Abstract
Many different parameters exist for the investigation of tear film dynamics. We present a new tear meniscus segmentation algorithm which automatically extracts tear meniscus area (TMA), height (TMH), depth (TMD) and radius (TMR) from UHR-OCT measurements and apply it to a data set including repeated measurements from ten healthy subjects. Mean values and standard deviations are 0.0174 ± 0.007 mm2, 0.272 ± 0.069 mm, 0.191 ± 0.049 mm and 0.309 ± 0.123 mm for TMA, TMH, TMD and TMR, respectively. A significant correlation was found between all respective tear meniscus parameter pairs (all p < 0.001, all Pearson’s r ≥ 0.657). Challenges, limitations and potential improvements related to the data acquisition and the algorithm itself are discussed. The automatic segmentation of tear meniscus measurements acquired with UHR-OCT might help in a clinical setting to further understand the tear film and related medical conditions like dry eye disease.
1. Introduction
Dry eye disease (DED) is a highly prevalent [1,2] disease of the tears and ocular surface, yet remains not fully understood due to its complex and multifactorial aspects. Generally it can be divided into aqueous-deficient dry eye (ADDE) and evaporative dry eye (EDE), according to the different causes of the disease. In both cases, the precorneal tear film plays an essential role, often characterized by instability and a loss of homeostasis [3]. The tear film provides protection, nutrition and lubrication for the ocular surface as well as a smooth surface for the optics of the eye and is composed of four layers [4]: the glycocalyx, the mucous layer, the aqueous layer and the lipid layer, which is the outermost layer and stabilizes the tear film while preventing loss of aqueous layer by evaporation. Due to its extremely thin nature, measuring 4-6 µm [5,6] in central thickness in healthy subjects, its visualization and quantification are quite challenging. Past studies have primarily looked into tear related parameters that are more easily accessible than tear film thickness (TFT) to evaluate the tear film and its associated diseases. Schirmer I test [7,8] and tear film breakup time (TBUT) [7,9] are clinical tests to assess the tear quantity and tear film stability respectively. However, these methods give only an indirect measure of the integrity of the tear film and suffer from limited reproducibility [10]. The tear meniscus, the concave surface of tear fluid situated at the upper and lower lid margins, is of equal interest. Here, various tear meniscus parameters like tear meniscus volume (TMV) [11], depth (TMD) [12,13], area (TMA) [11,14,15], height (TMH) [16,17] and curvature/radius (TMR) [18] have been measured with a variety of devices and methods, including optical coherence tomography (OCT). Optical coherence tomography is a non-invasive imaging modality based on interferometric principles that has revolutionized ophthalmologic diagnostics. Its high resolution and non-invasive nature made it an important instrument in retinal and corneal imaging [19,20]. With the advances in light source technology it became possible to directly visualize the tear film with OCT [21–23]. Employing an ultrahigh-resolution (UHR-)OCT system based on a Ti:Sapphire laser [6,24], we achieved an axial resolution of approximately 1.2 µm in tissue, allowing not only to image the tear meniscus, but also to fully resolve and measure the thickness of the much thinner precorneal tear film. The lipid layer, whose thickness range is far below the resolution limit of OCT [25,26], can be visualized and quantified using white light interferometry, a technology that has lately been implemented in a commercially available system. Recently, an approach using the OCT reflectance in UHR-OCT measurements was used to visualize the tear film lipid layer [27]. Considerable efforts have been made in the past to study the above mentioned parameters and find possible correlations between them in order to better understand tear film dynamics and to better characterize diseases like DED [28,29].
We present a fully-automated algorithm for the segmentation of the tear meniscus parameters TMA, TMH, TMD and TMR. In complement to our already existing TFT estimation algorithm, we tested the new algorithm on an UHR-OCT data set of the anterior eye of ten healthy subjects, for which lipid layer thickness (LLT), Schirmer I test and fluorescein breakup time (FBUT) were also measured. While tear meniscus parameters and TFT have been studied in the past [22], to the best of our knowledge, it is the first time that these parameters are assessed with an UHR-OCT and automatic segmentation.
2. Material and methods
2.1 Subjects
Measurements in ten eyes of ten healthy subjects (5 female, 5 male, age 31 ± 10 years) with no signs of DED were included in the study. The study protocol was approved by the Ethics Committee of the Medical University of Vienna and the study was performed in adherence to the guidelines of the Declaration of Helsinki as well as Good Clinical Practice guidelines. All participants gave their written informed consent and were instructed not to use any eye drops 24 hours prior to the study day.
2.2 Measurement systems
Measurements for the assessment of the precorneal tear film, tear meniscus and tear film lipid layer were performed with two different systems. Volumetric OCT data of the precorneal tear film and the tear meniscus was acquired using a custom-built UHR-OCT system, described in detail elsewhere [6,24]. The system is based on a Ti:Sapphire laser with a central wavelength of 800 nm as light source, providing an axial resolution of approximately 1.2 µm in tissue. The lateral resolution, given by the scanning optics and the probe beam geometry, is 21 µm. Using a telecentric scanning configuration, measurements from this system provide TFT, TMA, TMH, TMD and TMR values.
In addition, LLT measurements based on white light interferometry were performed non-invasively with a commercially available LipiView II Ocular Surface Interferometer system (Tear Science Inc, NC, USA). The device analyses the optical interference pattern produced by the reflected light and estimates the LLT based on the dominantly reflected colors [25,30].
2.3 Data acquisition
One measurement cycle consisted of two measurements per eye performed with the UHR-OCT system (tear film and lower tear meniscus) and one measurement per eye using the LipiView II system. The timeline and duration of the measurement cycle were explained to the subjects before the experiment. Subjects were advised to blink normally during the alignment of the UHR-OCT. After finalization of this procedure, which took five to ten seconds, subjects were asked to blink once and keep the eye open thereafter. The measurement then started immediately after this blink. Seven measurement cycles were completed at ten minute intervals leading to a total study period of one hour. After the last imaging cycle, FBUT was measured and a Schirmer I test was performed.
