They assessed the effects of treatment with 15 min incubation with BMP‐2 on osteogenic differentiation of AT‐MSCs. Their data indicate that incubation with BMP‐2 for 15 min induces osteogenic differentiation and they concluded that AT‐MSCs that have been triggered for only 15 min with BMP‐2 provide a viable source for bone tissue regeneration29. Hollinger et al. studied a combination of recombinant human bone morphogenetic protein‐2 (rhBMP‐2) and collagen for regenerating bone. Unilateral CSDs were treated with 35 μg of rhBMP‐2 combined with absorbable collagen (rhBMP‐2 and collagen) and compared with untreated CSDs. Their study showed that combined rhBMP‐2 and collagen can be an effective therapy for restoring segmental bone defects30. Keib et al. have reported that a combination of ADSCs and BMP‐2 in a fibrin matrix induce significantly less callus formation than BMP‐2 alone31. However, Lin et al. reported that, compared with ADSCs transiently expressing BMP‐2, ADSCs persistently expressing BMP‐2 not only accelerate healing of weight‐bearing segmental bone defects but also improve bone metabolism, bone volume, bone density, angiogenesis and mechanical properties32. However, Brown et al. have suggested that the strategy that is ideal for release of rhBMP‐2 for new bone formation includes both a burst and a sustained release. For large CSDs, a burst release helps to attract osteoprogenitor cells into the delivery system, whereas sustained release promotes osteoblastic differentiation33.
In addition to these above studies, Song et al. reported that BMP‐2 used alone can induce a surplus of callus formation (heterotopic ossification)34. However, they reported that BMP‐2 in combination with vitamin D3 promotes osteogenic differentiation of ADSCs, these agents can work synergistically and be used to achieve effective and economical osteogenic induction of ADSCs for bone tissue engineering.
The growth factor BMP‐2 is known to induce both osteogenic and chondrogenic commitment of human mesenchymal stem cells (hMSCs)35. However, factors influencing BMP‐2‐dependent chondrogenic and osteogenic differentiation have not been investigated. Kwon et al. demonstrated that extracellular microenvironments, in the form of cell‐derived matrices, play important roles in determining the specific lineage commitment of hMSCs in the presence of BMP‐2. They concluded their research by that cell‐specific ECMs are capable of modulating BMP‐2‐induced osteogenic and chondrogenic differentiation of hMSCs36.
All the strategies of using BMP‐2 alone have advantages and disadvantages37. Up to now a combination of BMP‐2 and stem cells for bone regeneration has shown promising results38. However the limitations and drawbacks39 need more investigation and research studies to obtain more complete answers regarding sequential release of growth factors for better bone tissue regeneration.
VEGF in Bone Tissue Regeneration
Successful bone formation and fracture healing is associated with osteogenesis and angiogenesis40. VEGF, a signal protein produced by cells, stimulates vasculogenesis and angiogenesis41. VEGF is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate42. Bone, a highly vascularized tissue, is reliant on a close connection between blood vessels and bone cells to maintain skeletal integrity43, 44. Angiogenesis thus plays a vital role in skeletal development and bone repair45, 46. VEGF's normal function is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels45, 47. Some studies suggest ADSCs participate in tissue regeneration through their production of angiogenic factors and mediation of endogenous vasculogenesis/angiogenesis47, 48. For example, both in vitro and in vivo studies suggest that ADSCs drive endothelial differentiation and stabilize it through paracrine action49.
Blood vessels are an important component of bone formation and maintenance and bone tissue differentiation requires the local presence of blood vessels50, 51. Liu et al. investigated in vivo vascularization and bone formation activity of tissue‐engineered bone constructed by using bone marrow MSCs transfected with VEGF. Growth of bone xenografts, clumps of cartilage cells, irregular bone‐like tissue and microvessels progressed with time. In the control mice, only small amounts of bone‐like and fibrotic tissue were observed. The differences between the control and experimental groups were significant. In conclusion, VEGF165‐transfected bone marrow MSCs promote vascularization of tissue‐engineered bone and ectopic osteogenesis52.
Bone regeneration and osseointegration of biological components are dependent on vascularization and angiogenesis. An angiogenic factor, VEGF has been shown to promote biomaterial vascularization and enhance bone formation. However, high local concentrations of VEGF induce the formation of malformed, nonfunctional vessels. Wernike et al. postulated that continuous delivery of low concentrations of VEGF from calcium phosphate ceramics may increase the efficacy of VEGF administration. The release kinetics of VEGF appear to be an important factor in promotion of biomaterial vascularization and bone formation. Sustained release of VEGF increases the efficacy of VEGF delivery, demonstrating that prolonged bioavailability of low concentrations of VEGF is beneficial for bone regeneration53.
Johannes et al. have studied the influence of controlled release of recombinant human vascular endothelial growth factor on angiogenesis and osteogenesis in a mandibular defect model. The area of newly formed bone was not significantly different from that of a control group; however, the bone regeneration was significantly more dense in the study group. Their study showed that use of recombinant human vascular endothelial growth factor leads to more intensive angiogenesis and bone regeneration54.
