Abstract
Objective: To investigate the expression and the clinical significance of basic fibroblast growth factor (b‐FGF) and endostatin in osteosarcoma.
Methods: From January 2003 to December 2005, expression of b‐FGF, endostatin and CD34 were detected in 30 osteosarcoma and 30 osteochondroma tissue specimens by the immunohistochemical Elivision method. All data were post‐processed with SPSS 13.0 software and prepared for investigation and analysis of these expressions and the relationships between the parameters.
Results: (i) The rates of expression of b‐FGF, endostatin and CD34 protein in osteosarcoma were 76.7%, 93.3%, and 96.7%, respectively, and in osteochondroma 43.3%, 40.0% and 16.7%, respectively. Each of the three expressions showed obvious differences between the osteosarcoma and the osteochondroma group. (ii) In the osteosarcoma group, expression of endostatin was positively correlated with that of CD34 (P < 0.05, γs = 0.528), and expression of endostatin in poorly differentiated osteosarcoma was much greater than that in highly differentiated osteosarcoma (P= 0.004). Expression of endostatin correlated with osteosarcoma metastasis (P= 0.036). (iii) There was no correlation between b‐FGF and endostatin expression rates (P= 0.182) in the osteosarcoma group.
Conclusion: Angiogenesis is the basis of tumor metastasis, as well as being an important factor in tumor growth. Expression of endostatin could be adopted as a parameter for the diagnosis of postoperative metastases and for assessing prognosis, and could act as an adjuvant indicator in the grading of osteosarcoma.
Keywords: Endostatins, Fibroblast growth factor, Osteosarcoma
Introduction
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents. About 80% of cases occur in the long bones of the limbs, the other 20% being in the axial skeleton and pelvis 1 . In young adults it usually occurs where there is rapid bone growth, such as in the distal femur, proximal tibia, and proximal humerus. Osteosarcoma is a primary malignant tumor of the skeleton characterized by the direct formation of immature bone or osteoid tissue by the tumor cells. Most osteosarcoma tumors are of high grade and tend to produce pulmonary metastases. Despite clinical improvements, patients with metastasis or recurrent diseases still have a poor prognosis. The growth and dissemination of osteosarcoma depends on angiogenesis. A number of measurable factors related to tumor angiogenesis have been studied in patients with osteosarcoma 1 , 2 , 3 , 4 , 5 .
Angiogenesis factors correlate with important clinical features in patients with sarcomas ranging from soft‐tissue sarcomas to bone sarcomas 6 , 7 . Angiogenesis factors may serve an important role in predicting a particular patient's clinical course and in identifying patients for possible antiangiogenic therapy. A delicate balance between positive and negative regulatory signals controls each step of angiogenesis. When the promotion function of angiogenesis regulatory factors is dominant, this leads to vascular proliferation, otherwise, vascular proliferation is inhibited.
Basic fibroblast growth factor (b‐FGF) is the most important angiogenic factor 8 , 9 . It can stimulate endothelial cells to proliferate, migrate, and alter their patterns of gene expression, and increase microvascular permeability. Up to now, endostatin is the most effective known inhibitory factor for angiogenesis, being capable of inhibiting vascular endothelial cell proliferation and controlling the growth of both metastatic and primary tumors 10 .
There has been little research on the vascular regulatory factors in solid osteosarcoma. In the present study, the expressions of b‐FGF, endostatin and CD34 in osteosarcoma and osteochondroma were evaluated by applying immunohistochemistry staining methods. The effects of b‐FGF and endostatin on the occurrence and growth of osteosarcoma are discussed. The relationships among b‐FGF, endostatin and CD34, as well as their relationships to clinical and pathological parameters were also studied. The results reported here provide a solid basis for further developments in pathogenesis, treatment and assessment of prognosis in osteosarcoma. Further work could elucidate the complex biology of tumor angiogenesis and define rational approaches to anti‐angiogenic cancer therapy.
