Abstract
Objective: To evaluate the biocompatibility of a new kind of prosthetic nucleus: a pectin/polyvinyl alcohol composite (CoPP) hydrogel.
Methods: According to Chinese national standard GB/‐T16886 documents, the toxicity of the CoPP prosthetic nucleus material was examined by cytotoxicity, sensitization, Ames, mice marrow micronucleus, chromosome aberration test of mammalian cell and implantation tests.
Results: Cell growth was similar in the CoPP culture and control groups. No significant difference was found between the CoPP culture and control groups at each time point (P > 0.05). The cell proliferation rate was greater than 100%. In accordance with the relationship between cytotoxicity to proliferation rate, it was confirmed that the cytotoxicity of CoPP was 0 grade. Mice had no allergic reaction when injected with an extract of CoPP. A reverse mutation test with Salmonella typhimurium showed that no significant effect on the number of histidine revertants of TA97, TA98, TA100 and TA102 strains after CoPP was added. The micronucleus rate in bone marrow cells was less than 5%; there was no significant difference compared with the negative control group (P > 0.05). The rate of chromosome aberration was less than 5%; no significant difference was found between the CoPP culture and the control groups. All experimental animal wounds achieved primary healing without exudation, infection or sinus formation. On macroscopic observation, no abscess or hematoma formed at the implantation site.
Conclusion: The CoPP prosthetic nucleus has good biocompatibility and can potentially be used as an implant material.
Keywords: Biocompatible materials, Intervertebral disk, Prostheses and implants
Introduction
Chronic discogenic back pain is a common ailment that affects a significant proportion of the general population. When conservative treatment fails to alleviate the patients' symptoms, operative treatment may be pursued. Currently, the gold standard for degenerative disk disease is spinal fusion. Though this procedure can be effective in relieving pain, this benefit does not come without consequence: adjacent segment post fusion disease has been well documented in the literature 1 , 2 , 3 . Since Fernstrom first implanted a stainless steel ball endoprosthesis in 1966 4 , spinal surgeons have been searching for a disk spacer that allows movement. Nucleus pulposus (NP) replacement is a non‐fusion technique currently being investigated to treat painful disc degeneration. The prototypical design for nucleus replacement is the prosthetic disc nucleus (PDN, Raymedica, Minneapolis, MN, USA), which is composed of a hydrogel polymer encased in a polyethylene jacket. The hydrogel absorbs 80% of its weight in water, allowing it to restore disk height 5 . In 2003 Shim reported 46 cases with the clinical follow‐up; radiographically 19.6% showed device subsidence into the endplates, 60.9% scleroses at the endplates, and 82.8% increased Modic changes of the vertebral body in subsequent lumbar MRI films 6 . These events may be correlated with the mechanical character and shape of prothesis 7 . Many scholars have pursued studies on prosthetic nucleus materials. Huang et al. prepared a new prosthetic material, a pectin/polyvinyl alcohol composite (CoPP) hydrogel 8 . Their studies indicated that CoPP is a soft, tenacious elastic material with good permeability 8 . In order to confirm the feasibility of clinical application of CoPP, we carried out the following biocompatibility tests.
Materials and methods
Preparation of hydrogel
CoPP was manufactured according to a compounding formula offered by Huang et al. 9 . After slicing into 1 mm thicknesses and routine cleaning, the material was sterilized with ethylene oxide. Afterwards, the hydrogel was dried in a vacuum to a constant weight and prepared for further use.
Preparation of CoPP standard leaching liquor
According to Chinese national standard of evaluation of medical device GB/T16886 documents, for the cytotoxicity test the culture medium was Dulbecco's modified Eagle's medium (DMEM) culture liquid containing 100 ml/L calf serum 10 . In the other tests, normal saline solution was used as a leaching medium for medical high grade polymer material. The material was immersed in 10 ml normal saline solution, which is considered to be a standard leaching liquor, and leached at 37°C for 24 h.
