Abstract
Objective
To investigate the efficiency of perforator pedicled propeller flaps for soft tissue coverage of lower leg and foot defects.
Methods
Twenty patients (12 male, 8 females; mean age 28 years, range, 5–75) with soft tissue defects of the lower leg and foot were retrospectively reviewed. Their defects had been repaired with perforator pedicled propeller flaps from September 2011 to October 2013 and included five cases of injuries caused by spokes, four of infection with postoperative skin necrosis, two of dorsal skin defects caused by heavy objects and nine caused by car accidents. The areas of soft tissue defect were from 2 cm × 8 cm to 10 cm × 20 cm. Fifteen cases had terminal branch of the peroneal artery perforator flaps and five posterior tibia artery perforator flaps, flap size ranging from 5 cm × 11 cm to 12 cm × 28 cm. Color Doppler ultrasound was used to locate all perforator vessels, the calibers of which ranged from 0.8 mm to 1.0 mm.
Results
The intraoperative coincidence rate of the color Doppler ultrasound was 96.7%. The donor sites were sutured directly in 12 cases and skin grafted in 8. One case had a venous crisis within 24 h that was treated by removal some sutures and drainage. All cases were followed up for 1–18 months; all flaps survived well and pedicles had a satisfactory appearance. The patients were extremely satisfied with the results for repair.
Conclusion
Perforator pedicled propeller flaps have the advantages over other pedicle flap of being simple, safe, and effective and not involving vascular anastomosis.
Keywords: Ankle, Foot, Soft tissue injuries, Surgical flaps
Introduction
Soft tissue defects, particularly in the lower leg and foot, are often associated with exposure of bone, joints, and tendons, create severe dysfunction, physical and mental suffering, and are difficult to manage.
At present, the flap transfer repair is the most popular means of managing foot and ankle soft tissue defects1, 2, commonly used ones including neurocutaneous, adjacent fascia, muscle, and free flaps. Although these flaps can produce satisfactory results, the range of defects they can repair is limited. Free flaps are preferable for large areas of skin damage, but require considerable technical skill, have significant operative risk, and the flaps can become engorged. Neurocutaneous, near fascia and muscle flaps can result in necrosis or engorgement of flap pedicle trim because of venous problems, requiring reoperation.
Perforator pedicled propeller flaps are relatively simple, safe and effective, postoperative engorgement does not occur, attractive in shape, and particularly suitable for soft tissue coverage of lower leg and foot defects. Hyakusoku et al. first introduced the term propeller flap in 1991 to define a method of elevating and rotating a flap that is much longer than wide and comprises two portions (the blades of the propeller), one on either side of the pedicle3. The flap is rotated 90° on the central pedicle, like a propeller, to resurface burn scar contractures at the elbow and axilla. In 2006, Aslan et al. reported using this kind of flap to repair elbow scar contraction; the flap rotation was limited to only 90° because of the generous size of the flap's subcutaneous pedicle4. In the same year, Hallock combined the perforator vessel and rotation techniques of propeller flap, designing a propeller flap with an eccentric adductor perforator vessel pedicle that rotated 180°. Part of the flap (the big paddle) was used to cover the wound and other part (the small paddle) to close the donor site. Because of the rich blood supply and easy rotation, it was used to treat two cases of ischial tuberosity or greater trochanter bedsores, achieving good results5.
The advisory panel of the first Tokyo meeting on perforator and propeller flaps in June 2009 reached a terminology consensus on propeller flaps, analogous to the “Gent” consensus on perforator flap terminology. It stipulated definitions of different propeller flaps with particular regard to perforator propeller flaps6. A propeller flap can be defined as an “island flap that reaches the recipient site through axial rotation.” All skin island flaps can become propeller flaps. However, this definition excludes island flaps that reach the recipient site through an advancement movement and flaps that move through a rotation but are not completely islanded. According to the type of nourishing pedicle, propeller flaps are subdivided into subcutaneous pedicled, perforator pedicled and supercharged propeller flaps. In 2011, Ayestaray et al. published a classification based on the type of pedicle, subdividing propeller flaps into subcutaneous‐pedicled, muscle‐pedicled, perforator‐pedicled, and vascular‐pedicled propeller flaps. Classification based on pedicle position can be divided into central axis and acentric axis propeller flaps7.
