Abstract
Fluorescence spectroscopy is a sensitive, fast and non-invasive tool for a diagnostics of cancerous gastrointestinal lesions. It could be applied for in situ detection of tumours during primary endoscopic observations or as add-on measurement modality during microscopic observations of tissue histology slides for their initial or retrospective diagnosis. Therefore, we are looking for diagnostically important features of normal and cancerous tissue areas in a broad spectral range for gastrointestinal tissues ex vivo using two steady-state macroscopic fluorescent spectroscopic modalities and by confocal fluorescent microscopic detection. Results obtained from autofluorescence spectroscopy of benign and malignant lower part gastrointestinal tract (GIT) lesions from freshly excised tissues during surgical removal of the lesions in 18 patients (22 lesions), were compared with the spectral measurements obtained during confocal fluorescent microscopy observations of unstained tissue slides using 405 nm excitation. Excitation–emission matrices (EEMs) were used for ex vivo measurements with applied excitation in 280-440 nm spectral region and emission observed between 300 and 700 nm. Synchronous fluorescence spectroscopy (SFS) approach was also applied to improve the spectral resolution of the observed complex emission spectra. Specific fluorescent features observed, related to presence of structural proteins, co-enzymes and endogenous porphyrins in the tissues investigated, allow discriminating normal mucosa from benign polyps and malignant carcinoma lesions with diagnostic accuracy up to 94.4%.
1. Introduction
Fluorescent analysis of gastrointestinal tissues recently became attractive mode for detection and differentiation of neoplasia in colon and rectum. Non-invasive and fast evaluation of the tissue state for development of an “optical biopsy”-based diagnosis could be a significant improvement of the current diagnostic modalities used in clinical practice [1,2]. Autofluorescence spectroscopy is one of the most intensively investigated add-on techniques for endoscopic gastrointestinal diagnostics. It is a powerful technique for noninvasive analysis of malignant tissues [3]. No requirements for application of exogenous contrast agents for visualization of neoplastic alterations make this technique a desired tool for endoscopic observations in situ during standard colonoscopy procedures. In general, this technique could be highly beneficial for initial diagnosis of suspicious tissue presence, for guided biopsy sampling, providing spectral data and images in vivo, in real time, characterized with different spectral peculiarities depending of benign or malignant lesions examined [4–8].
To evaluate the whole range of endogenous fluorophores existing in GIT tissues which contributes to the signal during fluorescent endoscopic observations one must applied multiple excitation wavelengths in the UV-VIS spectral range. Measurements of so called excitation-emission matrices (EEMs) of tissues could resolve this problem. EEM measurements are based on the scanning with a specific step of the excitation and detection of set of emission spectra corresponding to each excitation wavelength applied [9]. Obtained spectral maps reveal specific fluorescence intensity “islands”, which correspond to particular fluorophore with specific excitation and emission maximum, allowing addressing of the fluorophore content by type and quantity in the tissue investigated [6,7,9]. As endogenous fluorophores often have overlapping excitation or/and emission spectra, to improve the spectral resolution in such multicomponent system, such as biological tissues, a synchronous fluorescent spectroscopy (SFS) approach could be also applied, when the excitation and emission are scanned simultaneously. SFS leads to narrowing of the emission maxima detected, and improve in general resolution of the spectral maps developed [10–12]. Both steady-state fluorescent techniques are useful for tissue type evaluation and could bring information about diagnostically-important spectral features in different tissue types. Spectral mapping based on EEMs and SFS data also is useful in optimization of the number of used excitation wavelengths and spectral ranges of detection for discrimination of normal from abnormal areas, which would optimize in situ tumour detection and significantly reduces the required time for a single fluorescent measurement, a crucial parameter during clinical endoscopic observations of patients [10–13]. Up to nowadays, there is a limited number of autofluorescent properties of normal mucosa, benign and malignant GIT lesions’ studies using EEM and SFS techniques [9,12]. Some research groups used single excitation sources [4–6], few discrete excitation wavelengths [7], or proposed usage of exogenous fluorescent markers to allow good visualization and discrimination of GIT tissue types including usage of photosensitizers [14], or multiplex molecular fluorescent markers [15]. However, such approaches require application of exogenous markers, being more time-consuming and expensive, which decrease their applicability for initial endoscopic observation procedures in patients in situ [2,16,17].
