Abstract
Femtosecond laser pulses were applied for precise alphanumeric code engraving on the zona pellucida (ZP) of mouse zygotes for individual embryo marking and their identification. The optimal range of laser pulse energies required for safe ZP microsurgery has been determined. ZP was marked with codes in three different planes to simplify the process of embryo identification. No decrease in developmental rates and no morphological changes of embryos post laser-assisted engraving have been observed. ZP thickness of embryos post laser-assisted code engraving has been shown to differ significantly from that of control group embryos at the hatching stage. Due to moderate ZP thinning as compared to its initial width at 0.5 dpc (days post coitum), readability of the code degrades slightly and it still remains recognizable even at hatching stage. Our results demonstrate that application of femtosecond laser radiation could be an effective approach for noninvasive direct embryo tagging, enabling embryo identification for the whole period of preimplantation development.
1. Introduction
Lasers have become an efficient tool in assisted reproductive technologies (ART) [1–3]. Various laser sources are extensively employed for oocytes as well as for spermatozoa treatment and manipulations. Thus, for example, possibility of using a non-contact infrared diode laser (wavelength λ = 1.48 μm) for spermatozoa immobilization and permeabilization of the sperm membrane has been demonstrated in [4]. Low-power He-Ne laser has been used in in vitro fertilization for immature oocytes treatment and improving the system of in vitro embryo production [5,6]. Although first studies regarding spermatozoa movement stimulation have been conducted in 1980s, they are still underway. Earlier studies of Sato et al [7] and Lenzi [8] have demonstrated the stimulating effect of red (λ = 647 nm) and infrared (parameters of laser light were not clarified) laser light on sperm motility. Today, main attempts are made to develop methodology (usually based on optical tweezers) for safe and exact measurement of stimulating effects of laser light [9–11]. Optical tweezers have been successfully applied not only for sperm motility measurements, but also for sperm trapping and insertion into the perivitelline space of oocytes for in vitro fertilization [12], and for noncontact removal of polar bodies for their genetic analysis [13,14] (the so-called embryo biopsy). Openings in the outer shell surrounding oocytes and embryos required for sperm insertion during in vitro fertilization [12,15] or cell extraction during embryo biopsy can also be created by means of lasers [13].
Nowadays, infrared diode lasers (λ = 1.48 um) with milli- to microsecond pulse durations are the most popular lasers applied in the field of assisted reproduction for microdissection. Such systems are widely used for opening the ZP in assisted hatching [16–20]. Efficacy of various types of laser assisted hatching (LAH), for example, partial, quarter and total LAH has been analyzed [21]. The main danger of pointing a laser at an embryo is thermal damage. Infrared diode lasers seem to be an effective and safe tool, nevertheless strong recommendations regarding optimum regimes of embryo exposure should be taken into account to minimize possible laser-related thermal risks [22–24]. According to this, application area of infrared diode lasers is commonly limited to ZP dissection and spermatozoa immobilizing prior to use.
Recently, new approaches to assisted reproduction problems based on a novel, more delicate and effective laser systems generating laser pulses with shorter durations have been proposed. Femtosecond lasers have proven to be an excellent tool for noninvasive and precise microsurgery at cellular and even subcellular levels, for micromanipulation and optical modification of living biological objects. Femtosecond lasers have been successfully applied for fully noncontact optical microinjection and trapping of developing embryos [25], for oocyte enucleation by automated ablation of entire metaphase plates in porcine oocytes [26], and for blastomere fusion [27,28]. Efficacy of femtosecond laser use for laser-assisted hatching [29], noncontact polar body [30], and trophectoderm biopsy [31] (by simultaneous use of femtosecond laser and optical tweezers) has been previously shown by our group. We also used the unique ability of femtosecond lasers to perform precise microdissections to develop a novel technique for individual labeling of preimplantation embryos [32]. The technique is based on femtosecond laser microsurgery of ZP and “engraving” small (~5 µm in width and ~20 µm in length) alphanumeric codes in the depth of ZP. This technique may be useful in assisted reproductive technologies for preventing medical accidents relating to mix-ups. Although such errors are rare, several cases of mix-up in IVF laboratories have been reported [33–36] and various strategies, safety polices, and devices aimed at eliminating the risk of mistakes during the entire ART procedure are still being developed. By using femtosecond laser pulses relatively fast, precise, and delicate microsurgery can be performed with a minimal risk of thermal damage. The process of laser code engraving is performed in a contactless mode under sterile conditions and can be fully automated in the future. Moreover, only the ZP is subjected to laser microsurgery while leaving the embryo cells intact.
In our previous study [32] we performed one-plane code engraving (the code on ZP was created in a single, usually equatorial plane) as well as three-plane code engraving on 0.5 dpc (days post coitum) mouse embryos. No detrimental effects of laser-assisted code engraving on embryo developmental and hatching rates as well as on trophectoderm-to-inner cell mass ratio as compared to control group embryos have been observed. We have demonstrated that code created on the ZP could be clearly visualized at least until 3.5 dpc that allowed successful utilization of such technique for embryo identification from the day 0.5 to day 3 when embryo transfer could be done. However, the questions about code visualization at later stages of preimplantation embryo development and embryo identification during the entire preimplantation period were left unanswered. Our current study aims to answer these questions and provide interesting observations regarding features of ZP subjected to laser engraving and embryo hatching. Our observations allow us to suppose that the technique proposed may be used not only for embryo labelling but also for stimulating embryo hatching to start at prescribed location.
