Abstract
Objective: To investigate the effects of serum cobalt ion concentration on the liver, kidney and heart in mice.
Methods: Forty 4‐week‐old male ICR mice were randomly divided into four groups (n= 10 in each group) as follows: Group 1 (HD), high‐dose cobalt chloride group (3.28 mg/kg/day); Group 2 (MD), medium‐dose cobalt chloride group (1.64 mg/kg/day); Group 3(LD), low‐dose group cobalt chloride group (0.82 mg/kg/day); and Group 4(NC), normal control group (vehicle). Cobalt chloride and normal saline were given by intraperitoneal injection once per day for 3 weeks. The body weights of the mice were recorded every 3 days to ensure the correct doses of cobalt chloride. Blood samples for testing were taken at day 4, week 1, week 2 and week 3. Serum cobalt ion concentrations were measured in all samples whereas other serum biochemical variables, including aspartate aminotransferase (AST), aspartate aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (Cr), and creatine kinase (CK) were evaluated at week 1, 2 and 3. After killing the mice at week 3, the heart, liver and kidney were collected for pathological evaluation.
Results: Serum cobalt ion concentration was different between the groups. High‐dose cobalt chloride significantly increased AST, ALT and CK concentrations, the concentrations increasing in parallel with treatment duration. Pathological evaluation showed that high‐dose cobalt chloride had toxic effects on the heart and liver; however no significant effect was apparent in the kidney.
Conclusion: High‐dose cobalt ion concentration in serum has toxic effects on the heart and liver, but no significant effect on the kidney in mice.
Keywords: Cobalt, Ions, Toxic actions
Introduction
Clinical results have shown that metal‐on‐metal total hip resurfacing arthroplasty or large diameter metal‐on‐metal total hip arthroplasty has good outcomes in treating some related diseases in young subjects due to its special advantages, such as sparing femoral (and acetabular) bone stock, preservation of hip joint biomechanics (femoral offset, leg length), better recovery for high‐level sports activities, easier revision, and less risk of dislocation 1 . However, complications have also been reported in the past few years 2 . Among them, the effects on body health of degradation products, such as the metal ions produced by friction of metal‐on‐metal prostheses, have attracted research interest 3 , 4 , 5 . Some studies have reported the effects of cobalt ions on reproductive function and cancer induction 6 , 7 . However, the effects of cobalt ion on the liver, kidney and heart have not been adequately investigated. In this study, we set out to investigate the effects of different serum cobalt ion concentrations on the liver, kidney and heart in mice, as it is important to obtain experimental evidence for the complications of cobalt ions associated with metal‐on‐metal prostheses.
Materials and methods
Forty 4‐week‐old male ICR (Institute of Cancer Research) mice (weight, 20 ± 1 g) (purchased from the Laboratory Animal Service Center, Nantong University, Nantong, China) were used in this experiment. Purified powder of cobalt chloride was purchased from Merck (Whitehouse Station, NJ, USA). The experiment protocol was approved by the Animal Experiment Ethics Committee of the authors' institute.
Mice were housed at 22°C with a 12‐hour light and 12‐hour dark cycle in the animal house of the authors' institute, and received a standard rat chow with water ad libitum. Animals were randomly assigned to the following four groups (n= 10 in each group): Group 1 (HD), high‐dose cobalt chloride group (3.28 mg/kg/day); Group 2 (MD), medium‐dose cobalt chloride group (1.64 mg/kg/day); Group 3 (LC), low‐dose group cobalt chloride group (0.82 mg/kg/day); Group 4 (NC), normal control group (vehicle).
Cobalt chloride was dissolved in normal saline, and the vehicle and cobalt chloride were given daily by intraperitoneal injection for 3 weeks. The body weights of the mice were recorded every 3 days for adjustment of the dosage of cobalt chloride.
Blood samples were taken at day 4, week 1, week 2 and week 3 for serum testing and were stored at −80°C until use. After killing the mice at week 3, the heart, liver and kidney were collected for pathological evaluation.