Vascularization underlies the success of guided bone regeneration (GBR) processes. Kaigler et al. have evaluated the regenerative potential of GBR in combination with VEGF delivered via an injectable hydrogel system. CSDs were created in rat calvariae and GBR procedures performed with a collagen membrane alone (control), or plus bolus delivery of VEGF, or plus application of VEGF‐releasing hydrogels. They demonstrated that application of VEGF‐releasing hydrogels enhances early angiogenesis, whereas at a later stage it enhances bone regeneration. Approaches involving controlled delivery of angiogenic growth factors used adjunctively with GBR may be a promising strategy for enhancing outcomes of GBR55.
The use of single growth factors in bone regeneration has limitations and drawbacks56. BMP‐2 used alone in inappropriate amounts results in heterotrophic ossification and tumorigenesis57, 58. Even though angiogenesis is important for bone regeneration, VEGF plays more of a role in angiogenesis than in osteogenesis59. Single growth factors characteristically enhanced bone regeneration to a limited degree and greater doses are needed to achieve the desired outcome60. These high doses may lead to a variety of consequences and the unexpected outcomes. The synergy between BMP‐2 and VEGF is intimately related to bone development and healing that is advantageous for bone regeneration procedure. Thus, they may play important roles in enhancing the efficiency of cell‐based approaches to bone regeneration61, 62.
Combination of BMP‐2 and VEGF in Bone Tissue Regeneration
A potent angiogenic factor, VEGF has been shown to be essential for both intramembranous and endochondral bone formation63, 64 and for bone repair65, 66. Therefore, a combination of BMP‐2 and VEGF would be effective in bone regeneration and could be used for CSDs or compromised bones that are insufficiently vascularized.
Exogenous MSCs, VEGF and BMPs together with an osteoconductive scaffold are a very satisfactory means of enhancing bone repair. This concept has been incorporated into the development of new strategies for bone tissue engineering; significant advancements have been made in last 10 years. Contrary to a previous belief that VEGF modulates bone repair only by enhancing angiogenesis in the proximity of bone injury, recent evidence suggests that cross‐talk between VEGF and BMP signaling pathways in MSCs promotes osteoblastic differentiation of MSCs, which aids in fracture repair. Future studies should focus on cross‐talk between angiogenesis and osteogenesis, optimization of VEGF/BMP ratios, selection of the most potent BMPs, and optimization of delivery methods for VEGF and BMP. Recent discoveries from basic research, including effective delivery of growth factors and cells to the area of interest, will help bring VEGF plus BMP for bone healing from the bench to the patient's bedside67.
New approaches that focus on scaffold composition and the amount of growth factor released are being investigated68. Recent studies on the simultaneous release of combinations of several growth factors have demonstrated that they have a synergistic effect on bone healing. The findings of Geiger et al.54 and Peng et al.69 suggest that a combination of angiogenic and osteogenic factors can stimulate bone healing and regeneration. Therefore, development of a combined system for delivering growth factors derived locally from biodegradable scaffolds at different rate kinetics could enhance the mechanisms for repairing CSDs; thus mimicking in vivo bone repair conditions. Kanczler et al. have developed a polymeric system for tissue‐specific controlled‐release delivery from a structural polymer scaffold of two or more growth factors. They have shown that MSCs seeded onto these new generation combined growth factors can result in the co‐development of vessels and bone in situ, facilitating rapid development of vascularized engineered bone constructs70. Patel et al. studied dual release of VEGF and BMP‐2 and showed complete union of defects in 5/8 rats within 12 weeks, whereas BMP‐2 alone resulted in complete union in 3/8 rats and VEGF no union at all. The results were same as for VEGF alone in an experiment in which no growth factors were used. This indicates that delivery of both growth factors may enhance bone bridging and union of CSDs compared with delivery of one growth factor alone71.
However, the actions of growth factors are dependent on dose and vehicle of delivery according to Ehnert et al.72. Using VEGF/BMP‐2 ratios of >1, Young et al. failed to show a synergistic effect of BMP‐2 and VEGF on bone formation in CSDs compared with BMP‐2 alone73. They stated that use of high doses of VEGF results in stem cell differentiation towards an endothelial lineage, thereby reducing the number of cells available for osteogenic differentiation. All these studies suggest that the sequential release of both angiogenic and osteogenic growth factors can enhance natural healing and thus promote regeneration of bone tissue.