2. Experimental design
The femtosecond laser-based system for embryo microsurgery shown in Fig. 1 is based on our previously reported setup [32]. A femtosecond ytterbium 1028 nm wavelength laser 1 (TETA, Avesta LLC) that operates at 280 fs with repetition rate of 2.5 kHz was used. After power attenuation (beam attenuator 2 consists of half-wave plate and prism polarizer), the beam was sent through a second-harmonic generator 3. We employed second-harmonic radiation (514 nm wavelength) to perform microdissections on ZP in the form of arbitrary alphanumeric characters. The radiation was then directed through a telescope 4 (with magnification 1:3) to fill the entrance aperture of the microscope objective 8. After passing through an electro-mechanical shutter 5, laser beam was sent into an IX-71 microscope (Olympus) and was focused on the sample with a 20 × , NA = 0.5 UPLFLN objective (Olympus). The focal spot diameter was measured to be about 1.9 μm. The Petri dishes with experimental embryos were placed on a motorized X–Y stage (Märzhäuser) and moved with a speed of ~10 µm/s along prescribed path during laser-assisted code engraving. Images and videos were taken using a DFK 72AUC02 CMOS camera (Imaging Source). Notch filter 13 (NF514-17, Thorlabs) has been used to protect the camera and human eyes against laser radiation.
Fig. 1.
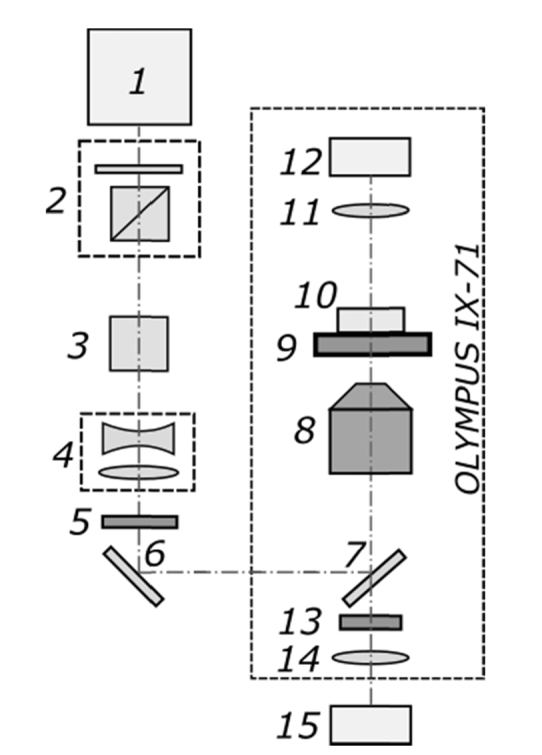
The setup sketch of the system for femtosecond-laser based embryo labelling: 1 – femtosecond ytterbium laser, 2 – beam attenuator, 3 – second-harmonic generator, 4 – telescope, 5 – electro-mechanical shutter, 6 – mirror, 7 – dichroic mirror, 8 – microscope objective, 9 – X-Y motorized stage, 10 – Petri dish, 11 – condenser lens, 12 – microscope lamp, 13 – Notch filter, 14 – tube lens, 15 – CMOS camera.
3. Materials and methods
3.1 Embryo collection, culture and monitoring
Female mice six-to-eight weeks old (C57BL/6J strain) were mated with males late in the evening and checked the following morning for a presence of a copulation plug. Later the same day mice were sacrificed by cervical dislocation. Oviducts were removed and placed into HEPES-containing medium (Human Tubal Fluid Medium, Irvine Scientific). Prior to this HEPES-containing medium was stored in a tightly closed bottles in the CO2-incubator (37 оС, 5% СО2/air) for approximately 15 min to equilibrate the temperature of the medium. Then, oviducts were dissected using insulin gauge needles and zygotes were collected (an average, 8 ± 2 zygotes per mouse were collected). Zygotes were collected from fresh oviducts according to standard protocol [37] with slight modifications. Briefly, to remove cumulus cells, cumulus-oocyte complexes (COCs) were placed into the HEPES-buffered solution containing 30IU/ml hyaluronidase (COOK Sydney IVF) for 2 minutes and placed into the CO2-incubator. Then zygotes were removed from cumulus cells by gentle pipetting and washed for three times in fresh HEPES-containing medium.
Embryos were collected and divided into three groups: group A (experimental embryos), group B (embryos kept out of the incubator for the same time as group A, but not exposed to laser radiation), and group C (fully intact control embryos stored in a CO2 incubator at 37 °C). Group A and B embryos were transferred to fresh drops of Human Tubal Fluid Medium in Petri dishes (glass-bottomed 170 μm thick, 5 embryos per dish) under mineral oil balanced with the medium. After the procedure, embryos of groups A and B were placed into 4-well plate (4–5 embryos per well), returned to the incubator, and cultured in Global Total medium (LifeGlobal Group) in the CO2 incubator until they hatched. Group C embryos after retrieval from mice were directly placed to the Global Total medium and cultured in the 4-well plate in the CO2 incubator. Embryos from the experimental group were treated with laser radiation in order to create the code in the ZP. Evaluation of embryonic development was performed and readability of laser engraved codes was checked every 24 hours post laser processing using AxioObserver.Z1 (Zeiss) or IX-71 microscopes (Olympus).
3.2 Ethics statement
All manipulations with animals were performed according to the Moscow State University Bioethical Committee recommendations (Ethical approval documentation registration number 72-j; date of registration March 26, 2018).