Therefore, new information about endogenous fluorescent specific spectral features could be beneficial for evaluation of the most diagnostically important and specific excitation and emission pairs. In our previous studies we demonstrated EEM and SFS maps of colon and rectum neoplasia, compared with the normal mucosa data to present the endogenous fluorophores appearance in the specific tissue areas [12]. However, spectral curves comparison is more informative, when diagnostically important features are searched for in the process of discrimination algorithms development based on endogenous fluorescent sources. The intensity levels, spectral shape and distribution of the emission signal give specific signatures of such complex samples as biological tissues. Such information is a basic one for a following development and optimization of discrimination algorithms themselves and for the design of novel spectroscopic add-on instruments for GIT neoplasia endoscopic initial diagnostics [2,3,7,9].
Another approach that could be used for diagnostic decision during pathological examination of the tissue slices is also based on fluorescent spectral features differentiation using confocal fluorescence microscopy (CFM) measurements. Histology is still the golden standard in cancer diagnostics and relays entirely on the pathologists’ experience and knowledge. The diagnostic accuracy gap between experienced and not so experienced pathologists is crucial. The ability of optical methods, and in particular fluorescent spectroscopy, to provide information about specific tissue properties could be beneficial for pre- or additional examination of tissue slides [3,4]. It could be especially useful for recognition of diagnostically important and confirmed tissue patterns and at general aiding diagnosis. Microscopic technique with such properties that provides fluorescence images with superior lateral and axial resolution is confocal fluorescence microscopy (CFM) [1–3,18]. Standard histology, based on microscopic examination of tissue biopsy samples, include multiple steps of time consuming preparation of tissue slides that includes formalin fixation, paraffin embedding, sectioning and staining (most frequently with hematoxylin and eosin (H&E)). Every step of this procedure alters the natural characteristics of the tissue that could be diagnostically valuable and multiplies the risk of formation of artefacts in the samples, which could lead to mistakes in the diagnosis [19]. Autofluorescent detection of such tissue slides, without need of tissue staining, remove part of the required preparatory steps in such histology analysis and could be used in image analysis mode of the fluorescent maps observed in automated discrimination algorithm, excluding human factor during histological verification procedure [20,21].
Comparison of spectral fluorescent features of normal mucosa, benign and malignant tissues of lower part GIT on macroscopic and microscopic level could be applied to evaluate the fluorophores distribution in different tissue types and to assess the diagnostically important spectral features of the emitted spectra in both detection modalities. Such addressing on macro level is useful for initial observations in situ to indicate presence of specific fluorescent features related to malignancy and to be used in add-on fluorescent diagnostic tool during endoscopy observation of novel, non-diagnosed in advance lesions, searching and mapping of lesions’ locations and boundaries, or during therapeutic procedures’ monitoring and follow-up of the treatment. On microscopic level, it could be applied during detailed histological verification in spectral imaging mode of detection, as an upgrading tool of standard histological examination, allowing objective mapping of tissue types, unrelated to the clinician professional experience, and allowing development of automated discrimination procedures in prospective and/or retrospective analysis of histological samples in hospital tissue samples’ depositories [1–4,13].
In the current study are compared fluorescent spectra’ properties of normal lower part GIT mucosa, colon polyps, as well colorectal carcinoma lesions on macroscopic and microscopic level, to evaluate the diagnostically important features in both modalities of detection, and their correlation with the fluorophores’ content in the tissues investigated.