Cytotoxicity test
In this test, the cytotoxicity of CoPP was investigated using L929 mouse fibroblasts. For each experiment, the cells were implanted into each well of 96‐well chambers (0.1 ml/well) at an initial concentration of 5 × 103 cells/ml. All cells were grown in RPMI‐1640 medium for 24 h at 37°C in an atmosphere of 5% CO2 in air. The cells were then divided into three groups (i.e. sample, positive and negative control groups) and each group allotted four wells. For the sample group the original culture liquid was replaced with extracts of the CoPP sample. For the positive control group, the culture medium was changed to one containing 0.64% phenol while fresh RPMI‐1640 medium was used for the negative control group. All tubes were incubated continuously at 37°C. Two, four, seven days later, 20 µm of 5% methyl thiazolyl tetrazolium (MTT) was added to each well. The cells were cultured for another 4 h and then the liquid in the wells was drained off. After that, 0.15 ml of dimethyl sulfoxide (DMSO) was added to each well, and after thorough mixing, the absorption value (A value) was recorded at 570 nm on an enzyme analyzer (Mode 680, Bio‐Rad, Hercules, CA, USA). Each group was tested three times and the average calculated. Then the relative growth rate (RGR) of cell was calculated according to the following equation and transformed into grade of cytotoxicity.
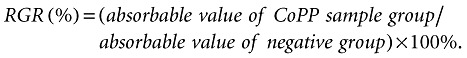 |
Thus, according to the GB/T161752‐1996 documents, the cytotoxicity of CoPP was evaluated.
Sensitivity test
Thirty guinea pigs, 300–500 g in weight and of random sex, were randomly divided into three equal groups: an experimental (CoPP standard leaching liquor), a negative control (physiological saline) and a positive control group (50 ml/L formaldehyde). The test consisted of three phases: inductive injection, topical patching and challenge. Twenty‐four h before the experiment, the pelage was shaved from a 3 cm × 3 cm area in the middle of the back of each pig. Four symmetrical points at an interval of 2 cm on each side of the spines of the pigs were chosen for injection. After sterilizing with alcohol (70%), 0.1 ml of liquor was injected into every animal in all three groups. One week later, the same area of pelage was shaved again, disinfected and covered with 100 g/L sodium dodecyl sulphate for 24 h. Next a 2 cm × 2 cm sheet of filter paper which had been fully immersed in CoPP leaching liquor, physiological saline or formaldehyde was pasted on for another 48 h. Two weeks later, the pelage on the lateral abdomens of the pigs was shaved, disinfected and pasted with the same filter paper for another 24 h. After removing the filter paper, any erythema and edema of the abdomen were recorded and graded for each pig according to a cutaneous reaction grading suggested by the GB/T161752‐1996 documents. Five scales were used: none, very slight, slight, moderate and severe.
Acute systemic toxicity test
Eighteen mice were weighed and randomly divided equally into experimental, negative control and positive control groups. Then the three groups of mice were injected intra‐abdominally with standard leaching liquor (50 ml/kg), saline and formaldehyde (5%), respectively. The general state, evidence of toxicity and number of deaths in each group were recorded 4, 24, 48 and 72 h after injection; each animal was also weighed 24, 48 and 72 h after injection. The toxicity grading system used was that suggested by the GB/T161752‐1996 documents.
Genetic toxicity test
Reverse mutation test (Ames test)
The procedure was carried out according to the Chinese national standard of evaluation of medical device GB/T16886 guidelines. CoPP was tested using four strains of Salmonella typhimurium: TA97, TA98, TA100 and TA102. The dosage of CoPP in the experimental group was 1, 1/2, 1/4, 1/8 and 1/16 CoPP solution per plate, respectively. Corresponding blank controls were set up. Physiological saline served as negative control and dexon, sodium azide (NaN3), 1, 4‐oxhydryl anthraquinone (1, 4‐OA) and 2‐aminofluorence (2‐AF) were used as positive controls. Three plates were used in each group and the test was repeated three times.
Mouse marrow micronucleus test
For the mouse micronucleus test, 50 NIH mice weighing 21 ± 3 g were divided into five groups according to a random number chart. Each group had 10 mice with half male and half female. Three different dosages of CoPP extract were used in the experimental group. Physiological saline served as negative control (50 ml/kg) and cyclophosphamide (CP, 80 mg/kg) as positive control. These were injected intraperitoneally. All mice in the test were injected twice, the second injection 24 h after the first, and the mice killed 6 h later to obtain femoral bone marrow smears, which were fixed with methanol and stained with Giemsa. We counted 1000 polychromatic erythrocytes (PEC) for each mouse. The number of PEC with micronuclei was recorded and the frequency of micronucleus was calculated.