4. Microsurgery of embryo shell with femtosecond laser pulses
4.1 Measuring optimal laser pulse energies for zona pellucida microsurgery
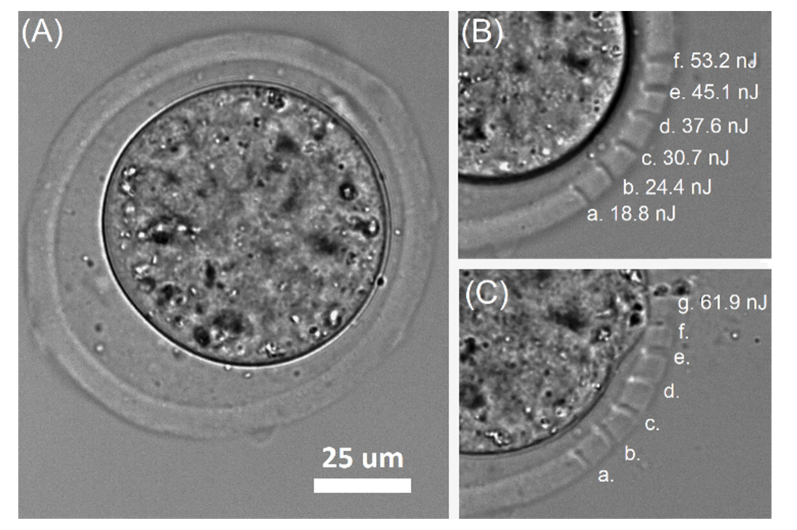
In order to determine optimal parameters for laser microsurgery of embryo outer shell we performed a set of microdissections on the ZP of mouse embryo (0.5 dpc, Fig. 2(A)) at various laser pulse energies (2.5 kHz pulse repetition rate, 20 × NA = 0.5 microobjective). Energy of laser pulses was varied in the range of several tens of nanojoules. We determined laser pulse energy of 18.8 ± 0.2 nJ to be a threshold, as far as microdissections on ZP could not be clearly visualized at lower pulse energies. Figures 2(B) and 2(C) demonstrate dependence of cut width on the energy of laser pulses varied in the range of 18.8 – 61.9 nJ. Minimal cut width was measured to be 1.55 ± 0.2 μm (Epulse = 18.8 ± 0.2 nJ), and maximal – 4.08 ± 0.3 μm (Epulse = 53.2 ± 1.2 nJ). At pulse energies of 53.2 ± 1.2 nJ formation of small cavitation bubbles did not lead to embryo membrane damage. Nevertheless, laser microsurgery at such energy levels should be performed with caution. Further energy increase (Epulse = 61.9 ± 1.4 nJ) resulted in formation of multiple cavitation bubbles of bigger sizes, which inevitably violated the embryo membrane integrity and caused irreversible embryo damage (Fig. 2(C)).
Fig. 2.
Determination of optimal range of laser pulse energies for ZP microsurgery: (A) 0.5 dpc mouse embryo prior to femtosecond laser microsurgery, (B) microdissections on the ZP at different laser pulse energies, (C) irreversible embryo damage during ZP microsurgery with laser pulse energies of 61.9 ± 1.4 nJ.
Thus, laser pulse energies in the range of 18 – 45 nJ were considered as safety for the experimental conditions used (laser wavelength of 514 nm, pulse duration of 280 fs, pulse repetition rate of 2.5 kHz). In our study laser pulse energies were chosen to be slightly above the threshold value in order to create thin, well-defined cuts on the ZP surface and prevent the formation of multiple, relatively large cavitation bubbles. For 280 fs laser pulses at 514 nm with the energy of 20 nJ per pulse, we calculated the peak power and power density to be about 71 kW and 2.5 TW/cm2.
4.2 Embryo marking with alphanumerical code by femtosecond laser microsurgery
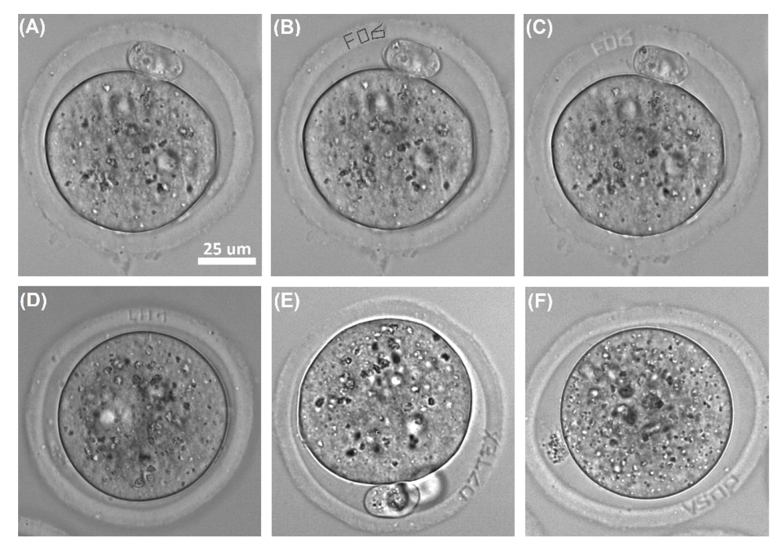
Basic principles of femtosecond laser-assisted microsurgery of ZP have been described in details in [32]. Briefly, alphanumerical characters were overlaid in the custom-built software by simply drawing the combination of primitive elements (like lines, curves etc.) on top of live image of embryo from the camera. Each drawn element was converted to a sequence of commands to the motorized stage. Start of the stage movement was synchronized with laser radiation “turn-on”. Figures 3(A) and 3(B) demonstrate images of embryo (0.5 dpc) prior to and post overlaying the “FO6” code in the software. Embryo with the code “FO6” engraved on its ZP is shown in Fig. 3(C). Examples of various codes engraved on ZP also presented in Figs. 3D-F).
Fig. 3.
Embryo labeling with alphanumerical codes by femtosecond microsurgery of ZP: (A) mouse embryo (0.5 dpc) prior to laser-assisted engraving, (B) creation of “FO6” code in the software, (C) mouse embryo with the code engraved on its ZP, (D – F) mouse embryos labelled with codes “LH1”, “07TEX”, and “V50P” correspondingly.
As far as embryo may change its orientation during the further ART cycle, we tried marking the egg coat of the embryo in three planes in order to simplify the process of code searching and embryo identification. Three-plane laser engraving was performed as follows. As soon as code was created in the first plane of each embryo’s ZP, the Petri dish with embryos (typically 5 embryos per dish) was gently shaken in order to cause embryo rotation. Then the process of laser-assisted code engraving was repeated in the second plane and Petri dish was shaken one more time in order to create the code in the third plane of ZP. Right after the procedure embryos were placed back into the incubator.