It has been demonstrated that periosteum contains mesenchymal progenitor cells that differentiate into osteoblasts, and that both osteogenic and angiogenic growth factors may play important roles in cell‐based approaches to bone regeneration74. Samee et al. evaluated the feasibility and efficacy of BMP‐2 and/or VEGF on periosteal cell differentiation to osteoblasts in vitro and ectopic bone formation in vivo. They showed that VEGF may enhance BMP2‐induced bone formation through modulation of angiogenesis75. Osteogenic growth factors are continuously expressed during bone formation and remodeling76, whereas angiogenic growth factors are predominantly expressed during the early phases of developing vascularity77. Because VEGF and BMP‐2 are key regulators of angiogenesis and osteogenesis during bone regeneration, Kempen et al. studied a combination of them with local sustained BMP‐2 release and found that VEGF significantly enhances ectopic bone formation compared with BMP‐2 alone. In orthotopic defects, they found that VEGF had no effect on vascularization and that bone formation was not greater with a combination of growth factors than with BMP‐2 alone. This study demonstrated that sequential angiogenic and osteogenic growth factor release may be beneficial for enhancing bone regeneration78.
Most of these above studies suggest that a combination of VEGF and BMP‐2 has better results than either used alone and also can be shown in diagram (Fig. 1 ). However, controlling the release of exogenous BMPs and VEGF for therapeutic application was initially motivated by early research. The drawbacks of growth factor delivery are as follows: (i) the in vivo half‐life of these proteins is very short; (ii) protein‐carrier devices rely on passive diffusion, which limits the capacity for reconstituting natural highly dynamic spatial and temporal patterns; and (iii) high (mg) doses of recombinant protein are required to elicit durable osteogenic responses79. Thus, creating well‐defined gradients and other physiologic patterns of expression remains a substantial challenge. Now we need to further understand how the growth factors interact with each other and with stem cells during their sequential release and engage in deeper study of their roles in bone tissue regeneration.
Figure 1.
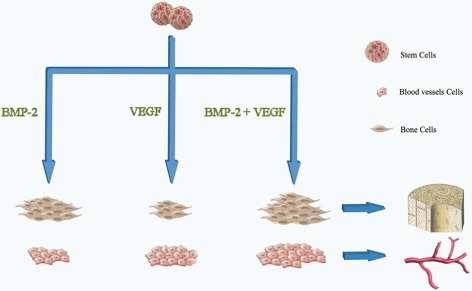
A combination of VEGF and BMP‐2 has better results on vascularization and bone formation than either used alone.
Role of Experimental Gene Therapy in Bone Tissue Regeneration
Gene therapy approaches to delivering BMPs have the potential to overcome these limitations, especially when we consider state‐of‐the‐art regulated expression systems80. In contrast to constitutive promoter‐driven expression constructs, which have some of the same limitations as protein‐carrier devices, chemically or physically activated expression systems provide substantial control over the level, duration and spatial localization of growth factors81. With the continued development of safe and efficient vectors, emergence of “same day” ex vivo gene delivery, and evaluation of bone tissue regeneration in large immunocompetent animal models, the technologies described below have tremendous potential for improving the clinical outcomes associated with growth factor therapy.
Controlling the Timing of BMP Expression
Tetracycline (Tet)‐dependent systems are sophisticated approaches to controlling the timing of transgenes that provide maximal control over the magnitude, timing, duration and spatial localization of expression of a target gene. The presence of tetracycline or its analog doxycycline (Dox) induces the TetON system to trigger transgene expression. In contrast, the transactivator in the TetOFF system cannot bind its target in the presence of antibiotics, which thereby inhibit expression. Moutsatsos et al. have reported that a murine MSC line that has been engineered to express BMP‐2 under the control of the TetOFF system promotes healing of non‐union of radius fractures in mice82. However, these researchers observed excess bone formation in some animals, which they attributed to expression of BMP beyond the desired degree. Gafni et al. reported that, after in vivo implantation of hBMSCs into critical‐sized calvarial defects, addition of Dox to the animals' drinking water resulted in expression of BMP2 and eventual closure of the defects83. A major concern with the clinical use of Tet systems is the risk that patients may develop resistance to tetracycline. Also, because Tet/Dox are bone‐seeking drugs, these compounds may accumulate in bone and interfere with regulated expression. Muthukuru and Sun recently reported that Dox counteracts BMP2‐induced osteogenic mediators in human periodontal ligament cells, suggesting that Tet systems may be particularly problematic in regard to regulation of BMP2 expression84. Finally, Tet regulated systems can be “leaky” in that they express significant amount of transgene in the uninduced state. Therefore, more stringent gene expression systems suitable for bone regeneration are required.
Dimerizer‐based gene switches use heterodimeric transcription factors composed of separate DNA‐binding and activation domains that interact only in the presence of a small dimerizer molecule such as rapamycin to form a functional transactivator. Because only the dimerized factor is capable of functioning as a transcription factor, this system provides stringent regulation of target gene expression85. A major advance occurred with the development of rapalogs, non‐immunosuppressive analogs of rapamycin that retain the ability to function as dimerizers. Koh et al. tested the ability of a rapamycin/rapalog‐based system to regulate BMP2 expression and heal critical‐sized calvarial defects. Rapamycin tightly regulated in vivo production of the growth factor; the system exhibited clear dose dependence, and amounts of BMP were shown to decline rapidly 4–6 days after a single rapamycin injection. Repeated rapamycin treatment over several weeks led to uniform new bone formation in the defect. The new bone was fully integrated with the host bone and showed no evidence of overgrowth. In contrast, when cells were transduced with an adenovirus encoding BMP2 under the control of a constitutive promoter, the new bone was highly irregular and discontinuous with the surrounding tissue. These differences may be attributable to the dynamics of BMP‐2 secretion driven by the inducible system, which provides sustained low‐level delivery of BMP over time versus the high (but transient) levels of transgene production with adenovirus. Using the former approach, precise temporal control over BMP delivery was achieved: a key factor for successful fracture healing and bone formation86.