Serum cobalt ion concentrations were measured at day 4, week 1, week 2 and week 3. Other serum biochemical variables, including aspartate aminotransferase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN), creatinine (Cr), and creatine kinase (CK) were evaluated at week 1, week 2 and week 3. Heart, liver and kidney samples were evaluated by histological section and electron microscopy.
Commercially available enzyme‐linked immunosorbent assay kits for serum biochemical parameters were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Testing was carried out according to the manufacturer's instructions.
Data were interpreted in mean ± standard deviation (SD). One‐way analysis of variances with Fisher's Least Significant Difference post hoc test was used for multiple comparisons between groups. All statistical analyses were two‐sided and a P‐value of less than 0.05 was regarded as statistically significant. SPSS version 11.0 software (SPSS, Chicago, IL, USA) was used for data analysis.
Results
Serum cobalt ion concentration
In the NC group, the serum cobalt ion concentration was so low that it could not be measured. Serum cobalt ion concentration was different between groups (P < 0.05). Serum cobalt ion concentration increased with the dose of cobalt chloride injected. Detailed data are presented in Fig. 1.
Figure 1.
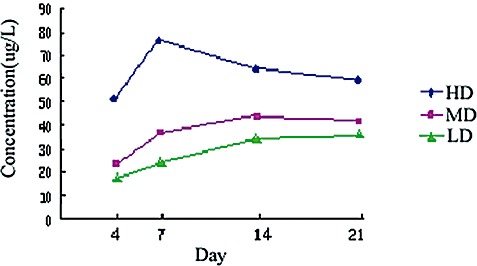
Changes over time in serum cobalt ion concentration in HD, MD and LD groups.
Effects of cobalt chloride on liver, kidney and heart
Serum AST, ALT and CK concentrations increased significantly in the HD compared with the NC group, the concentration increasing in parallel with treatment duration (F, 893.92, P < 0.05). Cobalt chloride apparently had no significant effect on serum BUN or Cr concentrations (P > 0.05). Serum AST, ALT, CK, BUN and Cr concentrations did not significantly increase in the MD and LD groups compared with the NC group (P > 0.05). Detailed data are presented in 1, 2, 3.
Table 1.
Concentrations of various serum biochemical variables at week 1
| Groups | AST (µ/l) | ALT (µ/l) | BUN (g/l) | Cr (µmol/l) | CK (µ/ml) |
|---|---|---|---|---|---|
| NC | 117.04 ± 2.95 | 37.51 ± 5.74 | 0.36 ± 0.03 | 54.32 ± 6.03 | 0.79 ± 0.10 |
| LD | 119.96 ± 3.25 | 40.17 ± 5.12 | 0.35 ± 0.01 | 52.47 ± 7.33 | 0.81 ± 0.09 |
| MD | 121.41 ± 7.23 | 41.72 ± 6.15 | 0.33 ± 0.04 | 53.49 ± 6.71 | 0.85 ± 0.10 |
| HD | 168.78 ± 7.15* | 81.4 ± 4.72* | 0.40 ± 0.07 | 56.74 ± 6.07 | 1.54 ± 0.08* |
| F value | 739.93 | 538.68 | 1.10 | 0.11 | 579.76 |
| P value | 0.00 | 0.00 | 0.36 | 0.95 | 0.00 |
Significant difference when compared with NC group.
Table 2.
Concentrations of various serum biochemical variables at week 2
| Groups | AST (µ/l) | ALT (µ/l) | BUN (g/l) | Cr (µmol/l) | CK (µ/ml) |
|---|---|---|---|---|---|
| NC | 129.89 ± 3.23 | 37.98 ± 6.31 | 0.38 ± 0.02 | 49.33 ± 5.10 | 0.84 ± 0.11 |
| LD | 125.28 ± 7.84 | 39.08 ± 5.52 | 0.37 ± 0.04 | 47.13 ± 6.21 | 0.82 ± 0.09 |
| MD | 132.33 ± 4.92 | 41.41 ± 5.72 | 0.40 ± 0.03 | 48.73 ± 4.57 | 0.89 ± 0.13 |
| HD | 234.57 ± 4.28* | 93.31 ± 5.17* | 0.42 ± 0.05 | 49.71 ± 4.72 | 1.87 ± 0.09* |
| F value | 8172.51 | 1042.22 | 0.47 | 0.01 | 1058.97 |
| P value | 0.00 | 0.00 | 0.71 | 1.00 | 0.00 |
Significant difference when compared with NC group.