Materials and methods
Sample collection
Thirty osteosarcoma tissue specimens were collected by the pathology department of the Second Hospital of Shanxi Medical University from January 2003 to December 2005. The selection criteria were as follows: (i) a diagnosis of malignant osteosarcoma; (ii) primary tumor in the limbs; (iii) no detectable lung metastases; (iv) no anti‐angiogenesis drugs had been used; (v) information on follow‐up could be obtained; (vi) complete records of the cases pre‐ and post‐operation, and samples of the primary tissue, had been preserved. There were 24 male and 6 female patients with a mean age of 12.4 years (range, 12–60 years). Among them, 12 cases were in the distal femur, 3 in the femoral shaft, 1 in the femoral trochanter, 11 in the proximal tibia, 1 in the distal tibia and 2 in the humerus and radius, respectively.
Sample staging
Hematoxylin‐eosin (HE) and immunohistochemical staining were performed. All stained sections were re‐examined by pathology experts. The specimens of osteosarcoma were classified as grade Ia, Ib, IIa, IIb and III according to the maximum diameter (two groups, one ≥8 cm and the other <8 cm), alkaline phosphatase (ALP, two groups, one ≥150 u/l and the other <150 u/l), ages (two groups, one ≥16 and the other <16 years old), postoperative survival time (two groups, one ≥1 year and the other <1 year), use of preoperative chemotherapy or not, the degree of differentiation, occurrence of metastasis or not, and the Enneking surgical staging system.
Thirty osteochondroma tissue specimens were collected from the same source as the controls. There were 17 male and 13 female patients with a mean age of 18.4 years (range, 6–65 years).
Immunohistochemistry
Immunohistochemistry was performed on sections of 5‐µm thickness from each specimen. The sections were dewaxed in xylene and rehydrated at concentration‐graded alcohol. Endogenous peroxide was blocked with 3% H2O2 for 20 minutes, and then pretreated in a microwave oven in 0.125% trypsin for 130 seconds. Thereafter, the slices were processed according to standard methods using the broad‐spectrum Elivision method plus an immunohistochemical staining kit. The primary antibodies used were mouse anti‐human CD34 monoclonal antibody, rabbit anti‐human endostatin polyclonal antibody, and rabbit anti‐human b‐FGF polyclonal antibody at dilutions of 1:50. Diaminobenzidine was used for the development of coloration. A negative control was generated by using phosphate buffered solution (PBS) to replace the primary antibody. Human tonsil cancer was used as the positive control for mouse anti‐human CD34 and rabbit anti‐human endostatin, and human esophageal cancer was used as a positive control for rabbit anti‐human b‐FGF.
Determination of micro‐vessel density
The tumors were usually inhomogeneous in their micro‐vessel density (MVD). MVD is a semi‐quantitative surrogate measure of angiogenesis. The micro‐vessels were immunohistochemically stained by using an endothelial cell‐specific antibody, namely anti‐CD34. For determination of MVD, the five most vascular areas (“hot spot” areas) within the same section were chosen under a light microscope with a 100× magnification and counted with a 400× magnification. A brown stain structure clearly separated from the adjacent micro‐vessels was regarded as a single countable micro‐vessel. The average count was recorded as MVD for each case.
Determination of results
Brown‐stained cell nuclei and cytoplasm are considered to be endostatin positive. Furthermore, brown‐stained cytoplasm is also b‐FGF positive. Ten high power fields were chosen randomly for each slice. The average number of positive cells was used to determine the overall percentage of positive cells as follows: 0 points were allocated for no positive staining cell, 1 point for <25% of positive staining cells, 2 points for between 25% and 50%, and 3 points for 50% or more. Scoring by staining intensity was as follows: 0 points for no staining, 1 point for yellow, 2 points for brown‐yellow, and 3 points for brown. The two types of score were added and classified as follows: score of 0, a negative result (−); between 1 and 2, a weak positive result (+); between 3 and 4, a moderately positive result (++); and between 5 and 6, a strongly positive result (+++).
Statistical methods
Statistical analysis was carried out with the SPSS 13.0 package (SPSS, Chicago, IL, USA). Cytokine concentrations between groups were compared by using the χ2 test, and the correlations among cytokine levels were assessed by the Spearman rank correlation and the Wilcoxon rank sum tests. P < 0.05 was considered statistically significant.