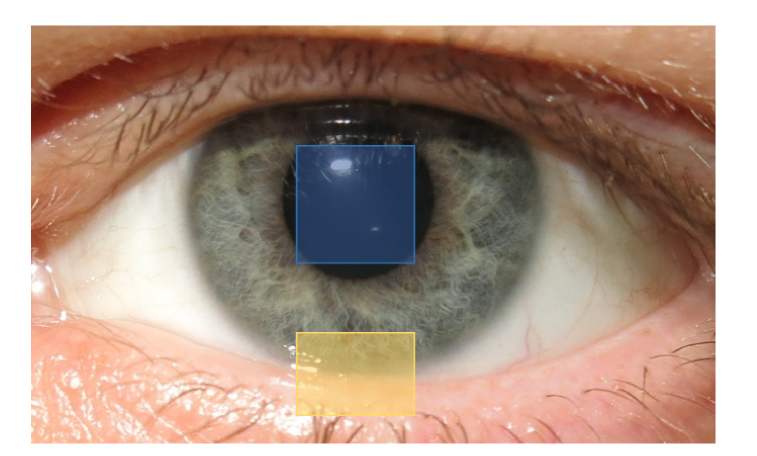
OCT data from the central cornea for the assessment of the TFT comprises three volumes with a size of 4x4x1.5 mm3 (height x width x depth, Fig. 1) and 256x256x2048 voxels each. Tear meniscus measurements were acquired at the center of the lower eyelid over an area of 2.9x4 mm2 (height x width, Fig. 1). A single measurement consisted of three volumes with 128 cross-sectional images (B-scans) each, resulting in a total of 384 images. The total acquisition time for each measurement was about three seconds.
Fig. 1.
Regions of measurement for central tear film thickness (blue square) and lower tear meniscus parameters (yellow rectangle).
TFT and tear meniscus parameters were extracted from the UHR-OCT data sets with custom algorithms written in Matlab (Matlab R2017a, The MathWorks, Inc., Natick, MA, USA). The LipiView II system provided LLT values as an average over five seconds of measurement time.
2.4 Custom algorithm for tear meniscus parameter estimation
In the following paragraphs, the tear meniscus segmentation algorithm will be described in detail. The custom algorithm provides an estimate of the four parameters TMA, TMD, TMH and TMR while handling image artifacts, e.g. due to camera saturation, floating debris and different meniscus morphologies.
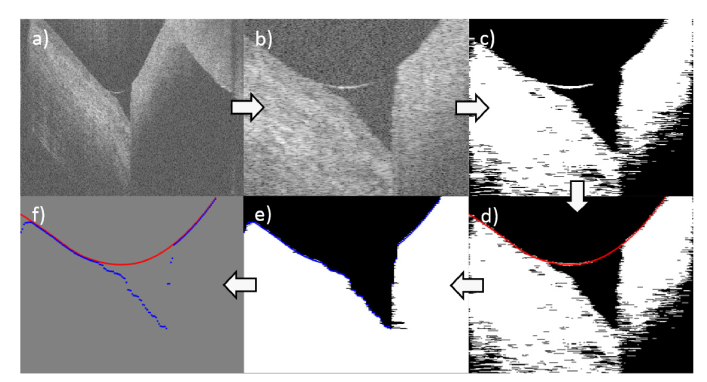
The initial B-scan is zero-padded in order to increase the pixel resolution. The region of interest (ROI) is detected in this 8192x256 pixel (depth x width) B-scan (Fig. 2(a)) using cross-correlation with a reference meniscus image. The B-scan is then cropped to the ROI with a size of 3000x130 pixels (Fig. 2(b)). These dimensions have been chosen empirically, leaving enough room for larger menisci, i.e. as consequence of topical applications, while restricting the area to the main structures of interest, simplifying automatic segmentation. Only images which, in the ROI, have less than 10% of A-scans with artifacts due to camera saturation are then used for further processing. In this case, the saturation affected A-scans are not used for the later described boundary detection, but their boundary positions are interpolated from neighboring boundary values. In the case of more than 10% affected A-scans, the image is discarded, since the tear meniscus boundaries usually cannot be detected with a high enough level of confidence when there are too many saturation artifacts.
Fig. 2.
Step-by-step illustration of the tear meniscus segmentation algorithm. (a) Initial measurement. Following images are after (b) detection of ROI, (c) Otsu’s thresholding, (d) first interface detection (red), (e) meniscus removal, islet removal, filling and second interface detection (blue). (f) Combination of both interfaces for parameter estimation (Fig. 3).
In a first step, the implemented algorithm uses Otsu’s method to obtain a single intensity threshold value [31]. The resulting binary image undergoes islet removal below 50 pixels, removing minor artifacts and floating debris without removing a possibly faint upper tear meniscus limit, as well as median filtering with a 7x7 window (Fig. 2(c)). The latter shows, in the current application, a better behavior than the usual opening and closing applied to binary images. The resulting image is then used to detect the upper boundary (Fig. 2(d)) by finding the first non-zero pixel from the top in each A-scan. If the pixel is followed (in axial direction) by more than 200 non-zero pixels, like it is the case for tissue, it is selected for the upper boundary. Alternatively, if there is a zero-value pixel within the next 200 pixels, like it is the case for the tear meniscus, then the pixel in the middle of this non-zero segment, representing the air-tear interface, is selected. Missing or erroneous points of the upper boundary, e.g. caused by remaining saturation artifacts, which strongly influence the thresholding, are corrected by means of a Hampel filter followed by a moving average filter. This also fills gaps in the upper tear meniscus boundary due to a weak reflectivity.
In the following step, the upper boundary is removed from the binary image by setting the upper boundary pixels and the surrounding pixels, which are considered part of the boundary, to zero. Additionally, islets with an area of less than 1000 pixels are removed in order to erase larger debris. Subsequently, the left, bottom and right border pixels are set to one and the image is flood-filled to close the tissue region and possible non-zero pixels therein. After this filling operation, the lower boundary is identified A-scan-wise by detecting the first non-zero pixel that is followed by a minimum amount of 200 non-zero pixels (Fig. 2(e)). This prevents most floating debris in the tear meniscus, which have not been removed with the islet filter, to falsify the detection of the lower boundary. The algorithm identifies the edge of the tear meniscus by starting from the biggest axial difference in pixels between the boundaries and going left and right from there, until the axial difference in pixels between the boundaries is below 30 pixels. Finally, the region of the two boundaries between those edges is used to estimate the different tear meniscus parameters, as described in section 2.5.
The segmentation of one B-scan, not including the preprocessing of the spectral data, takes 1.6 seconds on average.