Table 3.
Concentrations of various serum biochemical variables at week 3
| Groups | AST (µ/l) | ALT (µ/l) | BUN (g/l) | Cr (µmol/l) | CK (µ/ml) |
|---|---|---|---|---|---|
| NC | 127.75 ± 2.92 | 39.12 ± 5.69 | 0.37 ± 0.02 | 52.54 ± 4.67 | 0.71 ± 0.09 |
| LD | 129.71 ± 4.17 | 38.28 ± 0.23 | 0.34 ± 0.04 | 53.54 ± 5.67 | 0.67 ± 0.10 |
| MD | 131.73 ± 6.13 | 39.41 ± 4.28 | 0.37 ± 0.04 | 52.78 ± 6.21 | 0.69 ± 0.08 |
| HD | 368.78 ± 7.15* | 131.4 ± 4.72* | 0.42 ± 0.05 | 52.31 ± 5.30 | 2.17 ± 0.16* |
| F value | 98 493.26 | 7736.24 | 2.45 | 0.01 | 2555.96 |
| P value | 0.00 | 0.00 | 0.08 | 1.00 | 0.00 |
Significant difference when compared with NC group.
Pathological changes in the liver, kidney and heart
Liver
Macroscopic observation showed that high‐dose cobalt chloride caused significant hyperemia and swelling of the liver. There was no significant difference between the MD, LD and NC groups (2, 3).
Figure 2.
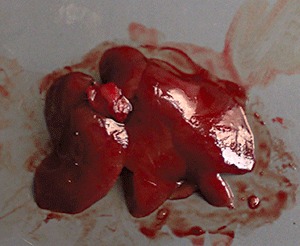
NC group: macroscopic observation of the liver.
Figure 3.
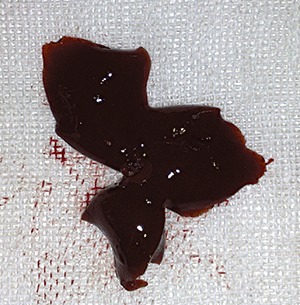
HD group: showing hyperemia and swelling of the liver.
Light microscopic examination showed that high‐dose cobalt chloride caused central vein displacement, abnormality and hyperemia, a single or few hepatocytes scattered in the hepatic lobules, condensation of the cytoplasm of hepatocytes, and increased reaction to eosinophilic staining. It also caused nuclear cataplasia, karyopyknosis and condensation. Inflammatory cell infiltration was also present (4, 5, 6).
Figure 4.
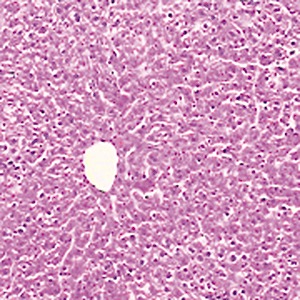
NC group; showing normal hepatocytes ×200.
Figure 5.
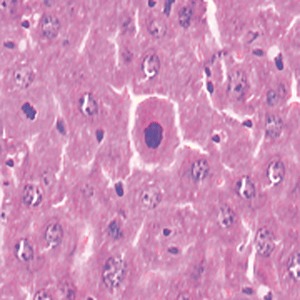
HD group: showing karyopyknosis of hepatocytes ×400.
Figure 6.