4.3 Analysis of code readability during preimplantation embryo development
We have previously demonstrated that the codes engraved on the zygote’s ZP (0.5 dpc) were also clearly recognizable on the 1.5-3.5 dpc. A total of 51 mouse embryos has been marked with codes (33 embryos were labelled in a single plane, and 18 embryos - in three planes (see Ref [32].). We have observed no decrease in the developmental rates of the embryos exposed to fs-laser radiation as compared to that of control group embryos (53 embryos in control group B and 43 embryos in control group C). All of the treated embryos (Group A) developed to the blastocyst stage, and hatching rate (81.8% and 77.7% in groups with single-plane and three-planes code engraving correspondingly) at the time of their assessment (at 4.5-5.0 dpc) was nearly the same as in parallel control group (≈80.8%) and slightly higher, than in the intact control group (70%).
Thus, the technique of femtosecond laser engraving on the ZP has been shown to be useful at least for embryo identification from the day 0.5 to day 3 when embryo transfer can be done. We have supposed that code readability could significantly degrade at late preimplantation developmental stages due to ZP thinning prior to hatching. In order to verify this assumption preimplantation development of 20 embryos subjected to three-plane laser-engraving procedure has been studied and compared with that of intact embryos group (a total of 20 embryos). Thus, a total of 40 mouse embryos was used in the study. We have measured the thickness of ZP at hatching stage and found that the thickness of the ZP subjected to laser-assisted code engraving was different from that of untreated embryos from control groups B and C. ZP thickness of each embryo was measured at 5 different points, then mean value and standard deviation were calculated. The same procedures were performed for control group embryos.
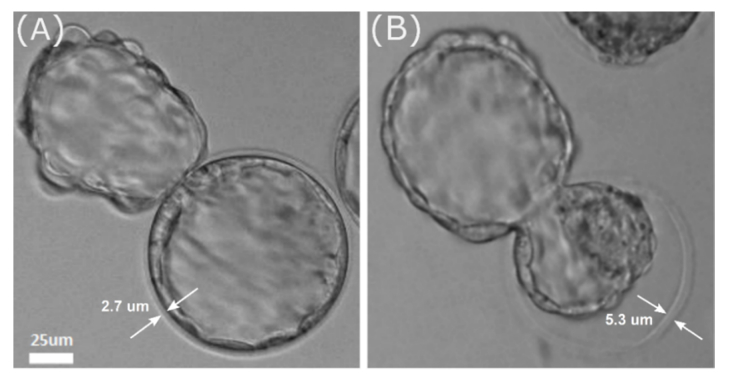
Average thickness of embryo ZP in the experimental and control groups was 5.84 ± 0.60 μm and 2.38 ± 0.40 μm respectively. The difference in the outer shell thickness for control and experimental group embryos is demonstrated in Fig. 4. Mean value for ZP thickness of intact embryo in Fig. 4(A) is 2.78 ± 0.30 μm, while it is equal to 5.26 ± 0.35 μm for experimental embryo shown in Fig. 4(B). Average thickness of ZP at 0.5 dpc (when laser-assisted code engraving was performed) did not differ significantly in experimental and control groups and was equal to 7.08 ± 0.26 μm and 7.19 ± 0.22 μm respectively. In case of proper choice of symbols for engraving as well as their size and a distance between them, given thickness values were considered to be enough for code to be clearly recognized even at the hatching stage.
Fig. 4.
Difference in ZP thickness between control group embryo (A) and experimental group embryo (B) at hatching stage.
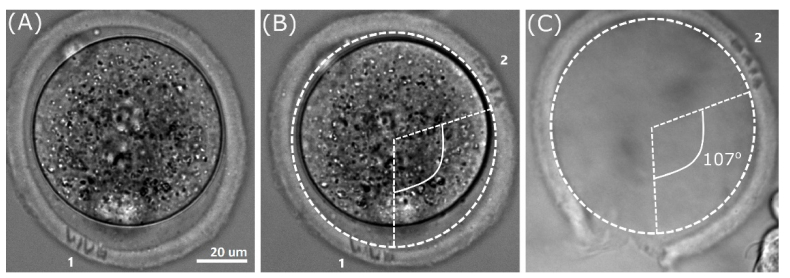
The possibility of embryo identification at a late developmental stage using the code engraved is demonstrated in Fig. 5. At first, the code “VIVO” (indicated as “1” in Fig. 5(A)) was engraved in the embryo ZP at 0.5 dpc stage, then procedure was repeated for two other planes. It should be noted that the code “VIVO” (indicated as “1” in Fig. 5(A)) is slightly out of focus in Fig. 5(B), whereas newly created code “VIVO” (indicated as “2”) is in focus and clearly seen. Figure 5(C) demonstrates the outer shell after embryo hatching. The code “VIVO” (created in the second plane) is still recognizable (nevertheless, it is slightly out of focus in the figure).
Fig. 5.
Checking the code readability at late developmental stage. (A) Mouse embryo (0.5 dpc). The code “VIVO” indicated as “1” is engraved in the first plane. (B) The same embryo. The code “VIVO” indicated as “2” is engraved in the second plane. (C) ZP after embryo hatching; the code “2” is still readable.
The second peculiar fact is that code marking in multiple planes did not decrease embryo hatching rate compared to control groups. Moreover, code engraving procedure similarly to assisted hatching can facilitate hatching to start right at the location of laser treatment. To demonstrate this, an angle between the code “1” and code “2” shown in Fig. 5(B) in the ZP of embryo at 0.5 dpc was measured. This angle equal to 107° is also shown in Fig. 5(C). One can see that hatching occurred in the rear part of ZP exactly where the code “1” had been engraved. This was not a single case, but determining the exact rate of embryo hatching through the code in the ZP was beyond the framework of current research; detailed studying of this phenomenon is a matter of further investigations.