2. Methods and materials
2.1 Benign and malignant tissue samples
Fluorescent spectra in EEM and SFS regimes were measured for the pairs of benign or malignant tissues and healthy tissue from the safety area of surgically excised samples from 18 patients (22 lesions). The procedure of obtaining the investigated samples included their excision during surgery for removal of colorectal neoplasia lesions. After the surgical removal lesions were divided on two parts – for histological and for spectral analysis. For spectral measurements, the biological samples were transported in isothermal conditions and safe-keeping solution from the hospital to the spectral laboratory, where their fluorescence properties were investigated. All patients received and signed written informed consent and this research was approved by the Ethics committee of University Hospital “Tsaritsa Yoanna-ISUL”, Sofia.
Confocal fluorescent microscopy (CFM) images were obtained from histological samples of the tissues, corresponding to the lesions investigated macroscopically. Two types of slices were made consequently from the paraffin blocks prepared from the tissues investigated – first group consisted of slides stained with eosin-haemoxylin and second group of unstained tissue slides. Consequent cuts were made to reproduce as closest as possible the distribution of the tissue morphology and histologic peculiarities for stained and unstained samples.
Stained samples were used for standard histological verification, as a “gold standard” in the current spectral study and in surgical excision procedure itself, as well as a landmark for unstained samples specific areas and comparison of the tissue type observed. Unstained samples group was used for CFM measurements, based on autofluorescent detection of fluorophores distributed in different areas of the slides – in normal and abnormal mucosa parts.
2.2 Fluorescence spectroscopy of colorectal tissue samples
Spectrofluorimeter FluoroLog 3 (HORIBA Jobin Yvon, France) was used for the fluorescence measurements of the surgically removed tissue samples. The excitation light source is a xenon lamp with 300 W output optical power, performance range of 200-650 nm. Detector is photomultiplier tube with performance range of 220-850 nm for fluorescence detection. Since our tissue samples ex vivo varied by shape and dimensions, their fluorescence properties were investigated with additional fiber-optical module F-3000, which allows investigation outside of the sample chamber of the spectrofluorimeter. Measurements of the fluorescence signals obtained in EEM regime were performed with excitation in 280-440 nm spectral range and emission observed between 300 nm and 700 nm. SFS measurements were performed with excitation wavelengths in the range of 280-440 nm with increment of 10 nm and wavelength interval (offset) in the range of 10-280 nm with increment of 10 nm between the scans. After the performing of both EEM and SFS spectroscopic measurements for healthy, benign and cancerous areas of the samples, they were stored in formalin solution.
2.3 Confocal fluorescent microscopy
Confocal fluorescent microscopy (CFM) images at 405 nm excitation were obtained with Leica TCS SP8 X microscope. Objective lens HCX PL APO CS 10x/0.40 were used in all measurements. Fluorescent images with diode laser excitation wavelength 405 nm and detection band 415-500 were encoded with blue pseudo-color, with detection band 500-600 with green and with detection band 600-700 with red, correspondingly. Photomultiplier tube (PMT) detector was used for all fluorescence images. Transmission images in all cases were detected by PMT in condenser beam path and encoded with gray pseudo-color. Scan rate was set to 100 Hz for all images.
Lambda scan (Leica Inc.) measurements on addressed areas as normal, benign and malignant ones, was carried out and the emission spectra received were compared from the point of view of the type of pathology observed and with the macroscopic spectral data received from the same lesion investigated.
2.4 Statistical analysis of the spectral data
Spectral data received during macroscopic measurements were averaged by tissue type and formed EEM and SFS matrices for each sub-type of GIT lesions. Emission spectra for single excitation wavelength applied were averaged and normalized with respect to the emission maxima for comparison of the spectral shapes in different types of tissues, classifying normal mucosa (22 samples), benign colon polyps (4 samples), malignant colon (13 samples) carcinoma and rectum (5 samples) carcinoma lesions. Similarly, the spectra obtained during microscopic observations in Lambda scan regimes of detection were averaged per area and normalized with respect to maximum of fluorescence, forming groups of normal mucosa, benign and malignant colon and malignant rectum tissue spectra. The statistical analysis and comparison of the spectral data was performed using the Mann-Whitney test, with the differences considered significant for p < 0.05.