Since September 2011, our hospital has used perforator pedicled propeller flaps to repair lower leg and foot soft tissue defects. In this study we reviewed and analyzed the procedures and outcomes of all cases, our aims being: (i) to clarify the indications for use of perforator pedicled propeller flaps; (ii) list key surgical points; (iii) compare naked and non‐naked perforator vascular pedicles; and (iv)summarize the advantages and disadvantages of perforator pedicled propeller flaps.
Materials and Methods
General Information
Twenty patients with soft tissue defects of the lower leg and foot were retrospectively reviewed and evaluated. Their defects had been repaired using perforator pedicled propeller flaps from September 2011 to October 2013. They included 12 male and 8 female patients with an average age of 28 years (range, 5–75 years). There were five cases of injuries caused by spokes (the “Achilles heel” of soft tissue defects), four of infection after internal fixation of calcaneal fractures had resulted in skin necrosis, two of dorsal skin defects caused by heavy objects, and nine of foot and ankle soft tissue defects caused by car accidents. The areas of the wounds and soft tissue defects ranged from 2 cm × 8 cm to 10 cm × 20 cm. The duration since injury ranged from 7–60 days.
According to the clinical indications set by Pignatti et al. for perforator pedicled propeller flaps8, no patient had a wound defect length of more than 20 cm. No patients had serious preoperative scar hyperplasia or vascular diseases. All wounds were swabbed, bacterial culture being positive in 14 cases and negative in 6; of the positive cases, 85.7% (12/14) were infected with Staphylococcus aureus.
Surgical Technique
Debridement
Patients with relatively little wound contamination underwent flap transfer after debridement. For extremely contaminated or infected wounds, flap transfer operation was deferred until fresh granulation tissue had been achieved by using vacuum sealing drainage or repeated dressing after debridement. It must be emphasized that it is necessary to thoroughly remove necrotic tissue until normal tissue has been exposed, and then to continue treatment for 1 week or so, to prevent secondary infection.
Flap Design, Incision, and Wound Coverage
Ipsilateral limb nearby transfer was chosen for all flaps, flap design depending on the size and shape of the wound, the flap areas exceeding the wound areas by about 5% so the flaps are not under tension. Preoperative color Doppler ultrasound was used to locate the perforator vessels and determine their diameters and blood flow velocity (perforator vessels located 2 to 10 cm from the wound, with diameters of 0.6 to 1.0 mm and maximal blood velocity were usually selected, bearing in mind that rotation point would wear the vessel), and to design the large and small paddles of the perforator pedicled propeller flaps. The large paddles of the propeller flap were designed close to the rotation point and used to cover the wound after rotating; whereas the small paddles of the propeller flaps were designed from beyond the rotation point to the skin wound and used to close part of the recipient area after rotating. Care was taken to ensure that the longitudinal length of the large blade was greater than the sum of the small paddle and longitudinal lengths of the wound (Fig. 1a).
Figure 1.
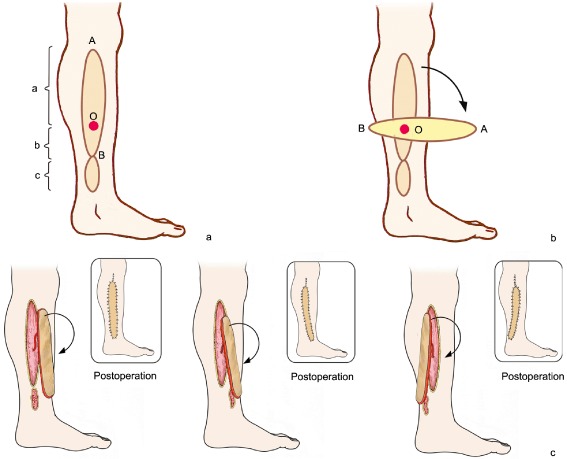
Schematic representation of perforator pedicled propeller flap. (a) Flap design. Preoperative use of color Doppler ultrasound to locate the perforator vessel and ascertain its diameter and blood flow velocity (perforator vessel 2 to 10 cm from the defect with diameter 0.6–1.0 mm, and maximal blood velocity usually selected). O is positioned preoperative of perforator vessel intended position, AB connect as the axis of the flap, a is the longitudinal length of the large paddle, b is the longitudinal length of the small paddle, c is wound defect longitudinal length. (b) The propeller flap is rotated clockwise and counterclockwise to determine the minimum angle required to cover the defect. (c) The donor and recipient area are closed.
