Abstract
The photothermal effect is undergoing great interest due to advances in new photosensitizing materials and better-suited light sources, but studies are frequently hampered by the need to employ exogenous photothermal agents and expensive irradiation devices. Here we present a simple strategy based on direct NIR irradiation of the melanin pigment with a commercial 808-nm laser pointer. Proof-of-concept studies showed efficient photothermal effects on melanin in vitro and in vivo. After NIR irradiation, BALB/c mice bearing B16-F10 melanotic melanoma tumors revealed severe histopathological damage and massive necrosis in melanin-containing tumor tissue, while surrounding healthy tissues showed no damage. Therefore, the feasibility of this approach may allow implementing direct procedures for photothermal therapy of pigmented tumors.
1. Introduction
Weakly pigmented people (Caucasians) have high incidence of skin melanoma [1,2]. In contrast, strongly pigmented human populations and African-Americans have fewer incidence of melanoma, which seems to be due to the protective effect of melanin against UV irradiation [3,4], although in these cases the disease appears more aggressive [5]. Obviously, experimental research in murine models involving melanotic cells is highly relevant to the biology and treatment of melanoma tumors in human patients.
The murine melanoma B16, originated and growing in the C57BL/6 strain of black mice, is one of the most popular and extensively used tumor models in oncological research. In 1980 Poste and Fidler isolated from it the established B16-F10 cell line featuring high metastatic capacity, which opened the broad field of subpopulations in the biology of metastasis [6]. It is known that murine cells and tissues from one strain are rejected by the immune system when inoculated in another strain (except in immunosuppressed mice). Thus, the melanoma B16-F10 from C57BL/6 mice is rejected by the immune system when inoculated in mice of the histo-incompatible strain, BALB/c [7], because the histocompatibility antigen (H2) of C57BL/6 is H2b, but that of BALB/c is H2d [8,9].
Recently, one of us (L.L.C.) obtained a transplanted form of the melanoma B16-F10. This new melanotic tumor has the capacity of growing in the subcutaneous tissue of white histo-incompatible BALB/c mice, and is being subjected to further characterization. In the present work we decided to take advantage of this new transplanted melanoma, because it is one step closer to what happens in human patients, where melanotic tumors generally have high incidence (mainly in geographical areas with intensive sun radiation, like Arizona or Australia, with weakly pigmented populations) [1–5].
From a therapeutic point of view, it is well known that photodynamic (PDT) and photothermal therapy (PTT) are approved and currently applied clinical antitumor treatments using appropriate photosensitizers and red or near infrared (NIR) irradiation. Light absorption and scattering of most soft biological tissues are very low in the red to NIR spectral region (between 600 and 1300 nm), in the so-called biological (diagnostic and therapeutic) window, with the lowest absorption at 810-820 nm [10–12]. At present, NIR-laser irradiation finds many applications in different fields, mainly cosmetics (hair and tattoo removal, skin resurfacing, and photo-treatment of vascular-related disorders [13,14]). Ruby (694 nm), alexandrite (755 nm), diode (800-810 nm), and Nd:YAG (1064 nm) lasers are the most used light sources for photothermal melanin-based hair removal [15–18], and PTT of cancer [19–24]. New NIR dyes and nanoparticles are also being investigated for biomedical imaging [25,26], as well as photothermal agents for PTT [27–31]. PTT is based on the local heat generation during light exposure. Thermal energy dissipation occurs from light-excited molecules by internal conversion and relaxation of vibrational energy levels, generating fast heating [32,33]. A key element in the photothermal effect is the efficient conversion of electronic excitation energy to vibrational energy and heat (Fig. 1).