Chromosome aberration test in mammalian cells
The test consisted of two independent experiments with and without metabolic activation by S‐9. Chinese hamster fibroblasts (CHL) were used. The dosages for the experimental group were 46, 23, and 11.5 g/L. In the experiments without S‐9, mitomycin C (MMC, 0.2 mg/l) served as positive control. CP (40 mg/L) served as positive control in the experiments with S‐9. DMSO served as negative control. In the experiment without metabolic activation, the cells were harvested 24 and 48 h after adding the sample liquid. In the experiments with metabolic activation, after the cells had been cultured with the sample liquid and S9‐mix for 6 h, a new culture liquid was used. Colchicine was added 4h before harvest. Slides were made and stained routinely. The structure and number of chromosome abnormalities in 100 cells in metaphase were counted and the frequency of aberration calculated.
In vivo implantation experiment
Thirty SD mice of body weight 182 ± 3 g were used for this test, half male and half female. Polyvinyl alcohol (PVA) was used for the negative control group. After anesthesia and disinfection, two symmetrical points on each side of the spines of the mice 2 cm above the anterior superior iliac spine were chosen for implantation. After removing the skin, blunt dissection was carried out on the subcutaneous tissues, fascia and muscles, creating a lesion measuring 0.3 cm × 0.3 cm × 1.0 cm. Then sterile CoPP or PVA was implanted intramuscularly in each dissected area. Finally, the tissues were sutured in order. Each mouse was injected with benzylpenicillin sodium (72 000 u) intraperitoneally postoperatively. At each of 1, 4 and 12 weeks after implantation, ten mice were killed to harvest the muscles of the spine bilaterally. The muscle from about 0.5 cm around the material was cut, fixed with 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin‐eosin and observed under a light microscope. Local response was graded according to the inflammatory cell reaction grading standard in the GB/T161752‐1996 documents.
Results
Cytotoxicity assay
Cell growth was similar in the CoPP culture and control groups. At day 7, inverted phase contrast microscopy and scanning electron microscopy showed good adherence between cells and materials in the CoPP group. With regard to detection of cell proliferation by MTT assay, with prolongation of time, the A value gradually increased in each group, reaching a peak at day 7. No significant difference was found between the CoPP culture and PVA control groups at each time point (P > 0.05) (Table 1). The cell proliferation rate was greater than 75% in the CoPP group, while in the positive group it was less than 25%. In accordance with the relationship between cytotoxicity and proliferation rate, we confirmed that the cytotoxicity of CoPP was 0–1 grade.
Table 1.
A value of L929 cell culture at 2, 4 and 7 days
| Groups | 2 days | 4 days | 7 days |
|---|---|---|---|
| Normal control | 0.089 ± 0.021 | 0.710 ± 0.124 | 1.091 ± 0.152 |
| PVA | 0.140 ± 0.047 | 0.699 ± 0.061 | 1.082 ± 0.040 |
| CoPP | 0.163 ± 0.052 | 0.769 ± 0.151 | 1.088 ± 0.277 |
| Positive control | 0.003 ± 0.016 | 0.024 ± 0.018 | 0.004 ± 0.021 |
Sensitivity test
After leaching liquor or saline was injected, no cutaneous reaction such as erythema or edema occurred. In the positive group, however, there were midrange reactions in four animals and severe reactions in six. This result confirms that the CoPP material does not induce sensitization.
Acute systemic toxicity test
No symptoms of toxicity were found in either the CoPP or negative control groups. After injection, the general state of animals remained good, they fed routinely, and no abdominal irritation, exhaustion, cyanosis or death occurred, while six animals in the positive control group showed toxic reactions ranging from slight to serious.
Genetic toxicity test
Ames test
The results of the Ames test with and without the metabolic activation system (S‐9) are shown in Table 2. Using varying doses of the CoPP extract, reversion mutation frequencies did not increase in strains TA97, TA98, TA100 and TA102. This indicates that, under the test conditions, the CoPP extract was not mutagenic for histidine requiring strains of Salmonella typhimurium.
Table 2.