The incisions started from the front (or back) of the flap axis with an exploratory incision. The superficial fascia was then carefully separated, the skin retracted, the preoperatively determined position of the perforator vessel reached and its direction noted. This ensured the correct position for performing the skin incision at the back (or front) and cutting between the deep fascia and superficial fascia, then incising the deep fascia about 1 cm away from the perforator vessel, ensuring the deep fascia was continuous with the superficial fascia at the point at which the perforator vessel passed though the deep fascia. Next, the soft tissue fascia surrounding the perforator vessel pedicle was completely removed, freeing and making the perforating vessel pedicle completely naked; the free flap perforator vessel pedicle was then the sole connection with the rest of the body. To protect the vascular pedicle, the propeller flap was rotated clockwise or counterclockwise as appropriate to determine the minimum angle required to cover the wound (Fig. 1b). The flap was then sutured and fixed (Fig. 1c) to prevent rotation or stretching of the vascular pedicle.
Postoperative Management
Oral rivaroxaban 10 mg/d was routinely administered for about 2 weeks after surgery for anticoagulation. Papaverine was given i.m. (60 mg/t.d.s) for 4 to 5 days as an antispasmodic. Cefotiam was administered i.v. (2 g/b.d.) to prevent infection. A 40 to 60 W cradle heat lamp was used to keep the affected area warm. The stitches were removed 2 weeks postoperatively.
Evaluation of Efficacy of Treatment
The scoring system of Zhang et al., which assesses the five aspects of flap healing, sensation, shape, temperature and donor site scar9, was used to evaluate the efficacy of the procedure. Each item scores 2 points for superior, 1 point for good, 0 points for possible, −1 point for inferior. The total value is then calculated, 5–10 points being considered satisfactory, 0 to 4 points adequate, and 1 to −5 points unsatisfactory.
Results
General Points
All operations were successful; the operation time was 60 to 100 min, with an average of 70 min. Intraoperative blood loss was 50–100 mL, with an average of 70 mL. The propeller flaps utilized the terminal branch of the peroneal artery in 15 cases and the posterior tibial artery in 5 cases. The flap sizes ranged from 5 cm × 11 cm to 12 cm × 28 cm and the calibers of the perforator artery from 0.8 mm to 1.0 mm. The donor sites were sutured directly in 12 cases and required skin grafting in 8. One patient had a venous crisis in the 24 h postoperatively, which responded to removal of some of the sutures and drainage of blood.
Clinical Outcomes
The flaps were followed up for 1–18 months (mean, 13.5 months). All flaps survived well, the pedicles were smooth, did not ulcerate, become engorged or develop “cat ears” malformation. The texture and color of the flaps were similar to those of adjacent tissue and their shape were good (Fig. 2). In cases where the donor skin had been directly sutures, the surgical scars were small. There were no obvious scars at the donor sites of free skin grafts. The donor site wounds healed well, 19 cases (95%, 19/20) had good postoperative ankle function with dorsiflexion of 10° to 25° and plantar flexion of 10° to 45°. At the last follow‐up, scores on the Zhang scale9 were 8 to 10 points and all patients were satisfied with the results.
Figure 2.