5. Discussion and conclusions
Although events of eggs, sperm or oocytes switching occur very rare at fertility clinics, sample mix-up has been reported in the literature several times. According to the latest research, 90.4% of the respondents (patients undergoing IVF treatment in a single private infertility center in Europe) expressed significant concerns relating to biological sample mix-up [36]. To eliminate the risk of any mix-up, strong recommendations and protocols by leading ART-related organizations (ESHRE and HFEA (Europe), FLASEF (Latin America) have been developed [38,39]. According to their guidelines, accurate labelling of all labware for correct patient identification and “double-witnessing” procedure are mandatory. Recently, novel electronic witness systems have been developed. Systems based on Radio Frequency Identification technology [40,41], barcode labels [42], and even direct embryo tagging system based on silicon barcode injection into zygotes/embryos [43] have been proposed. However, nearly all of these approaches have some limitations. Thus, for example, additional equipment is required (such as label printer or code reader) when using safety systems based on QR (quick response) code generation and recognition [44]; volatile organic components in the printing and adhesive materials should be thoroughly selected so as not to be toxic to embryo development [44]. Moreover, possible effects of polysilicon barcodes proposed in [43] on fetal growth and development should be studied in future. Thus, optimisation of existing methods aimed at preventing biological sample mix-up and development of new alternative devices and techniques are still required.
In this paper the possibility of direct embryo tagging with femtosecond laser microsurgery as well as possibility of embryo identification during the whole preimplantation period have been demonstrated. Due to highly localized effect during the action of fs-laser pulses, which fades away for out-of-focus cellular structures, relatively low pulse energy, and ultrahigh intensity, fs-lasers could be used for precise and delicate microsurgery of ZP with minimal risk of thermal damage to the adjacent embryo cells. The advantages of femtosecond laser-assisted microsurgery over milli-/microsecond or even nano-/picosecond duration pulses for minimizing collateral damage have been discussed in [45–47]. We have performed laser-assisted engraving of alphanumeric codes on mouse embryo’s ZP in three different planes in order to simplify the process of code searching and embryo identification. The codes engraved have been proven to be readable even after embryo hatching. No morphological changes of embryos subjected to three-plane laser-assisted engraving as compared to control group embryos have been observed. We have demonstrated that the average ZP thickness of embryos in experimental group was larger than ZP thickness of control group embryos. In spite of this fact, the blastocysts broke out of the shell successfully and hatching rates in the experimental and control groups embryos were nearly the same. A possible explanation for this is that formation of cuts on the ZP during laser-assisted code engraving leads to a weakening of the ZP stiffness and its easier rupture with no need for significant thinning.
In this study femtosecond laser pulses have been successfully applied for ZP microsurgery of mouse embryos. The thickness of the ZP at the time of code engraving (0.5 dpc) was measured to be ~7 µm. It was enough for creating high-quality, clearly readable codes. ZP of human embryos is usually wider than that of mouse embryos. Data regarding typical thickness of ZP of mouse and human embryos at various days of in vitro culture are summarized in Table 1. As can be seen, the thickness of the human embryo ZP usually lies within the range of 14 – 18 µm. Taking into account relatively higher ratio of ZP width to the size of focused laser beam in human embryo as compared to the mouse one, we suppose that the technique of femtosecond laser-assisted code engraving will be easier to implement for human embryo labelling as compared to mouse one.
Table 1. Zona pellucida thickness of mouse and human embryos.
| Embryo type | ZP thickness, µm | Details | Day of ZP measurement | Ref. No | |
|---|---|---|---|---|---|
| Mouse | 8 | – | – | [48] | |
| 4.3 ± 1.4 | C57BL/6J strain | 3.25 dpc | [49] | ||
| 10.8 ± 1.2 – 12.2 ± 3.2 | (ICR) strain | two-cell | [50] | ||
| Human (mean age of patients 33.9 ± 3.4) | 16.6 ± 3.2 | Fertilized | [51] | ||
| 18.9 ± 4.0 | unfertilized | ||||
| Human (mean age 33.8 ± 4.2) | 19.4 ± 2.7 | Patients with unexplained infertility | Day 1 of culture | [52] | |
| 17.7 ± 2.2 | Patients with endometriosis | Day 1 of culture | |||
| 17.5 ± 2.4 | Patients with tubal-factor infertility | Day 1 of culture | |||
| 16.4 ± 2.7 | Patients with male-factor infertility | Day 1 of culture | |||
| 18.2 ± 0.2 | Day 1 of culture | ||||
| 16.0 ± 0.2 | Day 2 of culture | ||||
| 13.9 ± 0.16 | Day 3 of culture | ||||
| Human (mean age 33.8 ± 4.2) | 17.7 ± 0.14 | Day 1 of culture | [53] | ||
| 16.3 ± 0.14 | Day 2 of culture | ||||
| 14.9 ± 0.14 | Day 3 of culture | ||||
| Embryo scope rangea: | |||||
| 14.4 ± 0.23 | 1.0-1.5 | Day 3 of culture | |||
| 15.1 ± 0.37 | 2.0- 2.5 | Day 3 of culture | |||
| 15.9 ± 0.60 | >3.0 | Day 3 of culture | |||
| Human (mean age 35.3 ± 4.2) | 14.29 ± 0.56 | Embryo scope rangea: 1.0-1.5 | Day 3 of culture | [54] | |
| 16.22 ± 0.61 | 1.6-2.2 | Day 3 of culture | |||
| Human (mean age 33.91 ± 5.91) | 16.18 ± 2.00 | Day 3 of culture | [55] | ||
In conclusion, we have demonstrated that femtosecond lasers could be employed as precise and effective tools for embryo microsurgery. Potential applications for laser-assisted code engraving technique would not be limited to safety systems aimed at preventing embryo mix-ups during the IVF treatment. The technique may be also useful in the field of developmental biology for studying the peculiarities of embryo development during their culture in groups.