Results of Ames test for CoPP hydrogel ( )
)
| Groups | TA97 | TA98 | TA100 | TA102 | ||||
|---|---|---|---|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | |
| CoPP solution | 140 ± 10 | 146 ± 20 | 40 ± 3 | 37 ± 3 | 139 ± 22 | 151 ± 21 | 272 ± 23 | 284 ± 19 |
| 1/2 CoPP solution | 145 ± 35 | 147 ± 19 | 39 ± 6 | 41 ± 8 | 146 ± 16 | 173 ± 21 | 258 ± 23 | 272 ± 24 |
| 1/4 CoPP solution | 107 ± 6 | 106 ± 3 | 24 ± 5 | 23 ± 5 | 187 ± 15 | 165 ± 17 | 272 ± 23 | 274 ± 28 |
| 1/8 CoPP solution | 112 ± 17 | 150 ± 26 | 6 ± 6 | 44 ± 6 | 145 ± 34 | 172 ± 29 | 250 ± 7 | 283 ± 22 |
| 1/16 CoPP solution | 130 ± 40 | 138 ± 26 | 39 ± 6 | 40 ± 3 | 145 ± 16 | 145 ± 24 | 275 ± 32 | 280 ± 17 |
| Solvent control | 124 ± 39 | 147 ± 19 | 42 ± 6 | 42 ± 7 | 154 ± 25 | 159 ± 16 | 273 ± 25 | 281 ± 26 |
| Spontaneous reversion | 142 ± 34 | 146 ± 20 | 39 ± 8 | 42 ± 6 | 141 ± 11 | 152 ± 14 | 261 ± 15 | 275 ± 21 |
| Dexon | 1120 ± 37 | — | 1007 ± 21 | — | — | — | 954 ± 32 | — |
| NaN3 | — | — | — | — | 1584 ± 27 | — | — | — |
| 1,4‐OA | — | — | — | — | — | — | — | 997 ± 57 |
| 2‐AF | — | 1159 ± 62 | — | 1004 ± 19 | — | 1564 ± 64 | — | — |
Mice marrow micronucleus test
The results of the mouse marrow micronucleus test are shown in the Table 3. Compared with the negative controls, the difference in frequency of micronuclei in the group in which different dosages of CoPP extract were used was not significant. This indicates that the material does not increase the number of micronucleated PEC.
Table 3.
Results of mice marrow micronucleus test for CoPP prosthetic nucleus (n= 10)
| Group | Counted PEC | Number of micronucleated PEC | Frequency of micronuclei (%) | P value |
|---|---|---|---|---|
| Negative control | 10 000 | 18 | 1.8 | — |
| Positive control | 10 000 | 206 | 20.6 | < 0.01 |
| CoPP (6000 mg/kg) | 10 000 | 26 | 2.6 | > 0.05 |
| CoPP (3000 mg/kg) | 10 000 | 22 | 2.2 | > 0.05 |
| CoPP (1500 mg/kg) | 10 000 | 19 | 1.9 | > 0.05 |
Chromosome aberration test
The results of the chromosome aberration test are shown in the Table 4. Compared with the negative controls, the difference in frequency of chromosome aberrations caused by different dosages of the CoPP extract solution was not significant with or without S‐9 (P > 0.05). This indicates that, under these test conditions, the CoPP extract does not induce chromosome aberration in CHL.
Table 4.