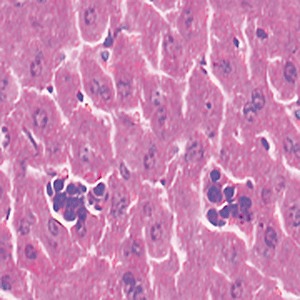
HD group: showing inflammatory cell infiltration ×400.
Transmission electron microscopic examination showed that high‐dose cobalt chloride caused particular apoptotic changes in the ultrastructure of hepatocytes, such as nuclear malformation, chromatin abnormality, decreased euchromatin, and increased heterochromatin, and clustering and lining along the nuclear membrane. Mitochondrial abnormalities were also present, the structure of the mitochondrial cristae being destroyed (7, 8, 9, 10).
Figure 7.
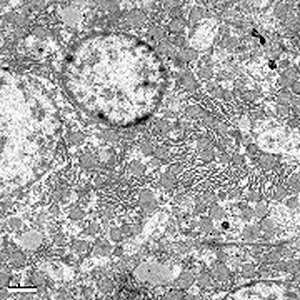
NC group: showing normal nucleus of a hepatocyte ×8000.
Figure 8.
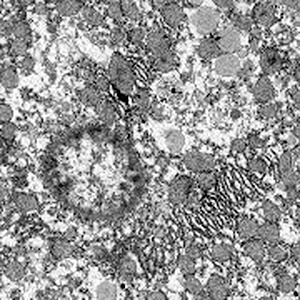
Nuclear malformations, increased heterochromatin, clustering and lining along the nuclear membrane ×8000.
Figure 9.

NC group: showing a normal mitochondrion ×15 000.
Figure 10.
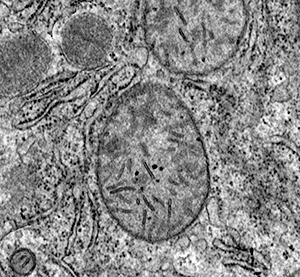
HD group: showing mitochondrial abnormality, the structure of the mitochondrial cristae has been destroyed ×15 000.
Kidney
Macroscopic observation and light microscopic examination showed that cobalt chloride had no significant effect on the kidney. Transmission electron microscopic examination also showed that cobalt chloride had no significant effect on the ultrastructure of the kidney.
Heart
Macroscopic observation showed that high‐dose cobalt chloride caused significant hyperemia and swelling of the heart. Spotty necrosis could also be seen. There was no significant difference between the MD, LD and NC groups (11, 12).
Figure 11.
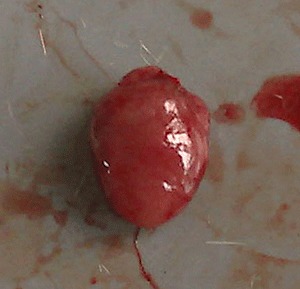
NC group: macroscopic observation of the heart.
Figure 12.
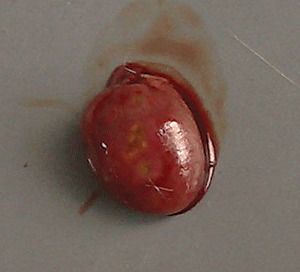
HD group: macroscopic observation of the heart showing spotty necrosis.
Light microscopic examination showed that no abnormal changes were found in the LD and MD groups. However, abnormal changes such as cardiac muscle edema, muscle fiber swelling, and muscle striation blurring were found in the HD group. Cardiac myocyte swelling, cytoplasmic granular degeneration, erythrophils, decreased nucleus stain reaction and glassy degeneration were also found. Intercellular spaces were swollen and an inflammatory cell infiltration was present (13, 14).
Figure 13.
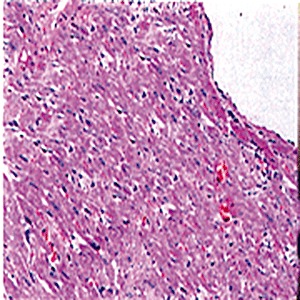
NC group: showing normal cardiac muscle ×200.
Figure 14.