2.5 Definition of parameters
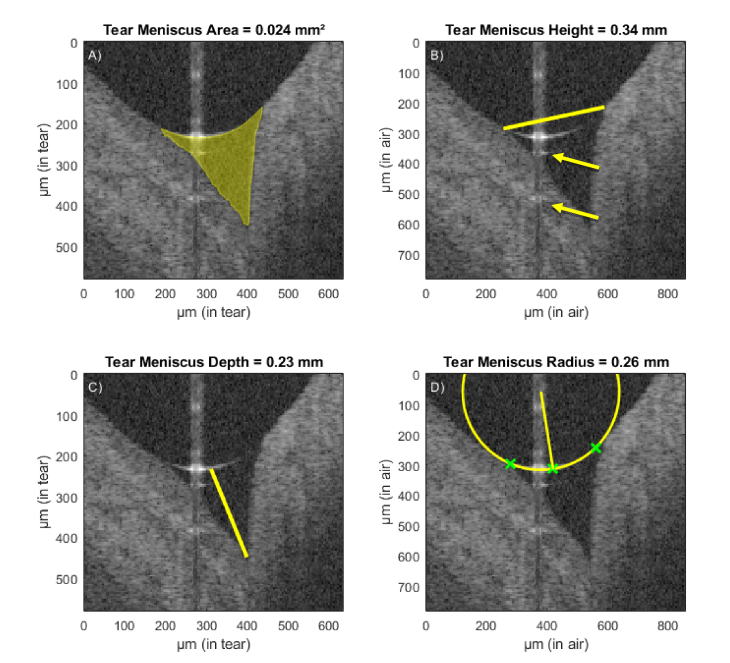
Tear meniscus parameters
The median value of a particular tear meniscus parameter yielded from all 384 cross-sectional images (excluding those with too many artifacts due to camera saturation) is considered the representative value for that measurement. The TMA is given in mm2 (Fig. 3(A)). Each pixel between the upper and lower boundary of the meniscus is counted. The geometrical meniscus area is calculated from the total amount of pixels, accounting for refraction of the probe beam and the group index of the tear fluid at the central wavelength of the used light source (Ng = 1.341 [24]). The TMH (Fig. 3(B)) is defined as the distance in mm between the two outermost points of the tear meniscus, where the curved tear surface joins the surrounding tissue. The TMD (Fig. 3(C)) is defined as the distance between the center of the upper boundary of the tear meniscus and the point at the bottom boundary where the cornea meets the eyelid. The TMR (Fig. 3(D)) is estimated by taking three points on the upper tear meniscus boundary and calculating the circumradius of the resulting triangle. Two of the selected points are close to the edges while the third one is at the center of the boundary.
Fig. 3.
Automatic segmentation of the lower tear meniscus in a healthy subject. Exemplary image with light camera saturation in the center region. Calculated parameters (represented in yellow) are (A) tear meniscus area, (B) height, (C) depth and (D) radius of curvature. Green crosses represent the points used for the estimation of the radius of curvature. The yellow arrows indicate mirror artifacts of the true upper meniscus boundary due to internal reflectors in the optical setup of the system.
Tear film thickness
For each of the three acquired volumes, the TFT is extracted from the 15 B-scans that are situated above the specular reflection at the apex of the cornea. The mean of these values is then taken as a measure for the central TFT value of the corresponding volume. Since the tear film still builds up and shifts immediately after the blink, only TFT values from volume 2 and 3 are considered for the final mean TFT value.
Lipid layer thickness
Lipid layer thickness values are provided by the LipiView II system based on white light interferometry. The measurement area is situated below the pupil and about 1 mm above the inferior tear meniscus in the lower third of the cornea.
Schirmer I test
Schirmer I test was performed following the guidelines published in the Report of the International Dry Eye WorkShop (DEWS) 2007 [32]. In brief, the Schirmer paper strips were inserted midway between the middle and outer third of the lower conjunctival sac of the unanaesthetized eye. The subjects were asked to close their eye and the paper strip was removed after five minutes. The Schirmer I test value represents the wetted distance of the Schirmer paper after this time period.
Fluorescein breakup time
FBUT, as an indicator for tear film stability, was measured following the guidelines published in the Report of the International Dry Eye WorkShop (DEWS) 2007 [32] and is considered a representative value for the TBUT. Briefly, Minims-Fluorescein Sodium 2.0% eye drops were applied into the conjunctival sac of the eye. For the fluorescein to distribute evenly, the subjects were asked to blink several times naturally and without squeezing of the eyelids. Within 10–30 s after fluorescein instillation, the subject was instructed to keep its eyes open without blinking, until told otherwise. The time between the last complete blink and the first appearance of a dry spot on the tear film was recorded. Once a break-up of the tear film was observed, the subject was instructed to blink naturally again.
2.6 Statistical analysis
Statistical analysis was performed in SPSS (IBM SPSS Statistics Version 25.0, NY, USA). Mean values and standard deviations were computed. Partial correlations between the parameters were analyzed using Pearson’s correlation coefficient r. A p-value <0.05 was considered significant. Intraclass correlation coefficients (ICC) and their 95% confidence intervals were calculated based on single-measures and average-measures (k = 7), absolute-agreement, 2-way mixed-effects models.
3. Results
The results of the evaluation of the different tear parameters in ten healthy subjects and the comparison to literature findings are given in Table 1. Mean values for TFT and LLT were 4.17 ± 0.50 µm and 63.8 ± 9.96 nm respectively. For the tear meniscus parameters, mean values were 0.0174 ± 0.007 mm2, 0.272 ± 0.069 mm, 0.191 ± 0.049 mm and 0.309 ± 0.123 mm for TMA, TMH, TMD and TMR, respectively. The mean value for Schirmer I test was 25.1 ± 12.2 mm and for FBUT 8.8 ± 2.4 seconds.