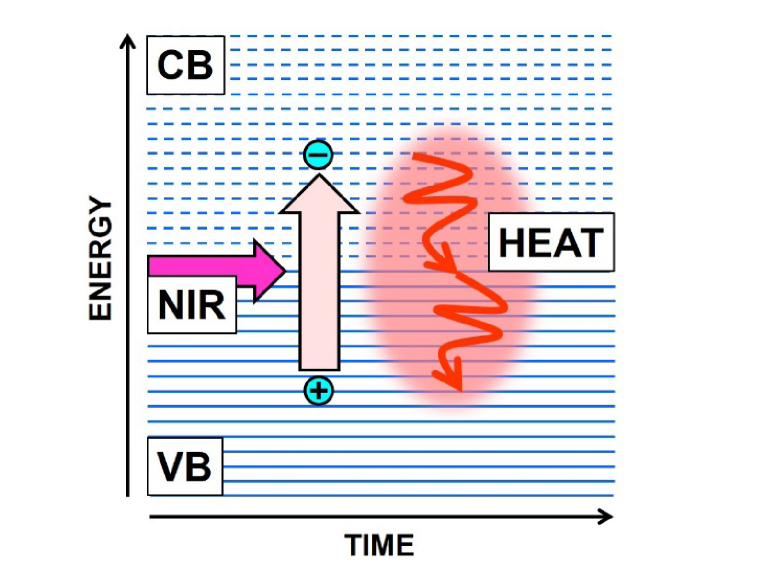
Fig. 1.
Scheme illustrating the photothermal effect. The energy of NIR photons is absorbed by electrons in the valence band (VB) of photothermal polymers or nanoparticles, pumping them to the conduction band (CB). Electronic excitation leaves positive charges (holes) in the VB, and after a very short time, electrons and holes recombine due to their mutual electrostatic attraction, converting a great part of the absorbed energy in lattice vibrations (wiggly arrows) and thermal energy.
In comparison with PDT, PTT has the advantage that it does not depend on O2 availability at the treated tumor, and only an efficient mechanism of fast light-to-heat conversion is necessary to induce a biological response. The photothermal effect (selective photothermolysis) was early applied by Anderson and Parrish [34] and Parrish et al. [35] on skin models, and relies on the selective absorption of brief laser pulses that generate and confine heat at certain pigmented cell and tissue targets by a radiation-less thermal decay. The laser pulse induces high temperature in the pigmented (absorbing) target, whereas no heating occurs in the surrounding tissue. In contrast, during a long irradiation heat transfer takes place and the entire tissue is damaged by coagulation necrosis [34,35].
In the last few years, carbon-based materials have been used to effectively generate a photothermal action on biological substrates, mainly experimental tumors [36]. We have recently reported that the pigment carbon black (CI: 77266) from China (Indian) ink is an efficient photothermal agent when injected to white mice tumors and irradiated with an 808-nm laser [37]. On the premise that melanotic melanoma cells indeed contain melanin as an intrinsic black chromophore, and that the structural and optical features of this biopolymer are similar to carbon black-containing materials with a quasi-graphitic structure [36,38–40], we decided to assess the photothermal effects of this endogenous and readily available intrinsic pigment, mainly in melanoma cells. In addition, the present murine model (white skin) is very adequate because if NIR laser irradiation of melanotic melanomas growing in black mice is used, the otherwise normal, but absorbing skin would also be strongly affected. In this work, proof-of-concept demonstrations and an easy-to-implement setup are applied to show the direct photothermal effect on melanotic tumors using NIR irradiation from a commercial laser pointer [37].
2. Materials and methods
2.1 NIR irradiation
A portable NIR commercial laser pointer (model GLP-808, Changchun New Industries Optoelectronics Technology Co., Ltd., P. R. China) was used for irradiation of model substrates and mice tumors. Emission wavelength as provided by the supplier is 808 ± 3 nm. The emission is cw and the emitted power is ~200 mW as measured with a thermopile (LM-10, Coherent Inc., USA). The beam diameter is ~1.2 mm which translates into an irradiance of ~17.68 Wcm−2. In all cases, the distance between the laser output and the different irradiated targets was kept constant at 10 cm. At this distance, the spot area of the laser beam was ~1.1 mm2.