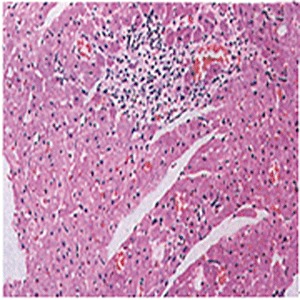
HD group: showing spotty necrosis of cardiac myocytes and inflammatory cell infiltration ×200.
Transmission electron microscopic examination showed that high‐dose cobalt chloride caused particular changes in the ultrastructure of cardiac myocytes such as vacuolar degeneration, mitochondrial swelling and reduction in mitochondrial cristae. Atrophy of myofibrils in cardiac myocytes, imbalance between mitochondria and myofibrils and broken sarcomeres were also found (15, 16).
Figure 15.

NC group: showing normal myofibrils in a cardiac myocyte ×10 000.
Figure 16.

HD group: showing atrophy of myofibrils of a cardiac myocyte, imbalance between mitochondrion and myofibrils and broken sarcomeres ×10 000.
Discussion
Wearing of the prosthesis is one of the complications of metal‐on‐metal resurfacing arthroplasty or total hip arthroplasty. Although improvements in the characteristics of the material used have decreased the rate of wear of prostheses, prosthesis wear cannot be totally eliminated. Prosthesis wear produces metal particles which persist in tissues around the prosthesis and release metal ion continuously, eventually increasing the serum metal ion concentration. It has been reported that serum cobalt and chromium ion concentrations increase significantly after metal‐on‐metal resurfacing arthroplasty, which results in higher concentrations of these ions than does metal‐on‐metal total hip arthroplasty 8 . In another study, the authors evaluated cobalt and chromium ion concentrations in serum and urine at day 5, 2 months, 6 months, 1 year, 2 years and 4 years after metal‐on‐metal resurfacing arthroplasty surgery. They found that cobalt and chromium ion concentrations in both serum and urine increased in parallel with time since surgery, reaching a peak value at around 6 months postoperatively and then decreasing slowly. However, even 4 years after surgery they were still higher than in a control group 9 . It has also been reported that in patients with Birmingham prostheses, serum cobalt ion concentration increases after surgery, reaching its peak value at around 6 months postoperatively, and then decreasing slowly. Serum chromium ion concentration reaches its peak value at around 9 months postoperatively, and then decreases 10 .
Recently, the biological effects of metal ions have attracted more research interest. Cobalt, which is necessary for maintaining normal physiological function of the human body, is one of its microelements. However it has an appropriate range of concentrations, above this range it will have toxic effects. Pharmacokinetic tests after intravenous injection of cobalt chloride show that cobalt ion concentration is highest in the liver (20% cobalt ions gathered in the liver), followed by the heart, which suggests that cobalt ions have significant effects on the liver and heart. In this study, we found that AST, ALT and CK concentrations were significantly higher in the HD group than in the NC group. Histological evaluation also showed toxic changes in the liver and heart in the HD group. The severity of these toxic effects seemed to be in parallel with treatment duration, suggesting that the toxic effects of cobalt ions are correlated with the length of contact. We found no significant differences between different groups at different time points in BUN and Cr concentrations, demonstrating that cobalt ions have no significant effect on kidney function. It should be noted that the treatment duration of our experiment was only 3 weeks. Long‐term experiments are needed to explore the nephrotoxicity of cobalt ions.