Table 1. Tear parameters in healthy subjects. Mean value and standard deviation (SD) over all time points of the investigated parameters in comparison to literature values in healthy subjects.
| Parameter | Mean ± SD | Literature values |
|---|---|---|
| Tear film thickness - TFT (µm) | 4.17 ± 0.50 | 3.4 ± 2.6 [33] 4.4 ± 0.97 (3 subjects) [34] 4.79 ± 0.88 [6] 6.0 ± 2.4 [5] |
|
| ||
| Lipid layer thickness - LLT (nm) | 63.8 ± 9.96 | 62.4 ± 14.5 (3 subjects) [34] 68.3 ± 13.7 [35] |
|
| ||
| Tear meniscus area - TMA (mm2) | 0.0174 ± 0.007 | 0.02 ± 0.001 (male) [36] 0.02 ± 0.03 (female) [36] 0.022 ± 0.008 [11] 0.031 ± 0.012 [13] 0.048 (median) [15] |
|
| ||
| Tear meniscus height - TMH (mm) | 0.272 ± 0.069 | 0.22 ± 0.065 [17] 0.234 ± 0.071 (male) [36] 0.256 ± 0.057 [11] 0.262 ± 0.122 (female) [36] 0.291 ± 0.062 [13] 0.30 (median) [37] 0.316 (median) [15] |
|
| ||
| Tear meniscus depth - TMD (mm) | 0.191 ± 0.049 | 0.185 ± 0.042 [38] 0.188 ± 0.057 [39] 0.22 ± 0.065 [13] |
|
| ||
| Tear meniscus radius - TMR (mm) | 0.309 ± 0.123 | 0.29 ± 0.09 [40] 0.33 ± 0.08 [41] 0.545 ± 0.259 [28] |
|
| ||
| Schirmer I test (mm) | 25.1 ± 12.2 | 15.5 ± 8.7 [42] |
|
| ||
| Fluorescein breakup time - FBUT (s) | 8.8 ± 2.4 | 7.6 ± 10.4 [43] 9.1 ± 3.51 [42] |
Partial correlation coefficients were computed for TFT, TMA, TMH, TMD, TMR and LLT (Table 2). This includes data from every time point which is adjusted for repeated measurements in the same subject. For the correlation of Schirmer I test and FBUT with other tear parameters, only the value of the last time point is used, since this value is the closest in time to the measurement of these two parameters (Table 3).
Table 2. Partial correlation coefficients and p-values of the investigated parameters over all eyes before correction for multiple testing.
| Parameter | TFT | TMA | TMH | TMD | TMR | LLT | |
|---|---|---|---|---|---|---|---|
| TFT | Pearson’s r | - | −0.143 | −0.065 | −0.126 | −0.130 | 0.106 |
| p-value | - | 0.271 | 0.618 | 0.331 | 0.317 | 0.415 | |
| TMA | Pearson’s r | - | - | 0.873a | 0.937a | 0.700a | −0.056 |
| p-value | - | - | < 0.001 | < 0.001 | < 0.001 | 0.669 | |
| TMH | Pearson’s r | - | - | - | 0.838a | 0.657a | −0.085 |
| p-value | - | - | - | < 0.001 | < 0.001 | 0.516 | |
| TMD | Pearson’s r | - | - | - | - | 0.770a | 0.014 |
| p-value | - | - | - | - | < 0.001 | 0.913 | |
| TMR | Pearson’s r | - | - | - | - | - | −0.138 |
| p-value | - | - | - | - | - | 0.290 |
Significant correlation
Table 3. Correlation coefficients and p-values of the investigated parameters over all eyes before correction for multiple testing.
| Parameter | TFT | TMA | TMH | TMD | TMR | LLT | Schirmer | FBUT | |
|---|---|---|---|---|---|---|---|---|---|
| Schirmer | Pearson’s r | 0.095 | −0.355 | −0,391 | −0.255 | −0.339 | −0.006 | - | −0.087 |
| p-value | 0.793 | 0.314 | 0.264 | 0.477 | 0.338 | 0.986 | - | 0.811 | |
| FBUT | Pearson’s r | 0.052 | 0.225 | 0.225 | 0.187 | 0.147 | 0.008 | −0.087 | - |
| p-value | 0.887 | 0.532 | 0.532 | 0.605 | 0.685 | 0.982 | 0.811 | - |
The significance values are given before correction for multiple testing. After application of a Holm-Bonferroni correction, a significant correlation remains between each respective pair of TMA, TMH, TMD and TMR (all p < 0.001, all r ≥ 0.657). No significant correlation was found between any of the tear meniscus parameters and TFT.
In Table 4, ICCs calculated based on single-measures and average-measures (k = 7), absolute-agreement, 2-way mixed-effects models are given.
Table 4. Intraclass correlation coefficients and 95% confidence intervals for the tear meniscus parameters based on single-measures and average-measures (k = 7), absolute-agreement, 2-way mixed-effects models.
| Parameter | Measures | ICC | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| TMA | Single | 0.608 | 0.366 | 0.851 |
| Average | 0.916 | 0.801 | 0.976 | |
| TMH | Single | 0.676 | 0.446 | 0.883 |
| Average | 0.936 | 0.850 | 0.981 | |
| TMD | Single | 0.659 | 0.425 | 0.875 |
| Average | 0.931 | 0.838 | 0.980 | |
| TMR | Single | 0.545 | 0.301 | 0.818 |
| Average | 0.894 | 0.751 | 0.969 | |
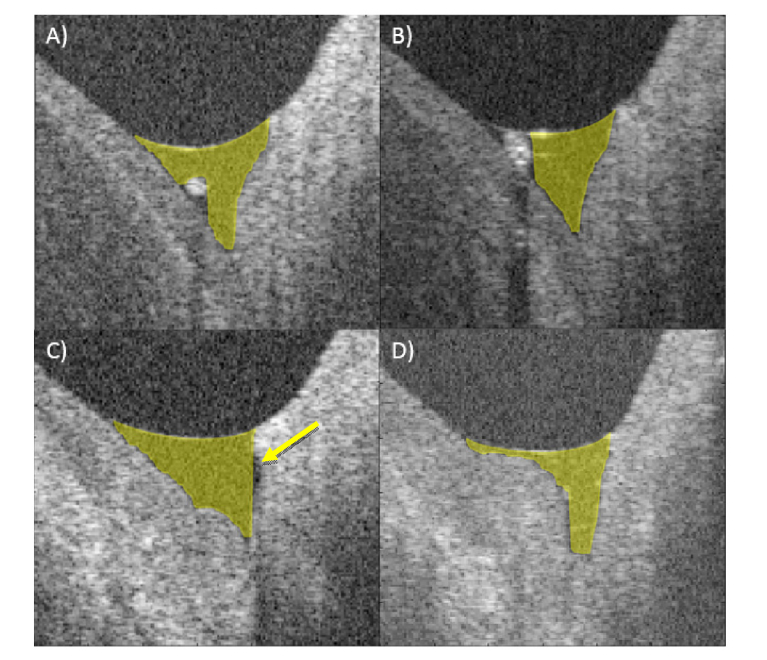
In Fig. 4, four particular segmentation cases are presented. They show the estimation of the TMA in the case of small and large debris in the tear meniscus, the limitation of the segmentation in the case of a cavity-like meniscus structure as well as the behavior of the algorithm in the case of an irregular meniscus shape.