Controlling the Temporal and Spatial Aspects of VEGF Expression
As demonstrated above, bone healing is a coordinated process that involves both osteogenesis and angiogenesis. The techniques of gene therapy listed above can only control one target gene expression dependently. The need to control two interest genes independently is encouraging researchers to investigate other control systems. There are some technologies that meet this demand; namely, genetic tools that are capable of providing 4D control of transgene expression in vivo.
Optogenetics systems exploit the ability of certain proteins to be activated by light. A light‐inducible synthetic gene switch was recently described by Wang et al.87. Upon exposure to blue light, the transactivator of this system binds synthetic promoters that rapidly initiate transcription of target transgenes. Withdrawal from light leads to the eventual inactivation of the transactivator and thus, to gene silencing. However, one challenge to the use of this system in vivo is related to difficulties in focusing light deep within the body. Light scattering, particularly of short wavelengths, substantially attenuates the ability of light to penetrate tissues. For this reason, it would be very difficult to activate transgene expression in deep tissue sites without also activating more proximal cells within the light path.
Inducible systems based on promoters that are activated by externally directed physical stimuli may be more generally useful for generating 4D patterns of transgene expression; these have been reviewed by Vilaboa et al.88. Promoters of this type include heat‐shock protein (hsp) gene promoters and radiation‐induced promoters that can be activated by heat and directed ionizing radiation, respectively. Both ionizing radiation and administered heat can be focused; however, because of its intrinsic toxicity, ionizing radiation should be utilized only in the context of cancer or ablative therapies. In contrast, localized heating of tissues can be achieved by many safe and non‐invasive methods, including ultrasound, microwaves and infrared radiation. Ultrasound is currently the most promising approach, because it exhibits low attenuation in biologic media over organ‐scale distances and can be focused to generate mm3–cm3 subvolumes of hyperthermia deep within the body. Rome et al. have reported localized, focused, ultrasound‐induced expression of target genes with the human hsp70B promoter in in vivo models, even in deep‐seated organs such as the liver89.
Moreover, hsp promoter‐controlled gene therapies are susceptible to non‐specific activation by hyperthermia associated with disease, local inflammation, strenuous exercise, pharmacological interventions or ischemic events. To overcome this problem, synthetic gene circuits that combine an hsp70B promoter and a small molecule‐dependent transactivator have been designed, as described by Vilaboa et al.90. These gene switches consist of: (i) a ligand‐activated transactivator expressed under the dual control of the hsp70B promoter; and (ii) a promoter that is responsive to the transactivator that controls the gene of interest. Steroid receptor‐derived and dimerizer‐controlled gene switches have been built and tested. These switches have been shown to stringently regulate the expression of reporter molecules such as luciferase and soluble factors, including VEGF. We have observed that brief application of focused ultrasound to cells harboring these switches in a fibrin scaffold results in dose‐dependent induction of a reporter transgene. Furthermore, this activation can be restricted to −30 mm3 subvolumes and used to create gradients.
In recent studies, the approach of 4D regulation of transgene expression has been adapted to in vivo applications91. With this technology, patterning the expression of BMP transgenes to establish physiologically relevant distributions of BMP signaling to promote the formation of bone with site‐specific composition and geometry has been visualized. This approach is predicated on the notion that morphogenic/regenerative signals induced by BMPs rely on their localization, persistence and amplitude and that such “context” for exogenous BMP activity will be critical to defining regions of bone formation and integration with surrounding tissue, particularly with high‐volume bone defects. Of interest for bone regeneration applications, heat‐activated gene switches based on different ligand‐activated transactivators may be used in combination for independent control of multiple transgenes91, 92.
Many of the tissue engineered products incorporate the use of growth factors to induce cell differentiation, migration, proliferation and/or matrix production93. However, current growth factor delivery methods are limited by poor retention of growth factors after implantation, resulting in low bioactivity94. These limiting factors have led to the use of high doses and frequent injections, putting patients at risk of adverse effects95. Although there have been great improvements in knowledge of bone tissue engineering, further steps are required to better understand what is needed to develop quality, healthy and affordable commercial tissue‐engineered bone96.