Results of chromosome aberration test in CHL cells for CoPP prosthetic nucleus
| Groups | S9 (±) | Time (h) | Chromosome aberration | Number of aberrant cells | Frequency of aberration (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Breakage | Gap | Ring centric | Triploid | Polyploid | |||||
| Negative control | − | 24 | 2 | 1 | 0 | 0 | 0 | 3 | 3 |
| Positive control (MMC) | − | 24 | 20 | 1 | 1 | 0 | 4 | 26 | 26 |
| CoPP (46 mg/ml) | − | 24 | 3 | 0 | 0 | 1 | 0 | 4 | 4 |
| CoPP (23 mg/ml) | − | 24 | 2 | 0 | 0 | 0 | 1 | 3 | 3 |
| CoPP (11.5 mg/ml) | − | 24 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Negative control | − | 48 | 3 | 0 | 0 | 0 | 0 | 3 | 3 |
| Positive control (MMC) | − | 48 | 36 | 0 | 0 | 0 | 0 | 36 | 36 |
| CoPP (46 mg/ml) | − | 48 | 4 | 0 | 0 | 0 | 0 | 4 | 4 |
| CoPP (23 mg/ml) | − | 48 | 1 | 0 | 0 | 0 | 1 | 2 | 2 |
| CoPP (11.5 mg/ml) | − | 48 | 2 | 0 | 0 | 0 | 0 | 2 | 2 |
| Negative control | + | 24 | 1 | 0 | 0 | 0 | 1 | 2 | 2 |
| Positive control (CP) | + | 24 | 18 | 0 | 0 | 0 | 2 | 20 | 20 |
| CoPP (46 mg/ml) | + | 24 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| CoPP (23 mg/ml) | + | 24 | 2 | 0 | 0 | 0 | 0 | 2 | 2 |
| CoPP (11.5 mg/ml) | + | 24 | 1 | 0 | 0 | 0 | 2 | 3 | 3 |
Experimental results of in vivo implantation
All experimental animal wounds achieved primary healing without exudation, infection or sinus formation. No experimental animal showed behavioral changes or local nerve dysfunction during the observation period, and all animals survived for the preset time. On macroscopic examination, no abscess or hematoma formed at the implantation site (Fig. 1A). On microscopic examination, reactions induced by the implant materials at 1 week after implantation included hemorrhage, edema, infiltration of lymphocytes and neutrophilic granulocytes (Fig. 1B,C), there were no significant differences between CoPP and PVA. The inflammatory cell reaction was grade IV. On macroscopic examination, the local reaction at 4 weeks was similar to that at 1 week (Fig. 1D). Fibroblastic proliferation is shown in Fig. 1E,F, the capsular space reaction was grade IV and the inflammatory reaction grade I in both groups. At 12 weeks, several layers of fibroblasts separated the materials and muscular tissue and no obvious inflammatory cell infiltration was found around the materials. Both the capsular space and inflammatory reactions were grade I in both groups (Fig. 1G–I).
Figure 1.
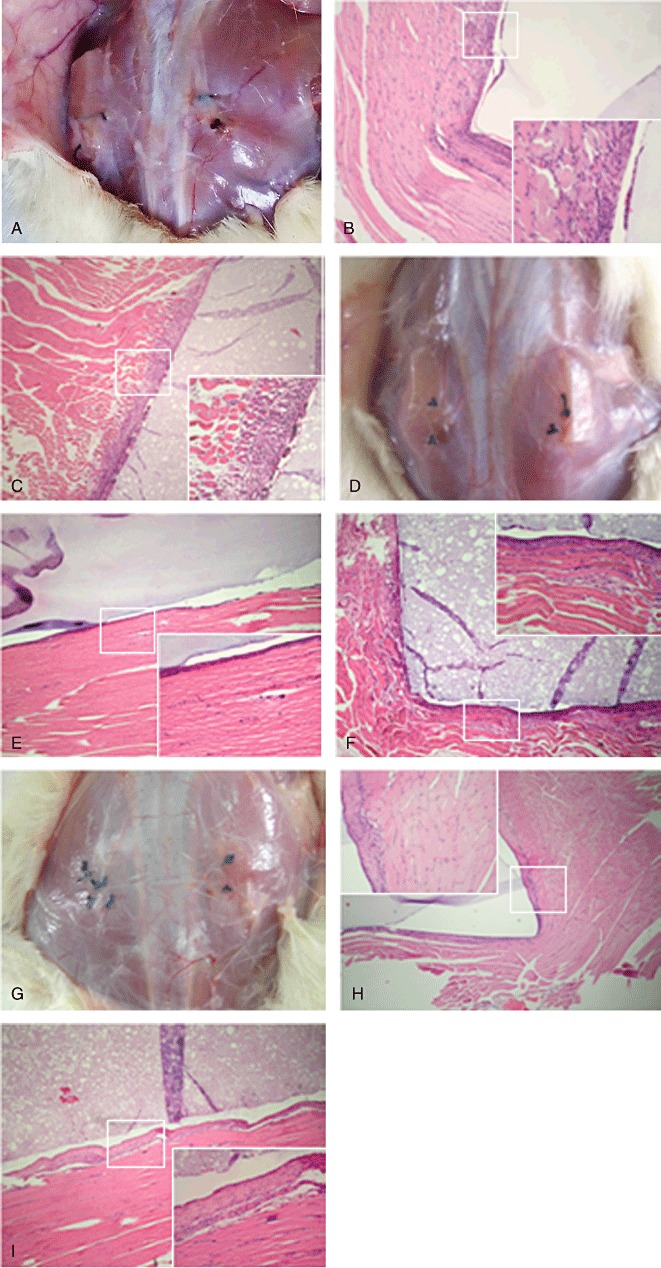
Macroscopic and microscopic reactions to PVA and CoPP hydrogel. A. Macroscopic appearance (1 week). B. Microscopic appearance of the tissues and PVA hydrogel with HE staining (1 week, ×40, ×200). C. Microscopic appearance of the tissues and CoPP hydrogel with HE staining (1 week, ×40, ×200). D. Macroscopic appearance (4 weeks). E. Microscopic appearance of the tissues and PVA hydrogel with HE staining (4 weeks, ×40, ×200). F. Microscopic appearance of tissue and CoPP hydrogel with HE staining (4 weeks, ×40, ×200). G. Macroscopic appearance (12 weeks). H. Microscopic appearance of the tissue and PVA hydrogel with HE staining (12 weeks, ×40, ×200). I. Microscopic appearance of the tissues and CoPP hydrogel with HE staining (12 weeks, ×40, ×200).