3. Results and discussion
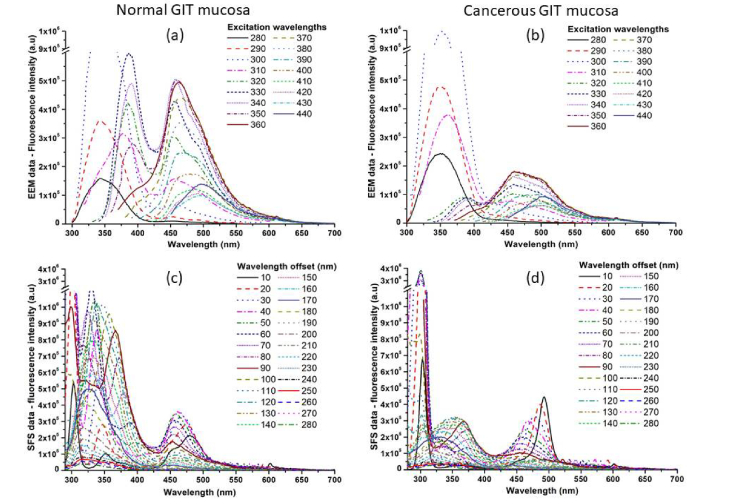
On Fig. 1 are presented excitation-emission spectral data for normal and malignant GIT mucosa, averaged by tissue type for all samples investigated, as well as synchronous fluorescent spectra for normal and abnormal tissue sites. The spectral intensity and shape differences are significant in both types of steady-state measurements of fluorescence emission of normal and cancerous mucosa. On the spectra could be recognized five specific spectral areas, related to the emission of endogenous fluorophores, as follow: amino acids (320-360 nm), collagen (380-420 nm), co-enzyme NADH (440-460 nm), protein cross-links (470-500 nm) and weak endogenous porphyrins fluorescence (630-650 nm) [22,23].
Fig. 1.
Excitation–emission spectral data of normal (a) and cancerous (b) GIT mucosa for excitation range from 280 to 440 nm and synchronous fluorescence spectral data of normal (c) and cancerous (d) GIT mucosa for spectral offset varying from 10 to 200 nm between excitation and emission spectra respectively.
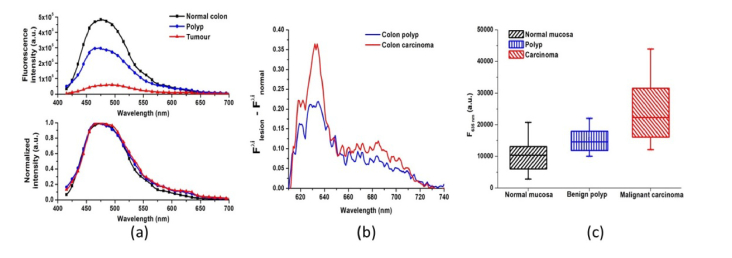
In the case of tumour lesion appearance, the amino acids fluorescent emission rapidly raised, due to increased growth of neoplasia itself, required more structural units for its development and due to demolition of some part of the structural proteins in the extracellular matrix of the lesion, due to uncontrolled growth of the pathology. This is correlated with the alterations foreseen in the emission peaks of collagen, and protein cross-links in longer wavelength part of the tissue spectra, which revealed significant decrease by intensity at 400 and 480 nm respectively. Alteration in the thickness of the mucosal layer during tumour growth also leaded to a decrease of protein fluorescent signal, which is addressed mainly with sub-mucosal layers of the colon wall. Weak signal observed from NADH is due to the work with ex vivo tissue samples, where the emission of this metabolic indicator rapidly decreased after surgical excision procedure and could not be used in the current study as diagnostically significant indicator for the tissue alterations, which could be done correctly only during in vivo tissue investigations, or if time between the excision and spectral measurement could be taken into account [22–25]. Appearance of endogenous porphyrins fluorescent emission at the region of 630 −700 nm, is typical for the tumour samples, but also has been found with lower intensity levels in benign lesions investigated (see Fig. 3(b)).