Conclusions
The sequential release of combinations of growth factors has a promising future in bone engineering. Because few reviews in the field of bone growth using stem cells have been published, carefully designed clinical trials are needed to test the efficacy of these strategies and enhance our understanding of the critical interplay between combinations of growth factors and the properties of the host environment to guide the application of genetic engineering to orthopaedics treatments. Thus, the ability to mimic natural bone healing characteristics, which has been proven to improve results of bone tissue regeneration, is making such treatment more appropriate and available. We expect that continued research and development at the confluence of developmental biology, synthetic biology and gene‐ and scaffold‐engineering will not only lead to the identification of spatiotemporal patterns of growth factors transgene expression that drive regeneration, but also provide the experimental and clinical tools for generating those patterns in vivo.
Disclosure: This research was supported by grants from the Natural Science Foundation of Tianjin, 12JCYBJC16400 and Science and Technology Foundation of Health Bureau of Tianjin, 2011KY24.
References
- 1. Reichert JC, Saifzadeh S, Wullschleger ME, et al The challenge of establishing preclinical models for segmental bone defect research. Biomaterials, 2009, 30: 2149–2163. [DOI] [PubMed] [Google Scholar]
- 2. Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury, 2005, 36: 1392–1404. [DOI] [PubMed] [Google Scholar]
- 3. Moreno LA, Valtueña J, Pérez‐López F, González‐Gross M. Health effects related to low vitamin D concentrations: beyond bone metabolism. Ann Nutr Metab, 2011, 59: 22–27. [DOI] [PubMed] [Google Scholar]
- 4. Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng, 2012, 40: 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee K, Silva EA, Mooney DJ. Growth factor delivery‐based tissue engineering: general approaches and a review of recent developments. J R Soc Interface, 2011, 8: 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res, 1987, 225: 7–16. [PubMed] [Google Scholar]
- 7. Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants, 2009, 24 (Suppl.): 237–259. [PubMed] [Google Scholar]
- 8. Mickiewicz P, Binkowski M, Bursig H, Wróbel Z. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank, 2013, 2013: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nauth A, Ristevski B, Li R, Schemitsch EH. Growth factors and bone regeneration: how much bone can we expect? Injury, 2011, 42: 574–579. [DOI] [PubMed] [Google Scholar]
- 10. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am, 2010, 41: 75–84. [DOI] [PubMed] [Google Scholar]
- 11. Zellin G. Growth factors and bone regeneration. Implications of barrier membranes. Swed Dent J Suppl, 1998, 129: 7–65. [PubMed] [Google Scholar]
- 12. Park do J, Yoon C, Thomas N, et al Prognostic significance of targetable angiogenic and growth factors in patients undergoing resection for gastric and gastroesophageal junction cancers. Ann Surg Oncol, 2014, 21: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 13. Eap S, Ferrand A, Schiavi J, et al Collagen implants equipped with “fish scale”‐like nanoreservoirs of growth factors for bone regeneration. Nanomedicine (Lond), 2013, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14. Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am, 2002, 84: 1032–1044. [DOI] [PubMed] [Google Scholar]
- 15. Geuze RE, Theyse LF, Kempen DH, et al A differential effect of bone morphogenetic protein‐2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large‐animal model. Tissue Eng Part A, 2012, 18: 2052–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez A, Reyes R, Sanchez E, Rodriguez‐Evora M, Delgado A, Evora C. In vivo osteogenic response to different ratios of BMP‐2 and VEGF released from a biodegradable porous system. J Biomed Mater Res A, 2012, 100: 2382–2391. [DOI] [PubMed] [Google Scholar]
- 17. Gorskaya YF, Danilova TA, Mezentseva MV, et al Effect of BMP‐2 protein on the count and osteogenic properties of multipotent stromal cells and expression of cytokine genes in primary cultures of bone marrow and spleen cells from CBA mice immunized with bacterial antigens. Bull Exp Biol Med, 2013, 155: 650–654. [DOI] [PubMed] [Google Scholar]
- 18. Winn SR, Uludag H, Hollinger JO. Carrier systems for bone morphogenetic proteins. Clin Orthop Relat Res, 1999, 367 (Suppl): S95–106. [DOI] [PubMed] [Google Scholar]
- 19. Suárez‐González D, Lee JS, Diggs A, et al Controlled multiple growth factor delivery from bone tissue engineering scaffolds via designed affinity. Tissue Eng Part A, 2013, [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bae MS, Ohe JY, Lee JB, et al Photo‐cured hyaluronic acid‐based hydrogels containing growth and differentiation factor 5 (GDF‐5) for bone tissue regeneration. Bone, 2013, 59C: 189–198. [DOI] [PubMed] [Google Scholar]