Fig. 4.
Exemplary cross-sectional images with particular segmentation cases. (A) Small debris, (B) larger debris cutting the tear meniscus area in two parts, (C) not segmented cavity (yellow arrow) and (D) irregular meniscus anatomy.
4. Discussion
We presented a new custom algorithm for the segmentation of the tear meniscus and estimation of four tear meniscus parameters without operator input. The software handles artifacts due to camera saturation as well as irregular anatomical shapes in a large array of cases. The algorithm was applied to a study data set and mean values and parameter correlations were investigated. The amount of images acquired during this study provided a large enough test data set to evaluate the segmentation thoroughly. The mean values are in good agreement with literature values, which is a first positive indication for the plausibility of the segmentation results. As second indicator, the single-measures ICCs were evaluated. These represent the similarity of the obtained parameter values within the subjects over the full measurement duration of one hour. These values encompass the possible variation of the segmentation algorithm as well as any intra-subject fluctuations over the given period and are slightly higher than previously published between-visit ICCs for TMA, TMD and TMH [12]. Average-measures ICCs are given for comparison and completeness. While these also evaluate the similarity, they consider an average of seven measurements for the basis of assessment instead of one single measurement, which usually should not be the case [44]. A third positive indicator for the reliability of the segmentation is the strong correlation between the different tear meniscus parameters TMA, TMH, TMD and TMR, that has also been reported in literature [11,14] and is primarily due to their geometric relationship. Considering these three findings, it can be stated with enough confidence that the algorithm provides good results.
The mean TFT value is in the same range as in our previously published work [6]. We did not, however, find a significant correlation between TFT and any of the tear meniscus parameters. Even though a mathematical model that predicts a relationship between TFT and TMR exists [45,46], we could not confirm the formula under the assumption that upper and lower TMR only differ slightly, which is controversial [22,47]. Past studies using OCT have also found that tear meniscus parameters are not predictive of TFT [22]. Hosaka et al. found a correlation between TFT and TMH but used a different estimation method and measured TFT only five seconds after a complete blink in their study [5].
Considering that the Schirmer I test measures the eye’s reaction to the inserted paper strip and evaluates the overall tear production, while tear meniscus parameters give a momentary value of the tear meniscus state, it is not evident that these values would correlate closely. While this lack of correlation has been identified before [48], it cannot be overlooked that larger studies have shown significant correlations between Schirmer values and TMH and TMA [14,49], although anesthesia or different measurement devices were used in these reports.
The measurement protocol consisted of three measurements in a single measurement cycle. Over the total time series, the repeated forced eye opening could have an effect on later time points and influence the tear meniscus parameters [50], although the effect should be very small in healthy subjects and with relatively short measurement times.
Image artifacts due to camera saturation (Fig. 3) as well as small debris (Fig. 4(A)) are handled well by the algorithm. Small debris is currently included in the area of the tear meniscus, leading to a very slight overestimation of the TMA in certain images. Large debris that fill out a considerable part of the tear meniscus are still a challenge, sometimes due to the lack of clear differentiation from the surrounding tissue or because they separate the tear meniscus in two parts (Fig. 4(B)). It also needs to be investigated if, in this case, the parameter of interest is the theoretically possible tear meniscus area or the actual occupied area of the tear fluid. The answer might greatly influence the estimation of the total tear volume [37]. In one particular subject, an anatomical cavity could be observed (Fig. 4(C)), which was not correctly segmented since the algorithm proceeds along the A-scan from the top and thus encounters tissue above the cavity. Given that the area difference is negligible over all images and that the case prevalence is rare, the handling of this particular case has a lower importance. In the same subject, an irregular meniscus anatomy was observed (Fig. 4(D)). Considering the impactful change on the overall tear meniscus shape of diseases like conjunctivochalasis, it might be possible to extend the algorithm in the future to automatically recognize the geometric change and identify these cases early, potentially helping with the monitoring of the disease [51].
On the hardware side, the investigation of the correlation between the different tear film and tear meniscus parameters would be more reliable with the simultaneous acquisition of the tear film and the tear meniscus in a single measurement [22]. Currently this is impeded by the limited depth range of the system and would require changes in the spectrometer setup as well as the scanning configuration. The algorithm could easily be adapted to these changes, since only a reliable detection of the initial ROI is required, while all further processing steps will be unaffected.
The Diagnostic Methodology report of the second Dry Eye Workshop of the Tear Film and Ocular Surface Society [10] stated that, while OCT meniscometry image acquisition is rapid and simple, the current disadvantage is the time consuming and operator-dependent processing of the image. The latter issue can be solved by means of automatic algorithms as presented in this manuscript, while the former challenge could be tackled by employing the results of these algorithms to train a neuronal network architecture [52], which is known to reduce the segmentation time manifold. It has to be noted that, for the present case, the optimization of the processing speed was not a priority during the development of the code. The automatization of the segmentation does not only eliminate the operator dependency, but can also minimize the possible instrument dependency [53,54].
In parallel to the aforementioned improvements, the next step should be to apply the algorithm to OCT data from larger scale studies, widening the range of encountered segmentation cases and also including the effect of topical lubricant applications [55,56] as well as patients with different stages and causes of DED [57,58]. Here, UHR-OCT and automatic segmentation of the tear fluid are promising tools for the further understanding of tear fluid dynamics and related diseases.
5. Conclusion
In this manuscript we presented a newly developed algorithm that extracts four major parameters from tear meniscus measurements without operator input. The algorithm was applied to measurements obtained from a study including ten healthy subjects and delivers results that are in good agreement with findings in the literature. Significant correlations were found between all tear meniscus parameters. In the future, the technology might further advance the understanding of the tear meniscus and tear film dynamics as well as tear film alterations in DED.
Acknowledgment
We would like to thank Dr. Alex Tumlinson and Dr. Ali Fard for fruitful discussions about dry eye topics.
Funding
Christian Doppler Research Association; Austrian Federal Ministry for Digital and Economic Affairs; National Foundation for Research, Technology and Development; Carl Zeiss Meditec Inc. as industrial partner of the Christian Doppler Laboratory for Ocular and Dermal Effects of Thiomers.