Results
Expression of b‐FGF in osteosarcoma and osteochondroma
The immune products of b‐FGF were brown and located in the cytoplasm. The positive staining cells in the tumor tissue were observed to be scattered and irregular. There were 23 positive expressions out of the 30 osteosarcoma cases, making a positive rate 76.7%, while it was 43.3% in the control group. As shown in Table 1, there were significant differences between the groups (χ2= 12.896, P < 0.01), expression of b‐FGF in osteosarcoma being greater than that in osteochondroma (Fig. 1a, b).
Table 1.
Semi‐quantitative results of b‐FGF, endostatin and CD34 positive staining in osteosarcoma and osteochondroma
| Group | Cases (n) | b‐FGF | Endostatin | CD34 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | Positive rate | − | + | ++ | +++ | Positive rate | − | + | ++ | +++ | Positive rate | ||
| Osteosarcoma | 30 | 7 | 3 | 12 | 8 | 76.7% | 2 | 2 | 13 | 13 | 93.3% | 1 | 4 | 19 | 6 | 96.7% |
| Osteochondroma | 30 | 17 | 7 | 4 | 2 | 43.3% | 18 | 7 | 3 | 2 | 40.0% | 25 | 3 | 1 | 1 | 16.7% |
The expressions of b‐FGF, endostatin and CD34 in osteosarcoma are greater than those in osteochondroma, the differences between the groups being significant (P < 0.01).
Figure 1.
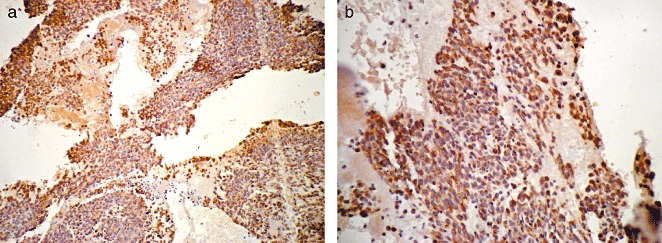
B‐FGF expression in osteosarcoma. The positive products of b‐FGF are brown and located in the cytoplasm: (a), ×100; (b), ×400.
Expression of endostatin in osteosarcoma and osteochondroma
The immune products of endostatin were brown and located in the cytoplasm and cell nuclei. The positive cells were irregular and scattered through the tumor tissue. There were 28 positive expressions among the 30 osteosarcoma cases, hence the positive rate was 93.3%, which contrasts markedly with 40.0% in the control group. As shown in Table 1, there were significant differences between these groups (χ2= 20.851, P < 0.01), expression of endostatin in osteosarcoma being greater than that in osteochondroma (Fig. 2a, b).
Figure 2.
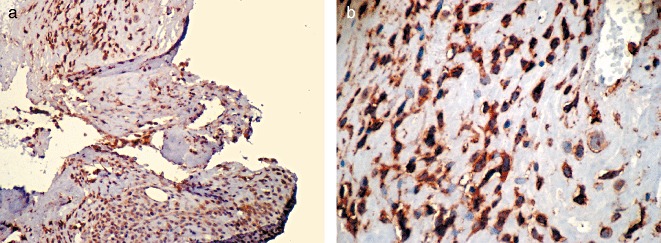
Endostatin expression in osteosarcoma. The positive products of CD34 are brown and located in the cell membrane: (a), ×100; (b), ×400.
Expression of CD34 in osteosarcoma and osteochondroma
The immune products of CD34 were brown and located in the cell membranes. The positive staining cells were endothelial cells. Micro‐vessels are mostly found at the periphery of tumors. The positive cells were irregular and scattered through the tumor tissue. There were 29 positive expressions out of the 30 osteosarcoma cases, making the positive rate 96.7%, as compared to 16.7% in the control group. As shown in Table 1, there were significant differences between the two groups (χ2= 25.127, P < 0.01), expression of CD34 in osteosarcoma being higher than that in osteochondroma (Fig. 3a, b).
Figure 3.