Fig. 3.
(a) Comparison of fluorescence spectra of normal mucosa, benign polyp and colon tumour’ lesions compared by fluorescence intensity and by spectral shape, when normalization with respect to emission maximum was applied; (b) resultant spectrum of the porphyrins’ emission in benign and malignant GIT tissues, when fluorescence of normal mucosa is subtracted from the lesions’ emission in the region 610-740 nm; (c) Box-whisker graph of the fluorescence emission values at 635 nm for normal, benign and malignant GIT mucosa.
In the case of SFS data comparison the differences in the emission lines for amino acids, structural proteins and endogenous porphyrins are even more pronounced than in EEM data, which is an indicator for their diagnostic importance as fluorophores’ compounds. Their fluorescent signatures, allowing comparison of their concentration in normal and abnormal tissue states, as well the dynamic spectral alterations of their emission observed during tumour development could be used as diagnostic parameters for evaluation of the presence and stage of malignant lesion growth in lower part GIT during fluorescent endoscopic examination of these organs.
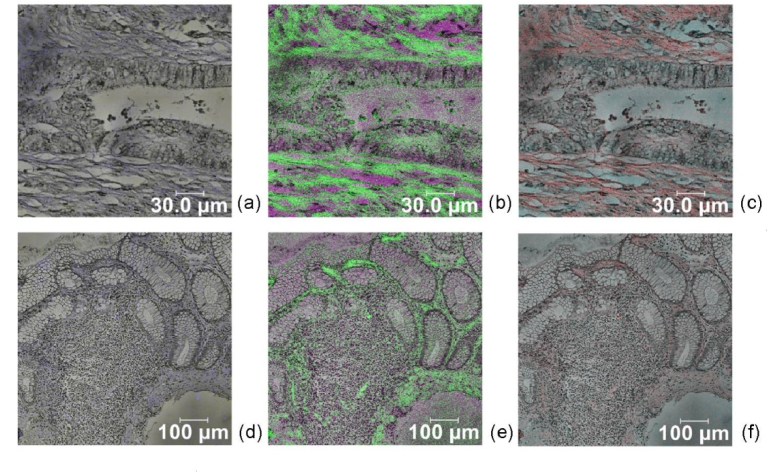
CFM images of colon and rectum carcinoma lesions are presented on Fig. 2. The highest intensity fluorescence was observed in the region of 500-600 nm for both locations, which correspond to the autofluorescence spectra of macroscopic measurements of the tissue investigated. Due to usage of 405 nm excitation in microscopic measurements we could not investigate the spectral range below this wavelength, where the typical endogenous fluorescence of amino acids was observed using macroscopic spectral detection. Low-level red fluorescence is observed in the range of 600-700 nm, well pronounced in colon malignancies and not observable in the polyp lesions investigated. In rectum carcinoma red shift is observable as well, but less pronounced in comparison with colon neoplasia spectra, and after processing of the spectral data could be used as diagnostic indicator for discrimination vs. normal mucosa. In Lambda scan regime was observed small shift of the fluorescent maximum for malignant tissue vs. healthy mucosa to longer wavelengths (5-7 nm for different samples), as well appearance of red fluorescent maxima at 630-650 nm, which could correspond to the accumulated endogenous porphyrins in the malignant areas, see Fig. 3. In macroscopic measurements of fluorescent properties of freshly excised tumour tissues are observed similar tendencies of red shift, as well accumulation of protoporphyrin IX in malignant parts of the excised tissue samples.
Fig. 2.
Confocal fluorescent images in pseudo-colours of colon (a, b, c) and rectum (d, e, f) carcinoma using excitation at 405 nm. Spectral window used for (a, d) 415-500 nm, (b, e) 500-600 nm and (c, f) 600-700 nm.
Significant differences in the fluorescence intensity were observed, correlated with the type of pathology - benign or malignant one vs. healthy mucosa. When normalized spectra with respect to the emission maximum are compared, a red signal, addressed to endogenous porphyrins is pronounced in the region of 635-650 nm, see Fig. 3 (a).