- 21. Sampath TK, Coughlin JE, Whetstone RM, et al Bovine osteogenic protein is composed of dimers of OP‐1 and BMP‐2A, two members of the transforming growth factor‐beta superfamily. J Biol Chem, 1990, 265: 13198–13205. [PubMed] [Google Scholar]
- 22. Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS, Lee SH. In vivo bone formation following transplantation of human adipose‐derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A, 2008, 14: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 23. Rivera JC, Strohbach CA, Wenke JC, Rathbone CR. Beyond osteogenesis: an in vitro comparison of the potentials of six bone morphogenetic proteins. Front Pharmacol, 2013, 4: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tirkkonen L, Haimi S, Huttunen S, et al Osteogenic medium is superior to growth factors in differentiation of human adipose stem cells towards bone‐forming cells in 3D culture. Eur Cell Mater, 2013, 25: 144–158. [DOI] [PubMed] [Google Scholar]
- 25. Arosarena OA, Collins WL. Bone regeneration in the rat mandible with bone morphogenetic protein‐2: a comparison of two carriers. Otolaryngol Head Neck Surg, 2005, 132: 592–597. [DOI] [PubMed] [Google Scholar]
- 26. Chu TM, Warden SJ, Turner CH, Stewart RL. Segmental bone regeneration using a load‐bearing biodegradable carrier of bone morphogenetic protein‐2. Biomaterials, 2007, 28: 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans KN, Potter BK, Brown TS, Davis TA, Elster EA, Forsberg JA. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res, 2014, 472: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose‐derived stromal cells expressing BMP‐2. Biochem Biophys Res Commun, 2007, 356: 836–842. [DOI] [PubMed] [Google Scholar]
- 29. Knippenberg M, Helder MN, Zandieh DB, Wuisman PI, Klein‐Nulend J. Osteogenesis versus chondrogenesis by BMP‐2 and BMP‐7 in adipose stem cells. Biochem Biophys Res Commun, 2006, 342: 902–908. [DOI] [PubMed] [Google Scholar]
- 30. Hollinger JO, Schmitt JM, Buck DC, et al Recombinant human bone morphogenetic protein‐2 and collagen for bone regeneration. J Biomed Mater Res, 1998, 43: 356–364. [DOI] [PubMed] [Google Scholar]
- 31. Keibl C, Fugl A, Zanoni G, et al Human adipose derived stem cells reduce callus volume upon BMP‐2 administration in bone regeneration. Injury, 2011, 42: 814–820. [DOI] [PubMed] [Google Scholar]
- 32. Lin CY, Lin KJ, Kao CY, et al The role of adipose‐derived stem cells engineered with the persistently expressing hybrid baculovirus in the healing of massive bone defects. Biomaterials, 2011, 32: 6505–6514. [DOI] [PubMed] [Google Scholar]
- 33. Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein‐2 release. Tissue Eng Part A, 2011, 17: 1735–1746. [DOI] [PubMed] [Google Scholar]
- 34. Song I, Kim BS, Kim CS, Im GI. Effects of BMP‐2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochem Biophys Res Commun, 2011, 408: 126–131. [DOI] [PubMed] [Google Scholar]
- 35. Liao X, Wu L, Fu M, et al Chondrogenic phenotype differentiation of bone marrow mesenchymal stem cells induced by bone morphogenetic protein 2 under hypoxic microenvironment in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2012, 26: 743–748. [PubMed] [Google Scholar]
- 36. Kwon SH, Lee TJ, Park J, et al Modulation of BMP‐2‐induced chondrogenic versus osteogenic differentiation of human mesenchymal stem cells by cell‐specific extracellular matrices. Tissue Eng Part A, 2013, 19: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simmonds MC, Brown JV, Heirs MK, et al Safety and effectiveness of recombinant human bone morphogenetic protein‐2 for spinal fusion: a meta‐analysis of individual‐participant data. Ann Intern Med, 2013, 158: 877–889. [DOI] [PubMed] [Google Scholar]
- 38. Fan J, Park H, Tan S, Lee M. Enhanced osteogenesis of adipose derived stem cells with Noggin suppression and delivery of BMP‐2. PLoS ONE, 2013, 8: e72474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chou YF, Zuk PA, Chang TL, Benhaim P, Wu BM. Adipose‐derived stem cells and BMP2: part 1. BMP2‐treated adipose‐derived stem cells do not improve repair of segmental femoral defects. Connect Tissue Res, 2011, 52: 109–118. [DOI] [PubMed] [Google Scholar]
- 40. Li Q, Wang Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co‐culture on osteogenesis and angiogenesis: an in vitro study. Arch Med Res, 2013, 44: 504–513. [DOI] [PubMed] [Google Scholar]
- 41. Detsch R, Stoor P, Grünewald A, Roether JA, Lindfors NC, Boccaccini AR. Increase in VEGF secretion from human fibroblast cells by bioactive glass S53P4 to stimulate angiogenesis in bone. J Biomed Mater Res A, 2014, doi: 10.1002/jbm.a.35069. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42. Hoffmann BR, Wagner JR, Prisco AR, Janiak A, Greene AS. Vascular endothelial growth factor‐A signaling in bone marrow‐derived endothelial progenitor cells exposed to hypoxic stress. Physiol Genomics, 2013, 45: 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med, 2011, 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Willems WF, Kremer T, Friedrich P, Bishop AT. Surgical revascularization induces angiogenesis in orthotopic bone allograft. Clin Orthop Relat Res, 2012, 470: 2496–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cooper ME, Vranes D, Youssef S, et al Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR‐2 in experimental diabetes. Diabetes, 1999, 48: 2229–2239. [DOI] [PubMed] [Google Scholar]