2.2 Spectra and model experiments in vitro
Absorption spectra and photothermal assessment were done using sepiomelanin, which is analogous to the eumelanin from mammals [41]. Sepiomelanin is the eumelanin pigment isolated from the ink sac of cuttlefish (Sepia officinalis L.). Cuttlefish or sepia ink (Freiremar, Las Palmas de Gran Canaria, Spain) consists of a suspension of small, dark melanin-containing granules in a colorless plasma. Absorption spectra were made with sepia ink diluted 1:2000 (v/v) with distilled water using a UV-VIS 1604 spectrophotometer (Shimadzu, USA). For comparative purposes, spectra of China ink (Pelikan black drawing ink Z, Pelikan AG, Hannover, Germany) diluted 1:2000 (v/v) with distilled water were also recorded.
Kinetic measurements of ignition point and temperature increase were made using different concentrations of sepia ink and irradiation times. In the first case, white paper stripes were impregnated with different dilutions of sepia ink in distilled water, air dried and irradiated with the 808-nm laser, recording the time elapsed before ignition. Temperature increase of sepia ink was also assessed using a chemical thermometer (Franklin, Buenos Aires, Argentina) placed at 10 cm of the laser output. The thermometer bulb was surrounded first by a sleeve of paper strip impregnated with 50% sepia ink, covered with transparent adhesive tape to prevent evaporation and dryness of the paper, and then subjected to NIR irradiation.
2.3 Melanotic tumor transplants
Melanotic tumor transplants in white mice were carried out with inbred female BALB/c mice, 2–4 months old, from the Breeding Area of the Institute of Oncology, kept in a temperature- (20 ± 2 °C) and light-controlled room with free access to water and dietary chow. Animal care was provided in full compliance with regulations for protection of animals at the Institute.
Along of a previous tumoral research line, several white BALB/c mice were intended to be immunized against the allogeneic melanotic melanoma B16-F10 (from black C57BL/6 mice) by subcutaneous (sc) injection of ~106 cultured cells. Unexpectedly, in one of the white mice, the black tumor grew up, without any sign of regression. Then pieces of about 1 mm3 of this tumor were transplanted (sc by trocar) in the flank of five BALB/c mice. Two mice never developed tumors, in other two cases tumors grew up but finally they regressed, and in one animal the tumor grew up without regression. From this mouse, black tumor pieces of about 1 mm3 were transplanted again in the flank of other five BALB/c mice. As the transplanted melanomas are not homogeneous in pigmentation, only the more pigmented (black) regions were used to make the transplants. This procedure was repeated for several months, gradually increasing the percentage of mice without tumor regression. Currently, we are close to obtain the 100% of successful in tumor growth without regression.
2.4 Photothermal tumor experiments
Black tumors around 10 mm in diameter in BALB/c mice were subjected to control (no treatment, n = 2), and experimental protocols (NIR irradiation, n = 5). The overlying hair on tumors and surrounding skin regions was clipped, and before laser treatment, anesthesia was administrated by intraperitoneal injection of 0.2–0.3 mL of a solution of xylazine (20 mg/kg) and ketamine (50 mg/kg) in saline. Tumors were irradiated for 4, 6 and 10 min with the 808-nm laser fixed to a metal frame to keep a constant distance to the target [37]. For an example of the experimental setup, with the position of the NIR laser pointer and a tumor-bearing mouse, readers are referred to Fig. 3(a) [37].
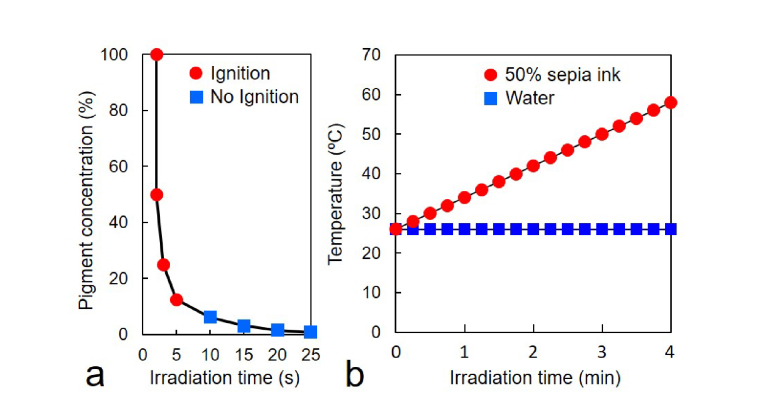
Fig. 3.