The most significant spectral shape alterations between normal and abnormal colon tissue areas were observed for the red spectral region and when compared by value (see Fig. 3 (b)) normal and benign tissues could be easily discriminated from the malignant one. However, using red fluorescence intensity values at 635 nm solely, where the endogenous porphyrins’ emission maximum in the tissues is situated, for appropriate discrimination between healthy and benign tissues, suboptimal discrimination had place, non-recognizing 2 from 4 benign polyps and 4 from 13 colon carcinoma samples as malignancies, see Fig. 3 (c). For improvement of the diagnostic accuracy, additional fluorescent parameters must be included – the integral fluorescence intensity levels for the spectral range from 420 to 740 nm, and fluorescence maximum intensity values at 480 nm, that correspond to the structural proteins emission, including collagen and elastin cross-links.
When these parameters were taken into account for discrimination of normal mucosa from benign and malignant GIT lesions only one from eighteen malignant lesions was misdiagnosed as a normal tissue, being colon carcinoma G2 by histological analysis. In such a way a diagnostic accuracy value of 94,4% was achieved for malignant lesion autofluorescent diagnosis in the ex vivo samples investigated.
Excitation at longer wavelengths for microscopic fluorescent observations did not allow receiving more informative CFM images than corresponding ones at 405 nm (data not presented). Using excitation higher than 440 nm in both modalities macroscopically and microscopically the observed differences between benign and malignant tissue sites, were related to alterations in emission intensity levels solely, but did not correspond to significant (p<0,05) spectral shape alterations. That fact did not allow achieving high diagnostic accuracy using fluorescent parameters solely for tissue type discrimination, as benign problems, such as, polyps and mucosal inflammations lead to decrease of the fluorescence emission as well. Most reliable results were obtained when short-wavelength excitation in the region of 365-420 nm in both macroscopic and microscopic fluorescence modalities, which correspond to our earlier observations on GIT neoplasia [26,27] and to the results obtained from other research groups using autofluorescence steady-state techniques for tumour initial diagnosis in GIT tissues [5–8].
4. Conclusions
Diagnostically valuable fluorescent images could be obtained from biopsy tissue slides without staining through CFM technique. Microscopic and macroscopic fluorescent measurements reveal similar tendencies in the fluorescent intensities alterations, appearance of specific maxima, addressed to endogenous fluorophores in the tissues and could cover both in vivo and histological verification of the tissue state. In the case of histological samples evaluation omitting of the staining procedure will reduce the time and cost of the procedure using fluorescent detection and evaluation, as well it could be beneficial for obtaining additional information from paraffin-embedded archived samples for retrospective studies or to gain more knowledge from and for rare diseases’ types or with small number of histological samples.
SFS and EEM data allow increasing the number of spectral discrimination parameters for clinically significant differentiation of normal, benign and malignant lower part GIT lesions. Diagnostic accuracy of 94,4% was achieved in the current study, using samples ex vivo, but verification with a larger set of samples, obtained after surgical excision of colorectal tumours is under way for the needs of development of diagnostic differentiation algorithm for in situ initial endoscopic observation for detection of colon neoplasia.
Acknowledgements
The EEM and SFS tissue analysis was supported by the National Science Fund of Bulgarian Ministry of Education and Science under grant #KP06-N28/11/2018. The measurements related to CFM investigations using excitation at 405 nm were supported from Russian Science Foundation project #18-15-00139. This work of Ts. G. was partially supported by the Bulgarian Ministry of Education and Science under the National Research Programme “Young scientists and postdoctoral students” approved by DCM # 577 / 17.08.2018.