- 46. Maes C. Role and regulation of vascularization processes in endochondral bones. Calcif Tissue Int, 2013, 92: 307–323. [DOI] [PubMed] [Google Scholar]
- 47. Fischer LJ, McIlhenny S, Tulenko T, et al Endothelial differentiation of adipose‐derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res, 2009, 152: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Froehlich H, Gulati R, Boilson B, et al Carotid repair using autologous adipose‐derived endothelial cells. Stroke, 2009, 40: 1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Traktuev DO, Prater DN, Merfeld‐Clauss S, et al Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res, 2009, 104: 1410–1420. [DOI] [PubMed] [Google Scholar]
- 50. Dirckx N, Van Hul M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today, 2013, 99: 170–191. [DOI] [PubMed] [Google Scholar]
- 51. Niklas A, Proff P, Gosau M, Römer P. The role of hypoxia in orthodontic tooth movement. Int J Dent, 2013, 2013: 841840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu B, Li X, Liang G, Liu X. VEGF expression in mesenchymal stem cells promotes bone formation of tissue‐engineered bones. Mol Med Rep, 2011, 4: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 53. Wernike E, Montjovent MO, Liu Y, et al VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo . Eur Cell Mater, 2010, 19: 30–40. [DOI] [PubMed] [Google Scholar]
- 54. Kleinheinz J, Stratmann U, Joos U, Wiesmann HP. VEGF‐activated angiogenesis during bone regeneration. J Oral Maxillofac Surg, 2005, 63: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 55. Kaigler D, Silva EA, Mooney DJ. Guided bone regeneration using injectable vascular endothelial growth factor delivery gel. J Periodontol, 2013, 84: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balseiro S, Nottmeier EW. Vertebral osteolysis originating from subchondral cyst end plate defects in transforaminal lumbar interbody fusion using rhBMP‐2. Report of two cases. Spine J, 2010, 10: e6–10. [DOI] [PubMed] [Google Scholar]
- 57. Carragee EJ, Chu G, Rohatgi R, et al Cancer risk after use of recombinant bone morphogenetic protein‐2 for spinal arthrodesis. J Bone Joint Surg Am, 2013, 95: 1537–1545. [DOI] [PubMed] [Google Scholar]
- 58. Dimar JN, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Clinical and radiographic analysis of an optimized rhBMP‐2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am, 2009, 91: 1377–1386. [DOI] [PubMed] [Google Scholar]
- 59. Helmrich U, Di Maggio N, Guven S, et al Osteogenic graft vascularization and bone resorption by VEGF‐expressing human mesenchymal progenitors. Biomaterials, 2013, 34: 5025–5035. [DOI] [PubMed] [Google Scholar]
- 60. Xiao C, Zhou H, Liu G, et al Bone marrow stromal cells with a combined expression of BMP‐2 and VEGF‐165 enhanced bone regeneration. Biomed Mater, 2011, 6: 15013. [DOI] [PubMed] [Google Scholar]
- 61. Furumatsu T, Shen ZN, Kawai A, et al Vascular endothelial growth factor principally acts as the main angiogenic factor in the early stage of human osteoblastogenesis. J Biochem, 2003, 133: 633–639. [DOI] [PubMed] [Google Scholar]
- 62. Deckers MM, van Bezooijen RL, van der Horst G, et al Bone morphogenetic proteins stimulate angiogenesis through osteoblast‐derived vascular endothelial growth factor A. Endocrinology, 2002, 143: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 63. Street J, Bao M, DeGuzman L, et al Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A, 2002, 99: 9656–9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med, 1999, 5: 623–628. [DOI] [PubMed] [Google Scholar]
- 65. Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology, 2000, 141: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 66. Pufe T, Wildemann B, Petersen W, Mentlein R, Raschke M, Schmidmaier G. Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: a study in the rat. Cell Tissue Res, 2002, 309: 387–392. [DOI] [PubMed] [Google Scholar]
- 67. Cui Q, Dighe AS, Irvine JJ. Combined angiogenic and osteogenic factor delivery for bone regenerative engineering. Curr Pharm Des, 2013, 19: 3374–3383. [DOI] [PubMed] [Google Scholar]
- 68. Glowacki J, Zhou S, Mizuno S. Mechanisms of osteoinduction/chondroinduction by demineralized bone. J Craniofac Surg, 2009, 20 (Suppl. 1): 634–638. [DOI] [PubMed] [Google Scholar]
- 69. Peng H, Usas A, Hannallah D, Olshanski A, Cooper GM, Huard J. Noggin improves bone healing elicited by muscle stem cells expressing inducible BMP4. Mol Ther, 2005, 12: 239–246. [DOI] [PubMed] [Google Scholar]