The possible toxic mechanism of cobalt ions on the liver and heart are as follows: cobalt is always present in the human body as radical ions, which can attack unsaturated fatty acids on the cell membrane and convert them to free radical lipid and free radical lipid peroxidation 11 . Lipid peroxidation can decrease cell membrane fluidity and increase cell membrane fragility. This in turn affects the function of receptors and enzymes anchored on the cell membrane, changes the permeability of the cell membrane, and eventually causes abnormalities in entry of calcium ions. This breaks the endoplasmic reticulum, destroys many enzymes and results in cell death. Cell death would increase ALT, AST and CK concentrations. Plasma membrane Ca2+ ATPase is a transport protein in the plasma membrane of cells which serves to remove calcium (Ca2+) from the cell. It is vital for regulating the amount of Ca2+ within cells 12 . A previous study has demonstrated that the diameter of the cobalt ion is similar to that of the calcium ion. Thus cobalt ions can occupy the combining site of calcium ions and inhibit the activity of Ca2+ ATPase 13 . Large amounts of cobalt ions entering liver cells may inhibit calmodulin activity, inhibiting Ca2+ ATPase, resulting in increased calcium ion concentrations inside the liver cells, and finally causing liver cell injury. On the other hand, cobalt ions can also occupy the combining site of calcium ions on calmodulin, forming a complex with it, thus destroying the regulatory function of calmodulin, resulting in uncontrolled calcium ion concentration inside the liver cells.
In conclusion, high‐dose cobalt ion concentration in serum has toxic effects on the heart and liver, but no significant effect on the kidney in mice. However, the toxic mechanism and the long‐term effects need to be studied further.
Disclosure
No benefits in any form related directly or indirectly to the subject of this article have been or will be received from any commercial party.
References
- 1. Girard J, Lavigne M, Vendittoli PA, et al Hip resurfacing: current state of knowledge. Rev Chir Orthop Reparatrice Appar Mot, 2008, 94: 715–730. [DOI] [PubMed] [Google Scholar]
- 2. Ru JY, Liu F. History and status quo of total hip surface replacement (Chin). Guoji Gu Ke Xue Za Zhi, 2006, 27: 36–37. [Google Scholar]
- 3. Brodner W, Bitzan P, Meisinger V, et al Elevated serum cobalt with metal‐on‐metal articulating surfaces. J Bone Joint Surg Br, 1997, 79: 316–321. [DOI] [PubMed] [Google Scholar]
- 4. Jacobs JJ, Skipor AK, Doorn PF, et al Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop Relat Res, 1996, 329 (Suppl.): S256–S263. [DOI] [PubMed] [Google Scholar]
- 5. Maezawa K, Nozawa M, Hirose T, et al Cobalt and chromium concentrations in patients with metal‐on‐metal and other cementless total hip arthroplasty. Arch Orthop Trauma Surg, 2002, 122: 283–287. [DOI] [PubMed] [Google Scholar]
- 6. Pedigo NG, Vernon MW. Embryonic losses after 10‐week administration of cobalt to male mice. Reprod Toxicol, 1993, 7: 111–116. [DOI] [PubMed] [Google Scholar]
- 7. Pedigo NG, George WJ, Anderson MB. Effects of acute and chronic exposure to cobalt on male reproduction in mice. Reprod Toxicol, 1988, 2: 45–53. [DOI] [PubMed] [Google Scholar]
- 8. Clarke MT, Lee PT, Arora A, et al Levels of metal ions after small‐ and large‐diameter metal‐on‐metal hip arthroplasty. J Bone Joint Surg Br, 2003, 85: 913–917. [PubMed] [Google Scholar]
- 9. Daniel J, Ziaee H, Pradhan C, et al Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four‐year results of a prospective longitudinal study. J Bone Joint Surg Br, 2007, 89: 169–173. [DOI] [PubMed] [Google Scholar]
- 10. Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal‐on‐metal hip resurfacing? Clin Orthop Relat Res, 2005, 438: 177–181. [DOI] [PubMed] [Google Scholar]
- 11. Misra M, Rodriguez RE, Kasprzak KS. Nickel induced lipid peroxidation in the rat: correlation with nickel effect on antioxidant defense systems. Toxicology, 1990, 64: 1–17. [DOI] [PubMed] [Google Scholar]
- 12. Wen YY, Zhang ZM, Hu P. Calcium and Calmodulin. Beijing: Chemical Industry Press, 1989; 106. [Google Scholar]
- 13. Chao SH, Suzuki Y, Zysk JR, et al Activation of calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol, 1984, 26: 75–82. [PubMed] [Google Scholar]