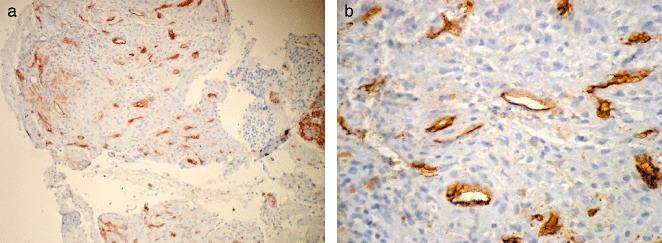
CD34 expression in osteosarcoma. The positive products of endostatin are brown and located in the cytoplasm and cell nuclei: (a), ×100; (b), ×400.
The relationships between b‐FGF, endostatin and clinical and pathologic parameters of osteosarcoma
The expressions of b‐FGF and CD34 in osteosarcoma were not correlated. There was no significant difference between the different groups (P > 0.05) in expressions of b‐FGF in tissue‐differentiation, tumor diameter, preoperative chemotherapy, age of patient, ALP, Ennecking stage, incidence of metastases and duration of postoperative survival. The expressions of endostatin and CD34 in osteosarcoma were positively correlated (P < 0.05), the correlation coefficient γs being 0.528. There was a significant difference between well and poorly differentiated carcinomas in expression of endostatin (P < 0.05), the expression of endostatin being greater in the low differentiation than in the high differentiation group. Expression of endostatin was correlated with osteosarcoma metastasis (P < 0.05). However, in regard to tumor diameter, pre‐operative chemotherapy, age of patient, ALP, Ennecking staging, incidence of metastases and duration of postoperative survival, there was no significant difference between the groups (P > 0.05) (Table 2).
Table 2.
The relationship of b‐FGF, endostatin and various clinical and pathologic parameters of osteosarcoma
| bFGF expression (cases) | Endostatin expression (cases) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | P values | − | + | ++ | +++ | P values | |
| Group | ||||||||||
| CD34 | ||||||||||
| − | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | ||
| + | 2 | 1 | 2 | 0 | 2 | 1 | 1 | 1 | ||
| ++ | 4 | 0 | 6 | 8 | 0 | 1 | 2 | 5 | ||
| +++ | 0 | 0 | 5 | 1 | 0.130 | 0 | 0 | 1 | 5 | 0.031 |
| Tissue differentiation | ||||||||||
| well differentiated | ||||||||||
| 4 | 1 | 3 | 2 | 2 | 2 | 5 | 1 | |||
| poorly differentiated | ||||||||||
| 2 | 0 | 10 | 8 | 0.054 | 0 | 0 | 10 | 10 | 0.004** | |
| Tumor diameter | ||||||||||
| ≥8 cm | 3 | 1 | 8 | 5 | 2 | 0 | 8 | 7 | ||
| <8 cm | 3 | 0 | 5 | 5 | 0.771 | 0 | 2 | 7 | 4 | 0.629 |
| Pre‐operative chemotherapy | ||||||||||
| Yes | 3 | 1 | 4 | 4 | 2 | 0 | 7 | 3 | ||
| No | 3 | 0 | 9 | 6 | 0.635 | 0 | 2 | 8 | 8 | 0.264 |
| Ages (years) | ||||||||||
| ≥16 | 4 | 1 | 10 | 10 | 2 | 2 | 13 | 8 | ||
| <16 | 2 | 0 | 3 | 0 | 0.090 | 0 | 0 | 2 | 3 | 0.188 |
| ALP | ||||||||||
| ≥150 u/l | 3 | 0 | 7 | 3 | 1 | 1 | 3 | 8 | ||
| <150 u/l | 3 | 1 | 6 | 7 | 0.460 | 1 | 1 | 12 | 3 | 0.069 |
| Enneking staging | ||||||||||
| Ia | 0 | 0 | 3 | 1 | 0 | 0 | 4 | 0 | ||
| Ib | 2 | 1 | 4 | 1 | 2 | 0 | 3 | 4 | ||
| IIa | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 1 | ||
| IIb | 3 | 0 | 4 | 5 | 0 | 0 | 5 | 6 | ||
| III | 1 | 0 | 1 | 1 | 0.302 | 0 | 2 | 1 | 0 | 0.610 |
| Metastasis | ||||||||||
| No | 5 | 1 | 12 | 9 | 2 | 0 | 14 | 1 | ||
| Yes | 1 | 0 | 1 | 1 | 0.796 | 0 | 2 | 1 | 0 | 0.036* |
| Postoperative survival | ||||||||||
| ≥1 year | 5 | 1 | 11 | 8 | 2 | 1 | 12 | 10 | ||
| <1 year | 1 | 0 | 2 | 2 | 0.766 | 0 | 1 | 3 | 1 | 0.426 |
P < 0.05;
P < 0.01.