(a) Ignition time (seconds) of paper stripes impregnated with sepia ink at different concentrations (circles and squares). Papers were air dried, and then irradiated with 808-nm laser for different times. The higher the ink content, the faster the paper ignition. Note that even low ink concentration lead to ignition after 5 s, proof of its very high photothermal efficiency. (b) Temperature increase (in °C) of water (squares) and 50% sepia ink in water (circles) during 808-nm irradiation for different times (min). The temperature increases significantly for the sepia ink solution (58 °C at 4 min), while the temperature remains constant for water alone (26 °C at 4 min).
To reduce light scattering by keratin layers, a drop of glycerol was placed on the shaved skin covering the tumor area. In one case, a buttonhole was made in the skin over the tumor, and the tumor surface was then directly irradiated, thus avoiding the overlying white skin. Twenty-four hours after treatment, mice were sacrificed, and tumors resected, fixed in 10% buffered formalin for 24 h, and embedded in paraffin. Histological sections were stained with Gill’s II hematoxylin and eosin Y (H&E), observed and photographed under a light microscope (Zeiss Corp., Germany).
3. Results and discussion
3.1 In vitro photothermal experiments
Mammalian melanosomes are spherical or ellipsoid granules about 0.5-1 µm with eumelanin as the major pigment [35]. Although the precise macromolecular organization of eumelanins is still controversial, there is consensus about their main chemical structure, which corresponds to linear indole-5,6-quinone and indole-5,6-quinone-2-carboxylic acid polymers (as well as 5,6-dihydroxy derivatives), with 4,7’ linkages and a high level of conjugated double bonds [41–46]. An example of linearly arranged indole-5,6-quinone (and 5,6-dihydroxy)-2-carboxylic acid units from eumelanin is shown in Fig. 2(a). This biopolymer is capable of dissipating >99.9% of absorbed UV and visible radiation through non-radiative decay [47] and therefore, it is a very suitable photothermal agent.
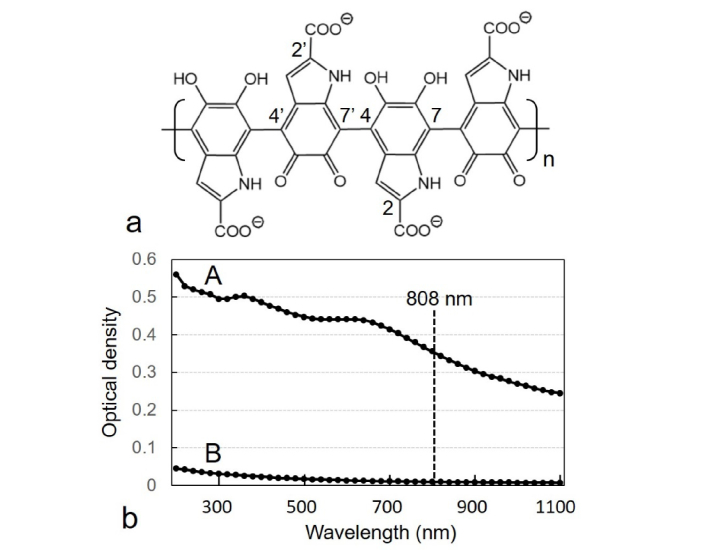
Fig. 2.
(a) Chemical structure of eumelanin, according to recent overviews [41,46], showing 4 units of the linear 4,7’-linked indole-5,6-quinone (and 5,6-dihydroxy)-2-carboxylic acid polymer, with atom numbering. (b) Comparative absorption spectra of sepia ink (A) and China ink (B), both diluted 1:2000 (v/v) with distilled water, showing the position of the 808-nm laser excitation (dashed line). The broad-band absorption curves decrease monotonically going from UV to NIR, a typical feature of these black polymeric chromophores.