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Gayton J. L., “Etiology, prevalence, and treatment of dry eye disease,” Clin. Ophthalmol. 3, 405–412 (2009). 10.2147/OPTH.S5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrand K. F., Fridman M., Stillman I. O., Schaumberg D. A., “Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older,” Am. J. Ophthalmol. 182, 90–98 (2017). 10.1016/j.ajo.2017.06.033 [DOI] [PubMed] [Google Scholar]
- 3.Craig J. P., Nichols K. K., Akpek E. K., Caffery B., Dua H. S., Joo C. K., Liu Z., Nelson J. D., Nichols J. J., Tsubota K., Stapleton F., “TFOS DEWS II Definition and Classification Report,” Ocul. Surf. 15(3), 276–283 (2017). 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 4.Dartt D. A., “Formation and Function of the Tear Film,” in Adler's Physiology of the Eye, Levin L. A., Nilsson S. F. E., Hoeve J. V., Wu S. M., eds. (Saunders Elsevier, 2011). [Google Scholar]
- 5.Hosaka E., Kawamorita T., Ogasawara Y., Nakayama N., Uozato H., Shimizu K., Dogru M., Tsubota K., Goto E., “Interferometry in the evaluation of precorneal tear film thickness in dry eye,” Am. J. Ophthalmol. 151(1), 18–23 (2011). 10.1016/j.ajo.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 6.Werkmeister R. M., Alex A., Kaya S., Unterhuber A., Hofer B., Riedl J., Bronhagl M., Vietauer M., Schmidl D., Schmoll T., Garhöfer G., Drexler W., Leitgeb R. A., Groeschl M., Schmetterer L., “Measurement of tear film thickness using ultrahigh-resolution optical coherence tomography,” Invest. Ophthalmol. Vis. Sci. 54(8), 5578–5583 (2013). 10.1167/iovs.13-11920 [DOI] [PubMed] [Google Scholar]
- 7.Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H. M., Alexander E. L., Carsons S. E., Daniels T. E., Fox P. C., Fox R. I., Kassan S. S., Pillemer S. R., Talal N., Weisman M. H., European Study Group on Classification Criteria for Sjögren’s Syndrome , “Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group,” Ann. Rheum. Dis. 61(6), 554–558 (2002). 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucca J. A., Nunez J. N., Farris R. L., “A comparison of diagnostic tests for keratoconjunctivitis sicca: lactoplate, Schirmer, and tear osmolarity,” CLAO J. 16(2), 109–112 (1990). [PubMed] [Google Scholar]
- 9.Lemp M. A., Hamill J. R., Jr., “Factors affecting tear film breakup in normal eyes,” Arch. Ophthalmol. 89(2), 103–105 (1973). 10.1001/archopht.1973.01000040105007 [DOI] [PubMed] [Google Scholar]
- 10.Wolffsohn J. S., Arita R., Chalmers R., Djalilian A., Dogru M., Dumbleton K., Gupta P. K., Karpecki P., Lazreg S., Pult H., Sullivan B. D., Tomlinson A., Tong L., Villani E., Yoon K. C., Jones L., Craig J. P., “TFOS DEWS II Diagnostic Methodology report,” Ocul. Surf. 15(3), 539–574 (2017). 10.1016/j.jtos.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Fukuda R., Usui T., Miyai T., Yamagami S., Amano S., “Tear meniscus evaluation by anterior segment swept-source optical coherence tomography,” Am J Ophthalmol 155, 620–624 (2013). 10.1016/j.ajo.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 12.Zhou S., Li Y., Lu A. T., Liu P., Tang M., Yiu S. C., Huang D., “Reproducibility of tear meniscus measurement by Fourier-domain optical coherence tomography: a pilot study,” Ophthalmic Surg. Lasers Imaging 40(5), 442–447 (2009). 10.3928/15428877-20090901-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park D. I., Lew H., Lee S. Y., “Tear meniscus measurement in nasolacrimal duct obstruction patients with Fourier-domain optical coherence tomography: novel three-point capture method,” Acta Ophthalmol. 90(8), 783–787 (2012). 10.1111/j.1755-3768.2011.02183.x [DOI] [PubMed] [Google Scholar]
- 14.Altan-Yaycioglu R., Sizmaz S., Canan H., Coban-Karatas M., “Optical Coherence Tomography for Measuring the Tear Film Meniscus: Correlation with Schirmer Test and Tear-Film Breakup Time,” Curr. Eye Res. 38(7), 736–742 (2013). 10.3109/02713683.2013.774422 [DOI] [PubMed] [Google Scholar]
- 15.Sizmaz S., Altan-Yaycioglu R., Bakiner O. S., Bozkirli E., Coban-Karatas M., Ulas B., “Assessment of tear meniscus with optical coherence tomography in thyroid-associated ophtalmopathy,” Curr. Eye Res. 39(4), 323–328 (2014). 10.3109/02713683.2013.847960 [DOI] [PubMed] [Google Scholar]
- 16.Oguz H., Yokoi N., Kinoshita S., “The height and radius of the tear meniscus and methods for examining these parameters,” Cornea 19(4), 497–500 (2000). 10.1097/00003226-200007000-00019 [DOI] [PubMed] [Google Scholar]
- 17.Uchida A., Uchino M., Goto E., Hosaka E., Kasuya Y., Fukagawa K., Dogru M., Ogawa Y., Tsubota K., “Noninvasive interference tear meniscometry in dry eye patients with Sjögren syndrome,” Am. J. Ophthalmol. 144(2), 232–237 (2007). 10.1016/j.ajo.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 18.Yokoi N., Bron A. J., Tiffany J. M., Maruyama K., Komuro A., Kinoshita S., “Relationship between tear volume and tear meniscus curvature,” Arch. Ophthalmol. 122(9), 1265–1269 (2004). 10.1001/archopht.122.9.1265 [DOI] [PubMed] [Google Scholar]