- 70. Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater, 2008, 15: 100–114. [DOI] [PubMed] [Google Scholar]
- 71. Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone, 2008, 43: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ehnert S, Zhao J, Pscherer S, et al Transforming growth factor beta1 inhibits bone morphogenic protein (BMP)‐2 and BMP‐7 signaling via upregulation of Ski‐related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Med, 2012, 10: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Young S, Patel ZS, Kretlow JD, et al Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein‐2 on bone regeneration in a rat critical‐size defect model. Tissue Eng Part A, 2009, 15: 2347–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. El BR, Zaky SH, Muraglia A, et al A platelet‐rich plasma‐based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng Part A, 2013, 19: 152–165. [DOI] [PubMed] [Google Scholar]
- 75. Samee M, Kasugai S, Kondo H, Ohya K, Shimokawa H, Kuroda S. Bone morphogenetic protein‐2 (BMP‐2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J Pharmacol Sci, 2008, 108: 18–31. [DOI] [PubMed] [Google Scholar]
- 76. Dai J, Li Y, Zhou H, Chen J, Chen M, Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci, 2013, 9: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zheng Y, Feng Z, You C, et al In vitro evaluation of Panax notoginseng Rg1 released from collagen/chitosan‐gelatin microsphere scaffolds for angiogenesis. Biomed Eng Online, 2013, 12: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kempen DH, Lu L, Heijink A, et al Effect of local sequential VEGF and BMP‐2 delivery on ectopic and orthotopic bone regeneration. Biomaterials, 2009, 30: 2816–2825. [DOI] [PubMed] [Google Scholar]
- 79. Kimelman BN, Kallai I, Lieberman JR, Schwarz EM, Pelled G, Gazit D. Gene therapy approaches to regenerating bone. Adv Drug Deliv Rev, 2012, 64: 1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pensak MJ, Lieberman JR. Gene therapy for bone regeneration. Curr Pharm Des, 2013, 19: 3466–3473. [DOI] [PubMed] [Google Scholar]
- 81. Liu F, Porter RM, Wells J, Glatt V, Pilapil C, Evans CH. Evaluation of BMP‐2 gene‐activated muscle grafts for cranial defect repair. J Orthop Res, 2012, 30: 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moutsatsos IK, Turgeman G, Zhou S, et al Exogenously regulated stem cell‐mediated gene therapy for bone regeneration. Mol Ther, 2001, 3: 449–461. [DOI] [PubMed] [Google Scholar]
- 83. Gafni Y, Turgeman G, Liebergal M, Pelled G, Gazit Z, Gazit D. Stem cells as vehicles for orthopedic gene therapy. Gene Ther, 2004, 11: 417–426. [DOI] [PubMed] [Google Scholar]
- 84. Muthukuru M, Sun J. Doxycycline counteracts bone morphogenic protein 2‐induced osteogenic mediators. J Periodontol, 2013, 84: 656–665. [DOI] [PubMed] [Google Scholar]
- 85. Nakai K. [Controlling signal transduction with synthetic ligands]. Tanpakushitsu Kakusan Koso, 2007, 52: 1794–1795. [PubMed] [Google Scholar]
- 86. Koh JT, Ge C, Zhao M, et al Use of a stringent dimerizer‐regulated gene expression system for controlled BMP2 delivery. Mol Ther, 2006, 14: 684–691. [DOI] [PubMed] [Google Scholar]
- 87. Wang X, Chen X, Yang Y. Spatiotemporal control of gene expression by a light‐switchable transgene system. Nat Methods, 2012, 9: 266–269. [DOI] [PubMed] [Google Scholar]
- 88. Vilaboa N, Boellmann F, Voellmy R. Gene switches for deliberate regulation of transgene expresiion: recent advances in system development and uses. J Genet Syndr Gene Ther, 2011, 2: 107. [Google Scholar]
- 89. Rome C, Couillaud F, Moonen CT. Spatial and temporal control of expression of therapeutic genes using heat shock protein promoters. Methods, 2005, 35: 188–198. [DOI] [PubMed] [Google Scholar]
- 90. Vilaboa N, Fenna M, Munson J, Roberts SM, Voellmy R. Novel gene switches for targeted and timed expression of proteins of interest. Mol Ther, 2005, 12: 290–298. [DOI] [PubMed] [Google Scholar]
- 91. Wilson CG, Martin‐Saavedra FM, Vilaboa N, Franceschi RT. Advanced BMP gene therapies for temporal and spatial control of bone regeneration. J Dent Res, 2013, 92: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Benazet JD, Zeller R. Vertebrate limb development: moving from classical morphogen gradients to an integrated 4‐dimensional patterning system. Cold Spring Harb Perspect Biol, 2009, 1: a1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Suarez‐Gonzalez D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, Murphy WL. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials, 2012, 33: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater, 2009, 21: 3269–3285. [DOI] [PubMed] [Google Scholar]
- 95. Li P, Bai Y, Yin G, et al Synergistic and sequential effects of BMP‐2, bFGF and VEGF on osteogenic differentiation of rat osteoblasts. J Bone Miner Metab, 2013, 2013: 1–9. [DOI] [PubMed] [Google Scholar]
- 96. Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev, 2012, 64: 1292–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]