Funding
Russian Foundation for Basic Research and Moscow City Government (Nº 19-32-70036).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Davidson L. M., Liu Y., Griffiths T., Jones C., Coward K., “Laser technology in the ART laboratory: a narrative review,” Reprod. Biomed. Online 38(5), 725–739 (2019). 10.1016/j.rbmo.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 2.Bedient C., Khanna P., Desai N., “Laser Pulse Application in IVF,” in Lasers-Applications in Science and Industry, Jakubczak K., ed. (InTech, 2011), pp. 193–214. [Google Scholar]
- 3.Montag M. H. M., Klose R., Köster M., Rösing B., van der Ven K., Rink K., van der Ven H., “Application of non-contact laser technology in assisted reproduction,” Med. Laser Appl. 24(1), 57–64 (2009). 10.1016/j.mla.2008.11.001 [DOI] [Google Scholar]
- 4.Montag M., Rink K., Delacretaz G., van der Ven H., “Laser induced immobilization and plasma membrane permeabilization in human spermatozoa,” Hum. Reprod. 15(4), 846–852 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Millan H., Ocana Quero J. H., “Preliminary results of the evaluation of the use of clinical laser He-Ne radiation in the process of bovine “in vitro fertilization”,” Bull. UASVM Vet. Med. 66(1), 495 (2009). [Google Scholar]
- 6.Soares C. A., Annes K., Dreyer T. R., Magrini T., Sonoda M. T., da Silva Martinho H., Nichi M., d’Ávila Assumpção M. E., Milazzotto M. P., “Photobiological effect of low-level laser irradiation in bovine embryo production system,” J. Biomed. Opt. 19(3), 035006 (2014). 10.1117/1.JBO.19.3.035006 [DOI] [PubMed] [Google Scholar]
- 7.Sato H., Landthaler M., Haina D., Schill W. B., “The effects of laser light on sperm motility and velocity in vitro,” Andrologia 16(1), 23–25 (1984). 10.1111/j.1439-0272.1984.tb00229.x [DOI] [PubMed] [Google Scholar]
- 8.Lenzi A., Claroni F., Gandini L., Lombardo F., Barbieri C., Lino A., Dondero F., “Laser radiation and motility patterns of human sperm,” Arch. Androl. 23(3), 229–234 (1989). 10.3109/01485018908986845 [DOI] [PubMed] [Google Scholar]
- 9.Nascimento J. M., Shi L. Z., Meyers S., Gagneux P., Loskutoff N. M., Botvinick E. L., Berns M. W., “The use of optical tweezers to study sperm competition and motility in primates,” J. R. Soc. Interface 5(20), 297–302 (2008). 10.1098/rsif.2007.1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow K. W., Preece D., Berns M. W., “Effect of red light on optically trapped spermatozoa,” Biomed. Opt. Express 8(9), 4200–4205 (2017). 10.1364/BOE.8.004200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preece D., Chow K. W., Gomez-Godinez V., Gustafson K., Esener S., Ravida N., Durrant B., Berns M. W., “Red light improves spermatozoa motility and does not induce oxidative DNA damage,” Sci. Rep. 7(1), 46480 (2017). 10.1038/srep46480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clement-Sengewald A., Schütze K., Ashkin A., Palma G. A., Kerlen G., Brem G., “Fertilization of bovine oocytes induced solely with combined laser microbeam and optical tweezers,” J. Assist. Reprod. Genet. 13(3), 259–265 (1996). 10.1007/BF02065947 [DOI] [PubMed] [Google Scholar]
- 13.Clement-Sengewald A., Buchholz T., Schütze K., Berg U., Berg E. D., “Noncontact, laser-mediated extraction of polar bodies for prefertilization genetic diagnosis,” J. Assist. Reprod. Genet. 19(4), 183–194 (2002). 10.1023/A:1014894029099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilina I. V., Rakityanskiy M. M., Sitnikov D. S., Ovchinnikov A. V., Agranat M. B., Khramova Y. V., Semenova M. L., “Biomedical and biotechnology applications of noncontact femtosecond laser microsurgery of living cells,” AIP Conf. Proc. 1464, 560–571 (2012). 10.1063/1.4739909 [DOI] [Google Scholar]
- 15.Obruca A., Strohmer H., Sakkas D., Menezo Y., Kogosowski A., Barak Y., Feichtinger W., “Use of lasers in assisted fertilization and hatching,” Hum. Reprod. 9(9), 1723–1726 (1994). 10.1093/oxfordjournals.humrep.a138781 [DOI] [PubMed] [Google Scholar]
- 16.Germond M., Nocera D., Senn A., Rink K., Delacrétaz G., Fakan S., “Microdissection of mouse and human zona pellucida using a 1.48-microns diode laser beam: efficacy and safety of the procedure,” Fertil. Steril. 64(3), 604–611 (1995). 10.1016/S0015-0282(16)57800-5 [DOI] [PubMed] [Google Scholar]
- 17.Rink K., Delacrétaz G., Salathé R. P., Senn A., Nocera D., Germond M., De Grandi P., Fakan S., “Non-contact microdrilling of mouse zona pellucida with an objective-delivered 1.48 mm diode laser,” Lasers Surg. Med. 18(1), 52–62 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Balaban B., Urman B., Yakin K., Isiklar A., “Laser-assisted hatching increases pregnancy and implantation rates in cryopreserved embryos that were allowed to cleave in vitro after thawing: a prospective randomized study,” Hum. Reprod. 21(8), 2136–2140 (2006). 10.1093/humrep/del097 [DOI] [PubMed] [Google Scholar]
- 19.Kanyo K., Zeke J., Kriston R., Szücs Z., Cseh S., Somoskoi B., Konc J., “The impact of laser-assisted hatching on the outcome of frozen human embryo transfer cycles,” Zygote 24(5), 742–747 (2016). 10.1017/S0967199416000058 [DOI] [PubMed] [Google Scholar]