- 19.Werkmeister R. M., Sapeta S., Schmidl D., Garhöfer G., Schmidinger G., Aranha Dos Santos V., Aschinger G. C., Baumgartner I., Pircher N., Schwarzhans F., Pantalon A., Dua H., Schmetterer L., “Ultrahigh-resolution OCT imaging of the human cornea,” Biomed. Opt. Express 8(2), 1221–1239 (2017). 10.1364/BOE.8.001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang M., Baskaran M., Werkmeister R. M., Chua J., Schmidl D., Aranha Dos Santos V., Garhöfer G., Mehta J. S., Schmetterer L., “Anterior segment optical coherence tomography,” Prog. Retin. Eye Res. 66, 132–156 (2018). 10.1016/j.preteyeres.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 21.Schmoll T., Unterhuber A., Kolbitsch C., Le T., Stingl A., Leitgeb R., “Precise thickness measurements of Bowman’s layer, epithelium, and tear film,” Optom. Vis. Sci. 89(5), E795–E802 (2012). 10.1097/OPX.0b013e3182504346 [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Aquavella J., Palakuru J., Chung S., Feng C., “Relationships between central tear film thickness and tear menisci of the upper and lower eyelids,” Invest. Ophthalmol. Vis. Sci. 47(10), 4349–4355 (2006). 10.1167/iovs.05-1654 [DOI] [PubMed] [Google Scholar]
- 23.Yadav R., Lee K. S., Rolland J. P., Zavislan J. M., Aquavella J. V., Yoon G., “Micrometer axial resolution OCT for corneal imaging,” Biomed. Opt. Express 2(11), 3037–3046 (2011). 10.1364/BOE.2.003037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aranha Dos Santos V., Schmetterer L., Gröschl M., Garhofer G., Schmidl D., Kucera M., Unterhuber A., Hermand J. P., Werkmeister R. M., “In vivo tear film thickness measurement and tear film dynamics visualization using spectral domain optical coherence tomography,” Opt. Express 23(16), 21043–21063 (2015). 10.1364/OE.23.021043 [DOI] [PubMed] [Google Scholar]
- 25.Goto E., Dogru M., Kojima T., Tsubota K., “Computer-synthesis of an interference color chart of human tear lipid layer, by a colorimetric approach,” Invest. Ophthalmol. Vis. Sci. 44(11), 4693–4697 (2003). 10.1167/iovs.03-0260 [DOI] [PubMed] [Google Scholar]
- 26.King-Smith P. E., Fink B. A., Fogt N., “Three interferometric methods for measuring the thickness of layers of the tear film,” Optom. Vis. Sci. 76(1), 19–32 (1999). 10.1097/00006324-199901000-00025 [DOI] [PubMed] [Google Scholar]
- 27.Dos Santos V. A., Schmetterer L., Triggs G. J., Leitgeb R. A., Gröschl M., Messner A., Schmidl D., Garhofer G., Aschinger G., Werkmeister R. M., “Super-resolved thickness maps of thin film phantoms and in vivo visualization of tear film lipid layer using OCT,” Biomed. Opt. Express 7(7), 2650–2670 (2016). 10.1364/BOE.7.002650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mainstone J. C., Bruce A. S., Golding T. R., “Tear meniscus measurement in the diagnosis of dry eye,” Curr. Eye Res. 15(6), 653–661 (1996). 10.3109/02713689609008906 [DOI] [PubMed] [Google Scholar]
- 29.Golding T. R., Bruce A. S., Mainstone J. C., “Relationship between tear-meniscus parameters and tear-film breakup,” Cornea 16(6), 649–661 (1997). 10.1097/00003226-199711000-00009 [DOI] [PubMed] [Google Scholar]
- 30.Markoulli M., Duong T. B., Lin M., Papas E., “Imaging the Tear Film: A Comparison Between the Subjective Keeler Tearscope-Plus™ and the Objective Oculus® Keratograph 5M and LipiView Interferometer,” Curr. Eye Res. 43(2), 155–162 (2018). 10.1080/02713683.2017.1393092 [DOI] [PubMed] [Google Scholar]
- 31.Bartuzel M. M., Szczesna-Iskander D. H., Iskander D. R., “Automatic dynamic tear meniscus measurement in optical coherence tomography,” Biomed. Opt. Express 5(8), 2759–2768 (2014). 10.1364/BOE.5.002759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DEWS , “Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007),” Ocul. Surf. 5(2), 108–152 (2007). 10.1016/S1542-0124(12)70083-6 [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Aquavella J., Palakuru J., Chung S., Feng C., “Relationships between central tear film thickness and tear menisci of the upper and lower eyelids,” Invest. Ophthalmol. Vis. Sci. 47(10), 4349–4355 (2006). 10.1167/iovs.05-1654 [DOI] [PubMed] [Google Scholar]
- 34.Bai Y., Ngo W., Gu B., Zhang Y., Nichols J. J., “An imaging system integrating optical coherence tomography and interferometry for in vivo measurement of the thickness and dynamics of the tear film,” Biomed. Eng. Online 17(1), 164 (2018). 10.1186/s12938-018-0597-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang H., Jeon H. J., Yow K. C., Hwang H. S., Chung E., “Image-based quantitative analysis of tear film lipid layer thickness for meibomian gland evaluation,” Biomed. Eng. Online 16(1), 135 (2017). 10.1186/s12938-017-0426-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raj A., Dhasmana R., Nagpal R. C., “Anterior Segment Optical Coherence Tomography for Tear Meniscus Evaluation and its Correlation with other Tear Variables in Healthy Individuals,” J. Clin. Diagn. Res. 10(5), NC01–NC04 (2016). 10.7860/JCDR/2016/18717.7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pult H., Riede-Pult B. H., “Impact of conjunctival folds on central tear meniscus height,” Invest. Ophthalmol. Vis. Sci. 56(3), 1459–1466 (2015). 10.1167/iovs.14-15908 [DOI] [PubMed] [Google Scholar]
- 38.Baek J., Doh S. H., Chung S. K., “Assessment of the Tear Meniscus Using Optical Coherence Tomography in Patients With Type 2 Diabetes Mellitus,” Cornea 34(12), 1534–1540 (2015). 10.1097/ICO.0000000000000651 [DOI] [PubMed] [Google Scholar]
- 39.Sarac O., Soyugelen G., Gurdal C., Bostancı-Ceran B., Can I., “Tear meniscus analysis with Fourier-domain optical coherence tomography in keratoconus,” Curr. Eye Res. 36(6), 528–533 (2011). 10.3109/02713683.2011.569869 [DOI] [PubMed] [Google Scholar]