Sepia melanin revealed a broad-band and structure-less optical absorption spectrum with decreasing values from the UV to NIR regions (Fig. 2(b)), in agreement with previous observations [35,39]. Comparative absorption curves of sepia ink (A) and China ink (B) showed the same broad-band features, which are characteristic of unsaturated polymeric chromophores with high level of double bond conjugation [12,34,35,39]. Experiments regarding the ignition time of paper stripes impregnated with sepia ink at different concentrations, dried and then irradiated with 808-nm laser for different times showed a very rapid ignition of melanin spots (Fig. 3(a)). The average ignition temperature of paper is 230 °C, and can be considered as a clear end point of the photothermal reaction. Likewise, kinetics of NIR-induced temperature increase of water and sepia ink also revealed striking differences. After 4 min 808-nm irradiation, the temperature of 50% sepia ink reached 58 °C, whereas that of water remained unchanged (Fig. 3(b)). Obviously, this large photothermal effect should generate a significant biochemical and mechanical tissue damage.
3.2 In vivo photothermal experiments
In histological H&E-stained sections, non-irradiated tumors from control animals showed the typical morphology of the B16-F10 melanotic melanoma, with large polyhedral cells containing a variable amount of melanosomes (Fig. 4(a)). Although the occurrence of melanin pigment in control sections was clearly observed within most cells from black tumor regions, big extracellular melanin deposits were also found.
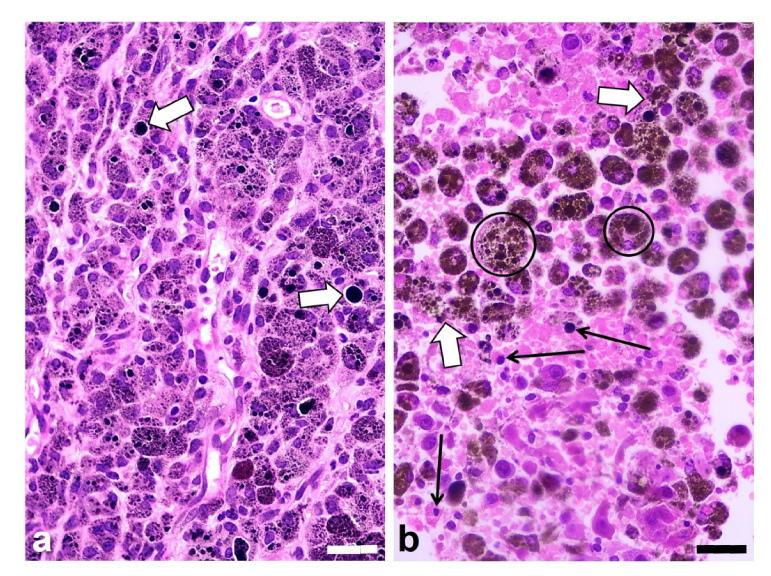
Fig. 4.
Histological H&E images of the melanotic melanoma B16-F10 growing in BALB/c mice, (a) without irradiation (control tumor), and (b) after irradiation with the 808-nm laser for 10 min and observed 24 h later. (a) Small and large black granules (melanosomes) appear within tumor cells, but sometimes they are located in the extracellular space (white arrows). The irradiated tumor section (b) shows extensive damaged and necrotic areas with rounded or disrupted tumor cells (white arrows), big brown-black melanophages (encircled), as well as pycnotic cell nuclei (black arrows), and cytoplasm fragmentation. Scale bars: 30 µm.
As expected from in vitro results, histopathological observations revealed that NIR irradiation induced a clear and extensive tissue damage, generally greater in tumor regions with large amounts of melanin. Sections of irradiated tumors showed massive necrosis, pycnotic nuclei, increased eosinophilia, severely disorganized stroma and edema, big and round brown-black melanophages, and disrupted tumor cells with release of melanin and cytoplasm fragments (Fig. 4(b)). Not only melanin-containing cells were damaged by NIR irradiation, but also neighboring cells (tumor cells with lesser or no melanosomes, stroma cells) appeared strongly damaged. This could represent a bystander effect due to the heat wave/mechanical wave (see below) affecting tumor regions near the melanin-containing laser target. However, scarce viable tumor cells showing mitosis were also observed. The degree of tissue damage was comparable after the different irradiation times used. Irradiation trough a skin buttonhole was also effective, and no appreciable differences were found compared with irradiation through the normal skin. In addition, the skin over the irradiated tumor area remained intact, without signs of cell damage. Blood vessels did not appear specifically damaged, and no hemorrhagic regions were observed within tumors.