- 20.Le M. T., Nguyen T. T. A., Nguyen T. T. T., Nguyen V. T., Le D. D., Nguyen V. Q. H., Cao N. T., Aints A., Salumets A., “Thinning and drilling laser-assisted hatching in thawed embryo transfer: A randomized controlled trial,” Clin. Exp. Reprod. Med. 45(3), 129–134 (2018). 10.5653/cerm.2018.45.3.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantoudis E., Podsiadly B. T., Gorgy A., Venkat G., Craft I. L., “A comparison between quarter, partial and total laser assisted hatching in selected infertility patients,” Hum. Reprod. 16(10), 2182–2186 (2001). 10.1093/humrep/16.10.2182 [DOI] [PubMed] [Google Scholar]
- 22.Douglas-Hamilton D. H., Conia J., “Thermal effects in laser-assisted pre-embryo zona drilling,” J. Biomed. Opt. 6(2), 205–213 (2001). 10.1117/1.1353796 [DOI] [PubMed] [Google Scholar]
- 23.Tucker M. J., Ball G. D., “Assisted hatching as a technique for use in human in vitro fertilization and embryo transfer is long overdue for careful and appropriate study,” Journal of Clinical Embryology 12(1), 5–8 (2009). [Google Scholar]
- 24.Taylor T. H., Gilchrist J. W., Hallowell S. V., Hanshew K. K., Orris J. J., Glassner M. J., Wininger J. D., “The effects of different laser pulse lengths on the embryo biopsy procedure and embryo development to the blastocyst stage,” J. Assist. Reprod. Genet. 27(11), 663–667 (2010). 10.1007/s10815-010-9461-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torres-Mapa M. L., Antkowiak M., Cizmarova H., Ferrier D. E. K., Dholakia K., Gunn-Moore F. J., “Integrated holographic system for all-optical manipulation of developing embryos,” Biomed. Opt. Express 2(6), 1564–1575 (2011). 10.1364/BOE.2.001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuetemeyer K., Lucas-Hahn A., Petersen B., Lemme E., Hassel P., Niemann H., Heisterkamp A., “Combined multiphoton imaging and automated functional enucleation of porcine oocytes using femtosecond laser pulses,” J. Biomed. Opt. 15(4), 046006 (2010). 10.1117/1.3463012 [DOI] [PubMed] [Google Scholar]
- 27.Ilina I. V., Ovchinnikov A. V., Sitnikov D. S., Rakityanskiy M. M., Agranat M. B., Khramova Y. V., Semenova M. L., “Application of Femtosecond Laser Pulses in Biomedical Cell Technologies,” High Temp. 51(2), 173–178 (2013). 10.1134/S0018151X13020089 [DOI] [Google Scholar]
- 28.Osychenko A. A., Zalessky A. D., Krivokharchenko A. S., Shakhbazian A. K., Ryabova A. V., Nadtochenko V. A., “Fusion of blastomeres in mouse embryos under the action of femtosecond laser radiation. Efficiency of blastocyst formation and embryo development,” Quantum Electron. 45(5), 498–502 (2015). 10.1070/QE2015v045n05ABEH015767 [DOI] [Google Scholar]
- 29.Il’ina I. V., Khramova Yu. V., Filatov M. A., Semenova M. L., Sitnikov D. S., “Femtosecond laser assisted hatching: dependence of zona pellucida drilling efficiency and embryo development on laser wavelength and pulse energy,” High Temp. 54(1), 46–51 (2016). 10.1134/S0018151X15060115 [DOI] [Google Scholar]
- 30.Ilina I. V., Sitnikov D. S., Ovchinnikov A. V., Agranat M. B., Khramova Y. V., Semenova M. L., “Noncontact microsurgery and micromanipulation of living cells with combined system “Femtosecond laser scalpel-optical tweezers,”,” Proc. SPIE 8427, 84270S (2012). 10.1117/12.922333 [DOI] [Google Scholar]
- 31.Ilina I. V., Khramova Y. V., Filatov M. A., Semenova M. L., Sitnikov D. S., “Application of Femtosecond Laser Scalpel and Optical Tweezers for Noncontact Biopsy of Late Preimplantation Embryos,” High Temp. 53(6), 804–809 (2015). 10.1134/S0018151X15060103 [DOI] [Google Scholar]
- 32.Ilina I. V., Khramova Y. V., Filatov M. A., Sitnikov D. S., “Femtosecond laser is effective tool for zona pellucida engraving and tagging of preimplantation mammalian embryos,” J. Assist. Reprod. Genet. (to be published), doi:. 10.1007/s10815-019-01424-x [DOI] [PMC free article] [PubMed]
- 33.Liebler R., “Are you my parent? Are you my child? The role of genetics and race in defining relationships after reproductive technological mistakes,” DePaul J. Health Care Law 5(1), 15–56 (2002). [PubMed] [Google Scholar]
- 34.Spriggs M., “IVF mixup: white couple have black babies,” J. Med. Ethics 29(2), 65 (2003). 10.1136/jme.29.2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bender L., “To err is human’. ART mix-ups: A labor-based, relational proposal,” J. Gend. Race Justice 9, 1–90 (2006). [Google Scholar]
- 36.Forte M., Faustini F., Maggiulli R., Scarica C., Romano S., Ottolini C., Farcomeni A., Palagiano A., Capalbo A., Ubaldi F. M., Rienzi L., “Electronic witness system in IVF-patients perspective,” J. Assist. Reprod. Genet. 33(9), 1215–1222 (2016). 10.1007/s10815-016-0759-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan B., Beddington R., Costantini F., Lacy E., Manipulating the Mouse Embryo: A Laboratory Manual (Cold Spring Harbor Lab, 2014). [Google Scholar]
- 38.de los Santos M. J., Ruiz A., “Protocols for tracking and witnessing samples and patients in assisted reproductive technology,” Fertil. Steril. 100(6), 1499–1502 (2013). 10.1016/j.fertnstert.2013.09.029 [DOI] [PubMed] [Google Scholar]