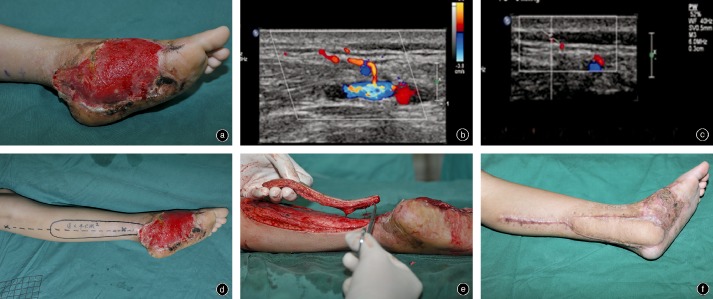
Illustrative case of female patient, aged 9 years. (a) Photograph of skin and soft tissue defect of left foot, the defect is 9 cm × 15 cm. (b,c) Preoperative Doppler ultrasound probe positioning shows posterior tibial artery perforator vessels in the skin 3 cm behind the medial malleolus, about 0.41 mm in diameter with for blood flow rate of 7.87 cm/s rate. (d) Design of a posterior tibial artery perforator flap. The location of the perforating vessel is indicated by the “X” behind the medial malleolus. (e) Construction of the flap, leaving only the perforator vessel pedicle connecting it to the rest of the body. (f) Three months postoperatively, the pedicle is smooth and attractive.
Discussion
Crucial Points Concerning Surgical Procedure
Attention should be paid to achieving the following: (i) vessel diameter ≥0.8 mm; (ii) free perforator vessel pedicle length ≥1 cm; and (iii) spin turn angle ≤180°. A perforator vessel 1 to 2 cm above the wound should usually be selected; if it is much further away from the wound, the overlap will be too long after rotating it 180°. This not only increases the length of the flap but makes it subject to ischemia and causes increased damage to the donor site. Intraoperatively, the flap generally needs to be separated from the fascia layer and the fascia layer and skin sewn together with a few stitches to prevent separation between the two parts affecting flap survival. In addition, there has been much research on how the rotation angle affects flap survival. The greater the angle of rotation, the greater the probability of acute postoperative complications, especially related to veins, which have thinner vessel walls and lower internal pressure and are more flexible than arteries, making them particularly sensitive to rotation10, 11, 12, 13. To optimally protect the vascular pedicle, surgeons should try turning the propeller flap both clockwise and counterclockwise to identify how to achieve the minimum angle of rotation flap that will cover the defect.
Perforator Vessel: Naked or Not?
At present, whether the vascular pedicle should be naked is controversial. Those who are in favor of a completely naked vascular pedicle believe that retaining surrounding fascia tissue can create “twisted turn stress” in the vessel wall after reversing, thus affecting venous return14. Those who support retention of some of the fascia believe that such retention reduces the probability of vascular separation pedicle injury and increases the vascular pedicle's ability to resist stretching and spasm15, 16. In our group of patients, the vascular pedicle vessels were completely naked and we achieved a high flap survival rate. We believe that if the surgeon constructs a long vascular pedicle (>3 cm), some of the fascia tissue surrounding the perforator vessel can be retained, because spin transfer torque is spread over a longer distance; thus, “twisted turn stress” is minimized. However, if the vascular pedicle is shorter (<2 cm), we prefer it to be thoroughly naked of vessels in order to ensure that fragile veins will not cause blood reflux as a result of “twisted turn stress”.
In conclusion, this study shows the advantages of perforator pedicled propeller flaps; namely, that their texture is similar to that of the adjacent tissue and the flap blood supply is reliable, allowing coverage of large defects. In addition, because the flaps cover the defects with their perforating vessels as the axis of rotation, they require no vascular anastomoses, making the operation relatively simple and safe, with a short operative time and low surgical risk, especially when repairing lower leg and foot soft tissue defects. It should be noted that there are anatomical variations in the precise location of flap perforator vessels; thus, preoperative assessment is necessary. Without such preparation, a suitable perforating vessel may not be found, leading to surgical failure.
From a research perspective, the following unresolved difficulties remain. (i) Exactly how should the flap be constructed? Both in the past and currently, we depend more on experience than objective indicators. Further basic research is required to clarify the importance of vascular diameter, blood flow speed, and many other microvascular variables and thus provide rational guidance rather than subjective experience. (ii) How best to locate optimal perforator vessels and determine how they run? Although color Doppler enables more accurate location of perforator vessels, the reproducibility and image continuity is poor. Computed tomography angiography provides continuous images and the images can be saved; however, angiography is problematic when the diameter is less than 1 mm. In addition, it cannot be positioned directly on the body surface over the perforator vessels. Thus, more exact positioning and imaging of perforator vessels requires developments in equipment and vascular detection technology.