Several experimental studies using NIR laser irradiation have been carried out on normal skin, and are widely applied for hair removal. As far as we know, attempts to treat melanotic melanomas directly by NIR laser irradiation have been rather overlooked, although the amelanotic melanoma B78-H1 cell line was sometimes applied in PTT studies using cyanines, naphthalocyanines and NIR irradiation [29,48]. In early studies using 351 nm-laser, degenerative and necrotic changes in normal melanin-containing human keratinocytes and disruption of melanosomes were observed [34]. It is well known that after strong and rapid heating by NIR laser pulses, protein coagulation causes “coagulation necrosis”, with subsequent hemostasis. The same process occurs by thermal diffusion during and after longer exposures to NIR irradiation [34,35]. It is known that tumor cells are more sensitive to a temperature increase than normal cells [49]. Currently, temperatures of 42-43 °C are considered lethal for tumor cells, and the smaller heat dissipation by impaired blood flow in tumors as compared to normal tissues contributes to thermal confinement and higher deleterious effect [50]. Interestingly, preliminary results showed that treatment of a non-pigmented murine tumor with synthetic dopamine-melanin followed by NIR irradiation induced lethal photothermal effects [51].
Other causes of tissue damage by photothermal effects could be the explosive vaporization of melanosomes in situ with severe damage of pigmented skin [52,53], and photothermally-induced generation of acoustic shock waves [21]. This seems important in our experimental context, as the observed tumor damage pattern at the cellular level is fully compatible with photothermally-driven mechanical damage to the irradiated sample (see white arrows in Fig. 4(b)). That this was achieved with a moderate intensity (200 mW) cw laser, not a pulsed one, deserves special notice, in our opinion. Indeed, the photothermal action in our experiments reminds of the similar thermocavitation phenomenon, whereby a cw laser is capable of driving cycles of bubble inception-cavitation-collapse under conditions of moderate light absorption by the irradiated volume [54]. Furthermore, “popping” sounds were audible during melanotic tumor irradiation, presumably proof of thermal (micro-)bubble evolution and collapse during laser exposure.
There are very few clinical trials about PTT and cancer (only 2 from 17,305), and no studies have been found in the case of melanoma tumors directly subjected to PTT protocols [55]. In addition to surgery, other therapeutical options for melanoma are now available (e.g., chemo-, radio-, immunotherapy, targeted therapy, PDT [56–58]), but further research and improved protocols are still necessary when pursuing the development and approval of new and more effective treatments for melanoma tumors and metastatic melanoma. As it also occurs in the case of PDT, the application of PTT is limited by the light penetration inside tissues, which is mainly due to light absorption and scattering. Although NIR irradiation penetrates deeper than visible light, tumors located in the depth of organs are difficult to be completely removed by these phototherapeutic procedures. Obviously, improvements such as NIR lasers with higher power and larger beam diameter must be achieved to increase the photothermal response of tumors. However, it has been our goal in this work to present a proof-of-concept procedure based in using melanin as an intrinsic chromophore within melanoma tumors, and direct irradiation with a cw 808-nm laser pointer to achieve a rapid, easy, and effective photothermal response. Interestingly, the photothermal treatment during or after surgical resection of melanotic tumors would be a suitable complement to remove some remaining tumor regions or scattered tumor cells. We hope that the presented methodology and results will allow further advances in the field of photothermal therapy.
4. Conclusions
Melanin is a very adequate infrared photothermal chromophore, and studies on model systems in vitro indicate that a very rapid and strong photothermal effect occurs in NIR-irradiated sepiomelanin, with almost immediate ignition of dried samples and considerable temperature increase of aqueous solutions. Histopathological observations reveal that a massive necrosis of the tumor tissue appears in NIR-irradiated regions of the B16-F10 melanotic melanoma successfully transplanted into white BALB/c mice. Taking into account our present results, the use of a cw 808-nm laser pointer as a light source supports the potential value of direct NIR irradiation for the therapy of pigmented skin tumors.