Funding
Bulgarian National Science Fund (KP06-N28/11/2018); Russian Science Foundation (18-15-00139); Bulgarian Ministry of Education and Science (DCM # 577 / 17.08.2018).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Polglase A. L., McLaren W. J., Skinner S. A., Kiesslich R., Neurath M. F., Delaney P. M., “A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract,” Gastrointest. Endosc. 62(5), 686–695 (2005). 10.1016/j.gie.2005.05.021 [DOI] [PubMed] [Google Scholar]
- 2.Subramanian V., Ragunath K., “Advanced Endoscopic Imaging: a Review of Commercially Available Technologies,” Clin. Gastroenterol. Hepatol. 12(3), 368–376 (2014). 10.1016/j.cgh.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 3.Jeon S. R., Cho W. Y., Jin S. Y., Cheon Y. K., Choi S. R., Cho J. Y., “Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study,” Gastrointest. Endosc. 74(4), 772–780 (2011). 10.1016/j.gie.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Song L. M. W. K., Banerjee S., Desilets D., Diehl D. L., Farraye F. A., Kaul V., Kethu S. R., Kwon R. S., Mamula P., Pedrosa M. C., Rodriguez S. A., Tierney W. M., “Autofluorescence imaging,” Gastrointest. Endosc. 73(4), 647–650 (2011). 10.1016/j.gie.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 5.Luo X. J., Zhang B., Li J. G., Luo X. A., Yang L. F., “Autofluorescence spectroscopy for evaluating dysplasia in colorectal tissues,” Z. Med. Phys. 22(1), 40–47 (2012). 10.1016/j.zemedi.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 6.Li B. H., Xie S. S., “Autofluorescence excitation-emission matrices for diagnosis of colonic cancer,” World J. Gastroenterol. 11(25), 3931–3934 (2005). 10.3748/wjg.v11.i25.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L., Nie Y., Lin L., Li W., Huang Z., Xie S., Li B., “Pattern recognition of multiple excitation autofluorescence spectra for colon tissue classification,” Photodiagn. Photodyn. Ther. 10(2), 111–119 (2013). 10.1016/j.pdpdt.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 8.DaCosta R. S., Andersson H., Cirocco M., Marcon N. E., Wilson B. C., “Autofluorescence characterisation of isolated whole crypts and primary cultured human epithelial cells from normal, hyperplastic, and adenomatous colonic mucosa,” J. Clin. Pathol. 58(7), 766–774 (2005). 10.1136/jcp.2004.023804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genova Ts., Borisova E., Penkov N., Vladimirov B., Zhelyazkova A., Avromov L., “Excitation–emission matrices and synchronous fluorescence spectroscopy for the diagnosis of gastrointestinal cancers,” Quantum Electron. 46(6), 510–514 (2016). 10.1070/QEL16112 [DOI] [Google Scholar]
- 10.Ebenezar J., Aruna P., Ganesan S., “Synchronous Fluorescence Spectroscopy for the Detection and Characterization of Cervical Cancers In Vitro,” Photochem. Photobiol. 86(1), 77–86 (2010). 10.1111/j.1751-1097.2009.00628.x [DOI] [PubMed] [Google Scholar]
- 11.Borisova E., Zhelyazkova A., Keremedchiev M., Penkov N., Semyachkina-Glushkovskaya O., Avramov L., “Endogenous synchronous fluorescence spectroscopy (SFS) of basal cell carcinoma – initial study,” Opt. Spectrosc. 120(1), 38–44 (2016). 10.1134/S0030400X16010057 [DOI] [Google Scholar]
- 12.Genova Ts., Borisova E., Zhelyazkova A., Penkov N., Vladimirov B., Terziev I., Semyachkina-Glushkovskaya O., Avramov L., “Colorectal cancer stage evaluation using synchronous fluorescence spectroscopy technique,” Opt. Quantum Electron. 48(8), 378–384 (2016). 10.1007/s11082-016-0634-7 [DOI] [Google Scholar]