The relationship between expressions of b‐FGF and endostatin in osteosarcoma
In this study, Wilcoxon's rank sum test was used to assess the relationship between b‐FGF and endostatin in the osteosarcoma specimens. No correlation between the groups was found (P > 0.05) (Table 3).
Table 3.
The relationship between expressions of b‐FGF and endostatin in osteosarcoma
| b‐FGF | |||||
|---|---|---|---|---|---|
| Endostatin | − | + | ++ | +++ | P values |
| − | 1 | 1 | 0 | 0 | |
| + | 1 | 0 | 1 | 0 | |
| ++ | 4 | 0 | 4 | 7 | |
| +++ | 0 | 0 | 8 | 3 | 0.182 |
Discussion
Expression of b‐FGF in osteosarcomas
Of the variety of factors secreted by tumors which have been identified as promoting tumor angiogenesis, b‐FGF (also known as FGF‐2), which induces endothelial cell proliferation, migration and capillary tube formation, was among the first to be characterized. The amount of b‐FGF is increased in patients with different cancers 11 , which shows that angiogenesis plays an important role in tumorigenesis. The multistep angiogenesis process can be divided into induction and resolution phases. The induction phase involves dynamic changes in endothelial cell‐cell and cell‐matrix interaction, which include degradation of the basement membrane, and proliferation, migration, and adhesion of endothelial cells. In the resolution phase, perivascular‐supporting cells are recruited, and a maturation process results in the assembly of fully functional new blood vessels 12 . All of these steps involve many growth factors, such as receptors, proteases, adhesion molecules, and ECM3 components. The fibroblast growth factor (FGF) family consists of at least 19 members, all being 18–30 kDa proteins with a strong affinity for heparin. Four different FGF receptors, all of which are tyrosine kinase receptors, have been identified 13 . B‐FGF can stimulate proliferation and migration of endothelial cells, alter their patterns of gene expression, increase microvascular permeability, cause extravasation of plasma proteins into the extravascular space, and also induce plasma‐derived matrix. B‐FGF plays a key role in tumor angiogenesis. It can induce neovascularization and is considered crucial in tumor biology 14 . Inhibition of b‐FGF decreases tumor growth in mice.
In their lung cancer study, Takanami et al. found that b‐FGF was correlated with microvessel density, histological grade and prognosis 15 . In the present data, 23 of 30 osteosarcoma cases showed positive expression of b‐FGF, the positive rate being 76.7%, while in the osteochondroma group it was 43.3%.Thus expression of b‐FGF in osteosarcoma was greater than in osteochondroma (P < 0.01). Because b‐FGF expression in osteosarcoma and osteosarcoma tissue is closely related to angiogenesis and b‐FGF is both an important factor in the occurrence and development of osteosarcoma and the most important factor on inducing angiogenesis, these findings suggest that occurrence and development of osteosarcoma occurs through promotion of tumor angiogenesis.