Discussion
NP replacement is a non‐fusion technique currently being investigated for treatment of painful disc degeneration. The typical replacement prosthesis is manufactured from an elastic material, such as a polyvinyl alcohol, polyacrylonitrile, polyurethane, and so on 11 , 12 . The use of PVA hydrogel, which is a three‐dimensional expandable polymer with variable water content and mechanical properties suitable for nuclear replacement, has been extensively explored. One of the most important characteristics of this material is the ability to absorb and release water depending on the applied load, similar to native NP tissue 13 . In addition, PVA molecules have been thought to be biocompatible and without toxicity, and are approved by the Food and Drug Administration (FDA) for wide biomedical application 14 . Unfortunately, imperfect mechanical properties (high elastic modulus) have adversely influenced the clinical outcomes. We have synthesized a new prosthetic nucleus material based on PVA: CoPP hydrogel. According to previous studies, we think CoPP is a better artificial material with regard to its mechanical characteristics 8 , 9 . The mechanical and chemical properties of a material used as an implant in the body should meet the physiological demands placed on it, and its biocompatibility should be excellent as well. Therefore we carried out strict tests to evaluate the biocompatibility of the material in order to ensure the safety and utility of CoPP for clinical use.
There are many well developed biological evaluation methods for evaluating material. For the present study, we adopted the Chinese national standard GB/T16886 documents as our criteria, and designed a series of classical biocompatibility tests to examine cytotoxicity, sensitivity, acute systemic toxicity, genetic toxicity, plus an implantation test to appraise the biocompatibility of CoPP. Mice and guinea pigs were selected for the above tests because they were considered to have the most suitable characteristics of various possible candidates for these experiments.
Cytotoxicity is one of the important factors which affect the use of polymers in medical engineering. In the present study, the continuous fibroblast cell line L929 was used to assess the cytotoxicity of CoPP deposited in a RPMI‐1640 medium. Scanning electron microscope observation showed that cell adhesion and spread on CoPP followed a similar pattern compared to that found on normal culture plates. In addition, assessment of mitochondrial function (MTT assay) further suggested CoPP's lack of cytotoxicity.
The aim of sensitivity testing was to determine whether CoPP could induce an allergic response in skin by forming a complete antigen with skin protein. In the present study, the results suggest that no hypersensitivity inducing micromolecular substance remains in the CoPP material. And, due to the non‐degradable nature of the material, we predict no allergic response with long‐term application.
The acute systemic toxicity test is a non‐specific test for judging the acute toxicity of materials by injecting a leaching liquor of the material intravenously or intraperitoneally. In this study, all animals in the leaching liquor group experienced no toxicity response, suggesting the safety of CoPP hydrogel.
For testing genotoxicity, we selected the Ames, micronucleus and chromosome aberration tests. They reflect changes in genes and chromosomes, and allow comprehensive assessment of the influence of materials on genotoxicity. In the latter two tests, mammalian cells were used as targets, so the tests closely approximated the real situation in the body. From the results of this study, we can make a preliminary conclusion that the CoPP material has no teratogenic or mutagenic effect.