- 13.Kim P., Puoris’haag M., Côté D., Lin C. P., Yun S. H., “In vivo confocal and multiphoton microendoscopy,” J. Biomed. Opt. 13(1), 010501 (2008). 10.1117/1.2839043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filonenko E., Kaprin A., Raszhivina A., Urlova A., Nechipai A., “Fluorescence Diagnostics of Colon Malignant and Premalignant Lesions Using 5-Aminolevulinic Acid,” Int. J. Photoenergy 2014, 378673 (2014). 10.1155/2014/378673 [DOI] [Google Scholar]
- 15.Bae S. M., Bae D. J., Do E. J., Oh G., Yoo S. W., Lee G. J., Chae J. S., Yun Y., Kim S., Kim K. H., Chung E., Kim J. K., Hwang S. W., Park S. H., Yang D. H., Ye B. D., Byeon J. S., Yang S. K., Joo J., Kim S. Y., Myung S. J., “Multi-Spectral Fluorescence Imaging of Colon Dysplasia InVivo Using a Multi-Spectral Endoscopy System,” Transl. Oncol. 12(2), 226–235 (2019). 10.1016/j.tranon.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broek F., Fockens P., Dekker E., “Review article: new developments in colonic imaging,” Aliment. Pharmacol. Ther. 26(Suppl 2), 91–99 (2007). 10.1111/j.1365-2036.2007.03494.x [DOI] [PubMed] [Google Scholar]
- 17.Croce A., Bottiroli G., “Autofluorescence Spectroscopy and Imaging: A Tool for Biomedical Research and Diagnosis,” Eur. J. Histochem. 58(4); 2461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragazzi M., Piana S., Longo C., Castagnetti F., Foroni M., Ferrari G., Gardini G., Pellacani G., “Fluorescence confocal microscopy for pathologists,” Mod. Pathol. 27(3), 460–471 (2014). 10.1038/modpathol.2013.158 [DOI] [PubMed] [Google Scholar]
- 19.Bindhu P., Krishnapillai R., Thomas P., Jayanthi P., “Facts in artifacts,” J. Oral Maxillofac. Pathol. 17(3), 397–401 (2013). 10.4103/0973-029X.125206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabbour J. M., Saldua M. A., Bixler J. N., Maitland K. C., “Confocal Endomicroscopy: Instrumentation and Medical Applications,” Ann. Biomed. Eng. 40(2), 378–397 (2012). 10.1007/s10439-011-0426-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dramićanin T., Lenhardt L., Zeković I., Dramićanin M. D., “Support Vector Machine on fluorescence landscapes for breast cancer diagnostics,” J. Fluoresc. 22(5), 1281–1289 (2012). 10.1007/s10895-012-1070-0 [DOI] [PubMed] [Google Scholar]
- 22.Ramanujam N., “Fluorescence spectroscopy in vivo”, in Encyclopedia of Analytical Chemistry, Meyers R. Chichester: John Wiley & Sons Ltd, (2000), pp. 20–56. [Google Scholar]
- 23.Mycek M., Pogue B., Handbook of Biomedical Fluorescence (Marcel Dekker, 2003). [Google Scholar]
- 24.Chen J., Zhuo S., Chen G., Yan J., Yang H., Liu N., Zheng L., Jiang X., Xie S., “Establishing diagnostic features for identifying the mucosa and submucosa of normal and cancerous gastric tissues by multiphoton microscopy,” Gastrointest. Endosc. 73(4), 802–807 (2011). 10.1016/j.gie.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 25.Zhuo S., Zhu X., Wu G., Chen J., Xie S., “Quantitative biomarkers of colonic dysplasia based on intrinsic second-harmonic generation signal,” J. Biomed. Opt. 16(12), 120501 (2011). 10.1117/1.3659715 [DOI] [PubMed] [Google Scholar]
- 26.Genova Ts., Borisova E., Angelova L., Zhelyazkova Al., Kermedchiev M., Penkov N., Vladimirov B., Avramov L., “Excitation-emission matrices for detection of colorectal tumors - initial investigations,” Bulg. Chem. Commun. 47, 464–468 (2015). [Google Scholar]
- 27.E. Borisova, L. Plamenova, M. Keremedchiev, B. Vladimirov, and L. Avramov, “Endogenous and exogenous fluorescence of gastrointestinal tumors – initial clinical observations,” Proc. SPIE, ISQ12–ISQ100–87 (2012). [Google Scholar]