Expression of endostatin in osteosarcomas
The process of angiogenesis is regulated by pro‐angiogenic factors rather than by a balance between pro‐ and anti‐angiogenic factors. Tumors are capable of the up‐down regulation of these factors, producing an environment in which new blood vessels can form (neo‐angiogenesis) and tumors can grow. Over the past decade, appreciation for the dependence of the growth of solid tumors on angiogenesis has increased. This recognition has led to the recent approach of using inhibitors of angiogenesis in the treatment of patients with cancers 16 . In addition to producing pro‐angiogenic cytokines, there is evidence that tumors also play a role in the generation of anti‐angiogenic proteins, one of the endogenous anti‐angiogenic factors being endostatin. Endostatin is a 20‐kD C‐terminal fragment of collagen XVIII, which was initially isolated from a murine hemangioendothelioma supernatant by O'Reilly et al. in 1997 17 . Systematic analysis in a variety of cell‐based angiogenesis assays revealed for the first time that endostatin inhibits a number of angiogenic processes, including proliferation, migration, tube formation, and endothelial cell adhesion 18 . Those effects were not observed in non‐endothelial cells. In preclinical animal efficacy models, administration of endostatin can lead to stable tumor dormancy without the development of acquired drug resistance. Currently, endostatin is being evaluated in clinical trials for a variety of human malignancies. The generation of endostatin by human tumors appears to depend on tumor histology. It has been shown that serum concentrations of endostatin are increased in patients with renal cell carcinoma 19 . In the present experiment, there were 23 positive expressions of endostatin out of 30 osteosarcoma cases, the positive rate being 86.7%, while it was only 40.0% in the osteochondroma group. These differences between the groups are significant (P < 0.01), the expression of the vascular inhibitory factor, endostatin, in osteosarcoma being greater than that in osteochondroma, suggesting that the occurrence of osteosarcomas is related to endostatin.
Interestingly, in this study it was also found that the amounts of endostatin were significantly decreased in patients with metastatic disease compared with those with localized disease, which may have significant implications in regard to the application of anti‐angiogenic therapies. Thirty patients were included in the present study. Analysis revealed that expression of endostatin is correlated with osteosarcoma metastasis (P < 0.05), lung metastases being more frequent in tumors with little endostatin expression than in those with strong expression. There are several possible explanations, as follows. Firstly, primary tumors may generate more endostatin than metastatic tumors. The only patients with metastases to have increased amounts of endostatin were those with local tumors as well as distant metastases. Secondly, amounts of endostatin may be directly correlated with gross volume of disease, and the patients in this study with metastases generally had a lower tumor burden. Lastly, primary sarcomas that generate high levels of endostatin may inhibit the growth of distant micrometastases, as described in animal models 20 . However, the last point is thus far only of scientific interest, as clinical trials of endostatin have not yet demonstrated significant efficacy against distant metastases 21 , 22 .
The relationship of b‐FGF and endostatin with osteosarcoma and MVD
It was suggested and demonstrated unequivocally by Folkman in the early 1970s that solid tumors can recruit new blood vessels through the elaboration of pro‐angiogenic factors 23 . Further studies have revealed that tumors cannot grow beyond a size of 2–3 mm3 in the absence of angiogenesis 24 . It is noteworthy that tumors are also capable of generating factors which inhibit angiogenesis. This phenomenon has been implicated as an explanation for the clinical observation that resection of primary tumors can lead to the rapid growth of distant micrometastatic disease 17 , support for this hypothesis having come from several murine models. Recent data have suggested that the suppressive effect of primary tumors on angiogenesis within metastases is due to the longer half‐life of anti‐angiogenic molecules than of pro‐angiogenic cytokines in the circulation 25 . Intratumoral MVD, which is commonly used to assess angiogenic activity, is a semiquantitative surrogate measure 26 . The microvessels are immunohistochemically stained using endothelial cell‐specific antibodies, such as anti‐CD34 27 . Defining and measuring MVD in residual tumor cells might help with assessing the blood supply of residual tumor tissue and indirectly allow a certain extent of prognostication on the activity of these cells. There is a close correlation between MVD and the growth and metastasis of prostate, bladder and colon cancers. The immune product of CD34 is brown and located on cell membranes. The positive staining cells are endothelial cells. In the present experiment, there were 29 positive expressions out of 30 osteosarcomas cases, the positive rate being 96.7%, while it was only 16.7% in the osteochondroma group. There were significant differences between the two groups (P < 0.01), the expression of CD34 in osteosarcoma being greater than that in osteochondroma. Angiogenesis is an inherent property of tumors, being important in their development and progression processes. Among the solid tumors, the degree of tumor angiogenesis is closely related to biological behaviors, correlating with their ability to grow, infiltrate, recur and metastasize. It has also been suggested that the anti‐angiogenesis method being adopted to treat osteosarcoma may well be an effective neo‐adjuvant therapy.