Disclosure: No funds were received in support of this work.
References
- 1. Mao HJ, Shi ZY, Yin WG, et al The anatomy and clinical applications of the reverse medialis pedis island flap to repair of the fore‐foot skin detects. Zhonghua Gu Ke Za Zhi, 2010, 30: 396–399 (in Chinese). [Google Scholar]
- 2. Yuan HJ, Cai PH, Cai YM, Fan CY. The reverse extended peroneal artery perforator flap for soft tissue defects of the ankle and fool. Zhonghua Gu Ke Za Zhi, 2009, 29: 873–876 (in Chinese). [Google Scholar]
- 3. Hyakusoku H, Yamamoto T, Fumiiri M. The propeller flap method. Br J Plast Surg, 1991, 44: 53–54. [DOI] [PubMed] [Google Scholar]
- 4. Aslan G, Tuncali D, Cigsar B, Barutcu AY, Terzioglu A. The propeller flap for postburn elbow contractures. Burns, 2006, 32: 112–115. [DOI] [PubMed] [Google Scholar]
- 5. Hallock GG. The propeller flap version of the adductor muscle perforator flap for coverage of ischial or trochanteric pressure sores. Ann Plast Surg, 2006, 56: 540–542. [DOI] [PubMed] [Google Scholar]
- 6. Pignatti M, Ogawa R, Hallock GG, et al The “Tokyo” consensus on propeller flaps. Plast Reconstr Surg, 2011, 127: 716–722. [DOI] [PubMed] [Google Scholar]
- 7. Ayestaray B, Ogawa R, Ono S, Hyakusoku H. Propeller flaps: classification and clinical applications. Ann Chir Plast Esthet, 2011, 56: 90–98. [DOI] [PubMed] [Google Scholar]
- 8. Pignatti M, D'Arpa S, Cubison TCS. Novel fasciocutaneous flaps for the reconstruction of complicated lower extremity wounds. Tech Orthop, 2009, 24: 88–95. [Google Scholar]
- 9. Zhang H, Zhang XD, Yu DC, Shi L, Chai Y. Reconstruction of skin and soft tissue defects by pedicle skin flaps. Zhonghua Gu Ke Za Zhi, 2012, 32: 260–264 (in Chinese). [Google Scholar]
- 10. Wong CH, Cui F, Tan BK, et al Nonlinear finite element simulations to elucidate the determinants of perforator patency in propeller flaps. Ann Plast Surg, 2007, 59: 672–867. [DOI] [PubMed] [Google Scholar]
- 11. Salgarello M, Lahoud P, Selvaggi G, Gentileschi S, Sturla M, Farallo E. The effect of twisting on microanastomotic patency of arteries and veins in a rat model. Ann Plast Surg, 2001, 47: 643–646. [DOI] [PubMed] [Google Scholar]
- 12. Topalan M, Bilgin SS, Ip WY, Chow SP. Effect of torsion on microarterial anastomosis patency. Microsurgery, 2003, 23: 56–59. [DOI] [PubMed] [Google Scholar]
- 13. Bilgin SS, Topalan M, Ip WY, Chow SP. Effect of torsion on microvenous anastomotic patency in a rat model and early thrombolytic phenomenon. Microsurgery, 2003, 23: 381–386. [DOI] [PubMed] [Google Scholar]
- 14. Pignatti M, Pasqualini M, Governa M, Bruti M, Rigotti G. Propeller flaps for leg reconstruction. J Plast Reconstr Aesthet Surg, 2008, 61: 777–783. [DOI] [PubMed] [Google Scholar]
- 15. Chang SM, Zhang F, Yu GR, Hou CL, Gu YD. Modified distally based peroneal artery perforator flap for reconstruction of foot and ankle. Microsurgery, 2004, 24: 430–436. [DOI] [PubMed] [Google Scholar]
- 16. Chang SM, Zhang F, Xu DC, Yu GR, Hou CL, Lineaweaver WC. Lateral retromalleolar perforator‐based flap: anatomical study and preliminary clinical report for heel coverage. Plast Reconstr Surg, 2007, 120: 697–704. [DOI] [PubMed] [Google Scholar]