The basis of the response to implantation of biological materials is a series of immune cell and repair cell activities, including aseptic inflammation and formation of a fibrous membrane. Because of differences in biocompatibility of different materials, the inflammatory reaction and process of forming a fibrous capsule vary. In the case of a material with excellent biocompatibility, the inflammatory reaction is light and of short duration, and a fibrous capsule forms over time. We used muscle implantation to check the biocompatibility of CoPP. In this test, all animal wounds achieved primary healing without infection; a mild inflammatory infiltration appeared at 1 week, fibroblast proliferation at 4 weeks and fibroblastic capsule formation at 12 weeks. The results of the implantation test were similar to that of other PVA composite hydrogels reported by some authors 15 , however less inflammatory cells were observed and a much thinner capsule had formed at the same time points, indicating better short‐term biocompatibility. The slightness of the tissue reaction might be attributable to the dimensions and nature of the implanted CoPP.
We carried out short‐term biocompatibility experiments to evaluate the properties of the CoPP material and the results indicate that this material has good biocompatibility, which provides a preliminary theoretical basis for clinical application. To assess its long‐term biocompatibility, we will conduct further research, such as carcinogenic, chronic toxicity and intervertebral space tests.
References
- 1. Abraham E, Manson N. Incidence of adjacent segment degeneration in thoracolumbar fusions of three or more levels. Spine J, 2007, 7 (Suppl.): S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacDougall J, Perra J, Pinto M, et al. Incidence of adjacent segment degeneration at ten years after lumbar spine fusion. Spine J, 2003, 3 (Suppl.): S67–S68. [Google Scholar]
- 3. Bhatia N, Ghiselli G, Wang J, et al. Proximal segment degeneration after posterior lumbosacral fusions (L4‐S1 and L5‐S1). Spine J, 2003, 3 (Suppl.): S91–S92. [Google Scholar]
- 4. Fernstrom U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl, 1966, 355: 154–159. [PubMed] [Google Scholar]
- 5. Bao QB, Yuan HA. New technologies in spine: nucleus replacement. Spine, 2002, 27: 1245–1247. [DOI] [PubMed] [Google Scholar]
- 6. Shim CS, Lee SH, Park CW, et al. Partial disc replacement with the PDN prosthetic disc nucleus device: early clinical results. J Spinal Disord Tech, 2003, 16: 324–330. [DOI] [PubMed] [Google Scholar]
- 7. Jin D, Qu D, Zhao L, et al. Prosthetic disc nucleus (PDN) replacement for lumbar disc herniation: preliminary report with six months' follow‐up. J Spinal Disord Tech, 2003, 16: 331–337. [DOI] [PubMed] [Google Scholar]
- 8. Huang C, Qu DB, Zhao WD, et al. Biomechanical evaluation of pectin/polyvinyl alcohol composite hydrogel (Chin). Zhongguo Ji Zhu Ji Sui Za Zhi, 2008, 18: 60–63. [Google Scholar]
- 9. Huang C, Lu L, Qu DB, et al. Preparation and swelling properties of pectin/poly (vinyl alcohol) composite hydrogel for prosthetic nucleus pulposus (Chin). Acta Materiae Compositae Sinica, 2008, 25: 69–74. [Google Scholar]
- 10. General Administration of Quality Supervision, Inspection and Quarantine of Peoples Republic of China . GB/T16886 China National Standard of Evaluation of Medical Device. Beijing: China national standard press, 2003; 1–6. [Google Scholar]
- 11. Di Martino A, Vaccaro AR, Lee JY, et al. Nucleus pulposus replacement: basic science and indications for clinical use. Spine, 2005, 30 (16 Suppl.): S16–S22. [DOI] [PubMed] [Google Scholar]
- 12. Ray CD. The PDN prosthetic disc‐nucleus device. Eur Spine J, 2002, 11 (Suppl. 2): S137–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas J, Lowman A, Marcolongo M. Novel associated hydrogels for nucleus pulposus replacement. J Biomed Mater Res A, 2003, 67: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 14. Seal BL, Otero TC, Panitch A. Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng, 2001, 34: 147–230. [Google Scholar]
- 15. Xue HB, Ma YZ, Zhou X, et al. Evaluation of biocompatibility of prosthetic disc nucleus materials (Polyvinyl Alcohol Hydrogel/Ultra‐high Molecular Weight Polyethylene) (Chin). Orthop Biomech Mater Clin, 2007, 4: 1–4. [Google Scholar]