The expression of b‐FGF in osteosarcoma is greater than that in osteochondroma, which shows that the occurrence and development of osteosarcoma is correlated with b‐FGF promoting tumor angiogenesis. B‐FGF is one of the most important angiogenic factors. There is no correlation between the expressions of b‐FGF and CD34 in osteosarcoma (P > 0.05), which also means that there is no obvious correlation between b‐FGF and MVD. Unfortunately, there are still insufficient cases to verify this result.
In the osteosarcoma group, expression of endostatin was positively correlated with that of CD34 (P < 0.05), the correlation coefficient being γs = 0.528. There were significant differences between expressions of endostatin in the poorly and well differentiated groups. Expression of endostatin in the poorly differentiated group was much greater than that in the well differentiated one (P < 0.05), which suggests a positive correlation of endostatin expression with osteosarcoma metastasis. There is also a correlation between endostatin expression and osteosarcoma tumor (P < 0.05), which may provide a certain auxiliary reference for assessing clinical prognosis. The present research shows that there is a close correlation between endostatin expression and MVD in osteosarcoma tissue. Lung metastases increase obviously with decrease in endostatin expression in osteosarcomas, which suggests that endostatin plays a very important role in the process of metastasis of osteosarcoma. With the increased degree of expression of endostatin protein, the value of MVD did not appear to decrease, which may be due to insufficient cases, the strong expression of b‐FGF, or some other factor. This study shows that there is no significant correlation between expressions of endostatin and b‐FGF protein, and endostatin and b‐FGF play roles from different angles in the complex process of angiogenesis. Their specific mechanisms require further discussion.
Conclusion
Tumor angiogenesis, a highly complex process involving precise communication between tumors cells and their host organ or tissue, is regulated by a wide variety of factors. Studies of such issues may give us a deeper insight into the subject of how angiogenesis inhibitors could be utilized as anticancer agents. In the present study, the angiogenic processes of osteosarcoma was examined by measuring the amounts of two different angiogenic factors, endostatin and b‐FGF, in samples of osteogenic sarcoma tissue. It was found that the amount of endostatin appears to be correlated with tumor aggressiveness, and that the amount of endostatin is significantly less in patients with metastases than in those without them. Further research will be carried out to elucidate the complex biology of tumor angiogenesis and to help define rational approaches that could opposet or slow down the growth of osteosarcoma by blocking the formation of new blood vessels.
Disclosure
The authors did not receive any outside funding or grants in support of their research for, or preparation of, this work.
References
- 1. Tsunemi T, Nagoya S, Kaya M, et al Postoperative progression of pulmonary metastasis in osteosarcoma. Clin Orthop Relat Res, 2003, 407: 159–166. [DOI] [PubMed] [Google Scholar]
- 2. Kreuter M, Bieker R, Bielack SS, et al Prognostic relevance of increased angiogenesis in osteosarcoma. Clin Cancer Res, 2004, 10: 8531–8537. [DOI] [PubMed] [Google Scholar]
- 3. Mikulić D, Ilić I, Cepulić M, et al Tumor angiogenesis and outcome in osteosarcoma. Pediatr Hematol Oncol, 2004, 21: 611–619. [DOI] [PubMed] [Google Scholar]
- 4. Lee YH, Tokunaga T, Oshika Y, et al Cell‐retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur J Cancer, 1999, 35: 1089–1093. [DOI] [PubMed] [Google Scholar]
- 5. Mantadakis E, Kim G, Reisch J, et al Lack of prognostic significance of intratumoral angiogenesis in nonmetastatic osteosarcoma. J Pediatr Hematol Oncol, 2001, 23: 286–289. [DOI] [PubMed] [Google Scholar]