- 40.Bandlitz S., Purslow C., Murphy P. J., Pult H., “Comparison of a new portable digital meniscometer and optical coherence tomography in tear meniscus radius measurement,” Acta Ophthalmol. 92(2), e112–e118 (2014). 10.1111/aos.12275 [DOI] [PubMed] [Google Scholar]
- 41.Bandlitz S., Purslow C., Murphy P. J., Pult H., “Time course of changes in tear meniscus radius and blink rate after instillation of artificial tears,” Invest. Ophthalmol. Vis. Sci. 55(9), 5842–5847 (2014). 10.1167/iovs.14-14844 [DOI] [PubMed] [Google Scholar]
- 42.Tian L., Qu J. H., Zhang X. Y., Sun X. G., “Repeatability and Reproducibility of Noninvasive Keratograph 5M Measurements in Patients with Dry Eye Disease,” J. Ophthalmol. 2016, 8013621 (2016). 10.1155/2016/8013621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols J. J., Nichols K. K., Puent B., Saracino M., Mitchell G. L., “Evaluation of tear film interference patterns and measures of tear break-up time,” Optom. Vis. Sci. 79(6), 363–369 (2002). 10.1097/00006324-200206000-00009 [DOI] [PubMed] [Google Scholar]
- 44.Streiner D. L., Norman G. R., Cairney J., “Chapter 8 - Reliability,” inHealth Measurement Scales - a practical guide to their development and use, 5th ed. (Oxford University Press, 2015). [Google Scholar]
- 45.Creech J. L., Do L. T., Fatt I., Radke C. J., “In vivo tear-film thickness determination and implications for tear-film stability,” Curr. Eye Res. 17(11), 1058–1066 (1998). 10.1076/ceyr.17.11.1058.5233 [DOI] [PubMed] [Google Scholar]
- 46.Wong H., Fatt I., Radke C. J., “Deposition and Thinning of the Human Tear Film,” J. Colloid Interface Sci. 184(1), 44–51 (1996). 10.1006/jcis.1996.0595 [DOI] [PubMed] [Google Scholar]
- 47.Shen M., Li J., Wang J., Ma H., Cai C., Tao A., Yuan Y., Lu F., “Upper and lower tear menisci in the diagnosis of dry eye,” Invest. Ophthalmol. Vis. Sci. 50(6), 2722–2726 (2009). 10.1167/iovs.08-2704 [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Palakuru J. R., Aquavella J. V., “Correlations among upper and lower tear menisci, noninvasive tear break-up time, and the Schirmer test,” Am. J. Ophthalmol. 145(5), 795–800 (2008). 10.1016/j.ajo.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei A., Le Q., Hong J., Wang W., Wang F., Xu J., “Assessment of Lower Tear Meniscus,” Optom. Vis. Sci. 93(11), 1420–1425 (2016). 10.1097/OPX.0000000000000986 [DOI] [PubMed] [Google Scholar]
- 50.Koh S., Ikeda C., Watanabe S., Oie Y., Soma T., Watanabe H., Maeda N., Nishida K., “Effect of non-invasive tear stability assessment on tear meniscus height,” Acta Ophthalmol. 93(2), e135–e139 (2015). 10.1111/aos.12516 [DOI] [PubMed] [Google Scholar]
- 51.Gumus K., Crockett C. H., Pflugfelder S. C., “Anterior segment optical coherence tomography: a diagnostic instrument for conjunctivochalasis,” Am. J. Ophthalmol. 150(6), 798–806 (2010). 10.1016/j.ajo.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dos Santos V. A., Schmetterer L., Stegmann H., Pfister M., Messner A., Schmidinger G., Garhofer G., Werkmeister R. M., “CorneaNet: fast segmentation of cornea OCT scans of healthy and keratoconic eyes using deep learning,” Biomed. Opt. Express 10(2), 622–641 (2019). 10.1364/BOE.10.000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan H. H., Zhao Y., Tun T. A., Tong L., “Repeatability of tear meniscus evaluation using spectral-domain Cirrus® HD-OCT and time-domain Visante® OCT,” Cont. Lens Anterior Eye 38(5), 368–372 (2015). 10.1016/j.clae.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 54.Arriola-Villalobos P., Fernández-Vigo J. I., Díaz-Valle D., Almendral-Gómez J., Fernández-Pérez C., Benítez-Del-Castillo J. M., “Lower Tear Meniscus Measurements Using a New Anterior Segment Swept-Source Optical Coherence Tomography and Agreement With Fourier-Domain Optical Coherence Tomography,” Cornea 36(2), 183–188 (2017). 10.1097/ICO.0000000000001086 [DOI] [PubMed] [Google Scholar]
- 55.Wozniak P. A., Schmidl D., Bata A. M., Fondi K., Witkowska K. J., Aranha Dos Santos V., Baar C., Room K. I., Nepp J., Baumgartner I., Popa-Cherecheanu A., Garhöfer G., Werkmeister R. M., Schmetterer L., “Effect of different lubricant eye gels on tear film thickness as measured with ultrahigh-resolution optical coherence tomography,” Acta Ophthalmol. 95(4), e307–e313 (2017). 10.1111/aos.13342 [DOI] [PubMed] [Google Scholar]
- 56.Schmidl D., Schmetterer L., Witkowska K. J., Unterhuber A., dos Santos V. A., Kaya S., Nepp J., Baar C., Rosner P., Werkmeister R. M., Garhofer G., “Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease,” Cornea 34(4), 421–426 (2015). 10.1097/ICO.0000000000000358 [DOI] [PubMed] [Google Scholar]
- 57.Szegedi S., Scheschy U., Schmidl D., Aranha Dos Santos V., Stegmann H., Adzhemian N., Fondi K., Bata A. M., Werkmeister R. M., Couderc C., Schmetterer L., Garhofer G., “Effect of Single Instillation of Two Hyaluronic Acid-Based Topical Lubricants on Tear Film Thickness in Patients with Dry Eye Syndrome,” J. Ocul. Pharmacol. Ther. 34(9), 605–611 (2018). 10.1089/jop.2018.0069 [DOI] [PubMed] [Google Scholar]
- 58.Schmidl D., Witkowska K. J., Kaya S., Baar C., Faatz H., Nepp J., Unterhuber A., Werkmeister R. M., Garhofer G., Schmetterer L., “The association between subjective and objective parameters for the assessment of dry-eye syndrome,” Invest. Ophthalmol. Vis. Sci. 56(3), 1467–1472 (2015). 10.1167/iovs.14-15814 [DOI] [PubMed] [Google Scholar]