- 39.“The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Revised guidelines for human embryology and andrology laboratories,” Fertil. Steril. 90(5), S45–S59 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Glew A. M., Hoha K., Graves J., Lawrence H., Read S., Ah-Moye M., “Radio frequency identity tags ‘RFID’ for electronic witnessing of IVF laboratory procedures,” Fertil. Steril. 86(3), S170 (2006). 10.1016/j.fertnstert.2006.07.454 [DOI] [Google Scholar]
- 41.Thornhill A. R., Brunetti X. O., Bird S., “Measuring human error in the IVF laboratory using an electronic witnessing system,” Proc. of 17th World Congress on Controversies in Obstetrics, Gynecology & Infertility (COGI) (2013), pp. 101–106. [Google Scholar]
- 42.Schnauffer K., Kingsland C., Troup S., “Barcode labelling in the IVF laboratory,” Hum. Reprod. 20(suppl.1), i79–i80 (2005). [Google Scholar]
- 43.Novo S., Nogués C., Penon O., Barrios L., Santaló J., Gómez-Martínez R., Esteve J., Errachid A., Plaza J. A., Pérez-García L., Ibáñez E., “Barcode tagging of human oocytes and embryos to prevent mix-ups in assisted reproduction technologies,” Hum. Reprod. 29(1), 18–28 (2014). 10.1093/humrep/det409 [DOI] [PubMed] [Google Scholar]
- 44.Hur Y. S., Ryu E. K., Park S. J., Yoon J., Yoon S. H., Yang G. D., Hur C. Y., Lee W. D., Lim J. H., “Development of a security system for assisted reproductive technology (ART),” J. Assist. Reprod. Genet. 32(1), 155–168 (2015). 10.1007/s10815-014-0367-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel A., Noack J., Huttman G., Paltauf G., “Mechanisms of femtosecond laser nanosurgery of cells and tissues,” Appl. Phys. B 81(8), 1015–1047 (2005). 10.1007/s00340-005-2036-6 [DOI] [Google Scholar]
- 46.Oraevsky A. A., Da Silva L. B., Rubenchik A. M., Feit M. D., Glinsky M. E., Perry M. D., Mammini B. M., Small W. I. V., Stuart B. C., “Plasma Mediated Ablation of Biological Tissues with Nanosecond-to-Femtosecond Laser Pulses: Relative Role of Linear and Nonlinear Absorption,” IEEE J. Sel. Top. Quantum. 2(4), 801–809 (1996). 10.1109/2944.577302 [DOI] [Google Scholar]
- 47.Feit M. D., Rubenchik A. M., Kim B. M., da Silva L. B., Perry M. D., “Physical characterization of ultrashort laser pulse drilling of biological tissue,” Appl. Surf. Sci. 127–129, 869–874 (1998). 10.1016/S0169-4332(97)00758-7 [DOI] [Google Scholar]
- 48.Wassarman P. M., Josefowicz W. J., “Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence,” J. Morphol. 156(2), 209–235 (1978). 10.1002/jmor.1051560206 [DOI] [PubMed] [Google Scholar]
- 49.Leonavicius K., Royer C., Preece C., Davies B., Biggins J. S., Srinivas S., “Mechanics of mouse blastocyst hatching revealed by a hydrogel-based microdeformation assay,” Proc. Natl. Acad. Sci. U.S.A. 115(41), 10375–10380 (2018). 10.1073/pnas.1719930115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park S. B., Kim H. J., Choi Y. B., Ahn K. H., Lee K. H., Yang J. B., Yu C. S., Seo B. B., “The effect of various assisted hatching techniques on the mouse early embryo development,” Clin. Exp. Reprod. Med. 41(2), 68–74 (2014). 10.5653/cerm.2014.41.2.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertrand E., Van den Bergh M., Englert Y., “Does zona pellucida thickness influence the fertilization rate?” Hum. Reprod. 10(5), 1189–1193 (1995). 10.1093/oxfordjournals.humrep.a136116 [DOI] [PubMed] [Google Scholar]
- 52.Loret De Mola J. R., Garside W. T., Bucci J., Tureck R. W., Heyner S., “Analysis of the Human Zona Pellucida During Culture: Correlation with Diagnosis and the Preovulatory Hormonal Environment,” J. Assist. Reprod. Genet. 14(6), 332–336 (1997). 10.1007/BF02765837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garside W. T., Loret de Mola J. R., Bucci J. A., Tureck R. W., Heyner S., “Sequential Analysis of Zona Thickness During In Vitro Culture of Human Zygotes: Correlation with Embryo Quality, Age, and Implantation,” Mol. Reprod. Dev. 47(1), 99–104 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Gabrielsen A., Bhatnager P. R., Petersen K., Lindenberg S., “Influence of Zona Pellucida Thickness of Human Embryos on Clinical Pregnancy Outcome Following in Vitro Fertilization Treatment,” J. Assist. Reprod. Genet. 17(6), 323–328 (2000). 10.1023/A:1009453011321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balakier H., Sojecki A., Motamedi G., Bashar S., Mandel R., Librach C., “Is the zona pellucida thickness of human embryos influenced by women’s age and hormonal levels?” Fertil. Steril. 98(1), 77–83 (2012). 10.1016/j.fertnstert.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 56.Van den Abbeel E., Van der Elst J., Van Waesberghe L., Camus M., Devroey P., Khan I., Smitz J., Staessen C., Wisanto A., Van Steirteghem A., “Hyperstimulation: the need for cryopreservation of embryos,” Hum. Reprod. 3(Suppl 2), 53–57 (1988). 10.1093/humrep/3.suppl_2.53 [DOI] [PubMed] [Google Scholar]
- 57.Staessen C., Van den Abbeel E., Carlé M., Khan I., Devroey P., Van Steirteghem A. C., “Comparison between human serum and Albuminar-20 (TM) supplement for in-vitro fertilization,” Hum. Reprod. 5(3), 336–341 (1990). 10.1093/oxfordjournals.humrep.a137100 [DOI] [PubMed] [Google Scholar]