- 6. Balasubramanian L, Evens AM. Targeting angiogenesis for the treatment of sarcoma. Curr Opin Oncol, 2006, 18: 354–359. [DOI] [PubMed] [Google Scholar]
- 7. Heymach JV. Angiogenesis and antiangiogenic approaches to sarcomas. Curr Opin Oncol, 2001, 13: 261–269. [DOI] [PubMed] [Google Scholar]
- 8. Ribatti D, Vacca A, Presta M. The discovery of angiogenic factors: A historical review. Gen Pharmacol, 2000, 35: 227–231. [DOI] [PubMed] [Google Scholar]
- 9. Linder C, Linder S, Munck‐Wikland E, et al Independent expression of serum vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in patients with carcinoma and sarcoma. Anticancer Res, 1998, 18: 2063–2068. [PubMed] [Google Scholar]
- 10. Folkman J. Antiangiogenesis in cancer therapy—Endostatin and its mechanisms of action. Exp Cell Res, 2006, 312: 594–607. [DOI] [PubMed] [Google Scholar]
- 11. Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol, 2001, 19: 1207–1225. [DOI] [PubMed] [Google Scholar]
- 12. Dhanabal M, LaRochelle WJ, Jeffers M, et al Angioarrestin: An antiangiogenic protein with tumor‐inhibiting properties. Cancer Res, 2002, 1: 3834–3841. [PubMed] [Google Scholar]
- 13. Cross MJ, Claesson‐Welsh L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci, 2001, 22: 201–207. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura T, Ozawa S, Kitagawa Y, et al Expression of basic fibroblast growth factor is associated with a good outcome in patients with squamous cell carcinoma of the esophagus. Oncol Rep, 2005, 14: 617–623. [PubMed] [Google Scholar]
- 15. Takanami I, Tanaka F, Hashizume T, et al Tumor angiogenesis in pulmonary adenocarcinomas: Relationship with basic fibroblast growth factor, its receptor, and survival. Neoplasma, 1997, 44: 295–298. [PubMed] [Google Scholar]
- 16. Talks KL, Harris AL. Current status of antiangiogenic factors. Br J Haematol, 2000, 109: 477–489. [DOI] [PubMed] [Google Scholar]
- 17. O'Reilly MS, Boehm T, Shing Y, et al Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell, 1997, 88: 277–285. [DOI] [PubMed] [Google Scholar]
- 18. Kruger EA, Duray PH, Tsokos MG, et al Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem Biophys Res Commun, 2000, 268: 183–191. [DOI] [PubMed] [Google Scholar]
- 19. Feldman AL, Tamarkin L, Paciotti GF, et al Serum endostatin levels are elevated and correlate with serum vascular endothelial growth factor levels in patients with stage IV clear cell renal cancer. Clin Cancer Res, 2000, 6: 4628–4634. [PubMed] [Google Scholar]
- 20. Yoon SS, Eto H, Lin CM, et al Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res, 1999, 59: 6251–6256. [PubMed] [Google Scholar]
- 21. Eder JP Jr, Supko JG, Clark JW, et al Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol, 2002, 20: 3772–3784. [DOI] [PubMed] [Google Scholar]
- 22. Herbst RS, Hess KR, Tran HT, et al Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol, 2002, 20: 3792–3803. [DOI] [PubMed] [Google Scholar]
- 23. Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med, 1971, 285: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 24. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst, 1990, 82: 4–6. [DOI] [PubMed] [Google Scholar]
- 25. Ramanujan S, Koenig GC, Padera TP, et al Local imbalance of proangiogenic and antiangiogenic factors: A potential mechanism of focal necrosis and dormancy in tumors. Cancer Res, 2000, 60: 1442–1448. [PubMed] [Google Scholar]
- 26. Rege TA, Fears CY, Gladson CL. Endogenous inhibitors of angiogenesis in malignant gliomas: Nature's antiangiogenic therapy. Neuro Oncol, 2005, 7: 106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liekens S, De Clercq E, Neyts J. Angiogenesis: Regulators and clinical applications. Biochem Pharmacol, 2001, 61: 253–270. [DOI] [PubMed] [Google Scholar]