Compliance with ethical standards
This study was conducted according with the Committee of Animal Ethics of the Institute of Oncology Angel H. Roffo, University of Buenos Aires.
Funding
Marie Skłodowska-Curie Action COFUND 2015 (EU project 713366 – InterTalentum).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References
- 1.Alexandrescu D. T., Maslin B., Kauffman C. L., Ichim T. E., Dasanu C. A., “Malignant melanoma in pigmented skin: does the current interventional model fit a different clinical, histologic, and molecular entity?” Dermatol. Surg. 39(9), 1291–1303 (2013), doi:. 10.1111/dsu.12251 [DOI] [PubMed] [Google Scholar]
- 2.Guy G. P., Jr., Thomas C. C., Thompson T., Watson M., Massetti G. M., Richardson L. C., Centers for Disease Control and Prevention (CDC) , “Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030,” MMWR Morb. Mortal. Wkly. Rep. 64(21), 591–596 (2015). [PMC free article] [PubMed] [Google Scholar]
- 3.Cress R. D., Holly E. A., “Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of california cancer registry data, 1988-93,” Cancer Causes Control 8(2), 246–252 (1997). 10.1023/A:1018432632528 [DOI] [PubMed] [Google Scholar]
- 4.Cormier J. N., Xing Y., Ding M., Lee J. E., Mansfield P. F., Gershenwald J. E., Ross M. I., Du X. L., “Ethnic differences among patients with cutaneous melanoma,” Arch. Intern. Med. 166(17), 1907–1914 (2006). 10.1001/archinte.166.17.1907 [DOI] [PubMed] [Google Scholar]
- 5.Kabigting F. D., Nelson F. P., Kauffman C. L., Popoveniuc G., Dasanu C. A., Alexandrescu D. T., “Malignant melanoma in African-Americans,” Dermatol. Online J. 15(2), 3 (2009). [PubMed] [Google Scholar]
- 6.Poste G., Fidler I. J., “The pathogenesis of cancer metastasis,” Nature 283(5743), 139–146 (1980). 10.1038/283139a0 [DOI] [PubMed] [Google Scholar]
- 7.Walter W., Lingnau K., Schmitt E., Loos M., Maeurer M. J., “MHC class II antigen presentation pathway in murine tumours: tumour evasion from immunosurveillance?” Br. J. Cancer 83(9), 1192–1201 (2000). 10.1054/bjoc.2000.1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holden R. J., Ferguson A., “Histopathology of cell mediated immune reaction in mouse colon--allograft rejection,” Gut 17(9), 661–670 (1976). 10.1136/gut.17.9.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nevala W. K., Wettstein P. J., “The preferential cytolytic T lymphocyte response to immunodominant minor histocompatibility antigen peptides,” Transplantation 62(2), 283–291 (1996). 10.1097/00007890-199607270-00022 [DOI] [PubMed] [Google Scholar]
- 10.Anderson R. R., Parrish J. A., “The optics of human skin,” J. Invest. Dermatol. 77(1), 13–19 (1981). 10.1111/1523-1747.ep12479191 [DOI] [PubMed] [Google Scholar]
- 11.DeRosa M. C., Crutchley R. J., “Photosensitized singlet oxygen and its applications,” Coord. Chem. Rev. 233, 351–371 (2002). 10.1016/S0010-8545(02)00034-6 [DOI] [Google Scholar]
- 12.Plaetzer K., Krammer B., Berlanda J., Berr F., Kiesslich T., “Photophysics and photochemistry of photodynamic therapy: fundamental aspects,” Lasers Med. Sci. 24(2), 259–268 (2009). 10.1007/s10103-008-0539-1 [DOI] [PubMed] [Google Scholar]
- 13.Anderson R. R., “Lasers in dermatology--a critical update,” J. Dermatol. 27(11), 700–705 (2000). 10.1111/j.1346-8138.2000.tb02262.x [DOI] [PubMed] [Google Scholar]
- 14.Murphy M. J., Torstensson P. A., “Thermal relaxation times: an outdated concept in photothermal treatments,” Lasers Med. Sci. 29(3), 973–978 (2014). 10.1007/s10103-013-1445-8 [DOI] [PubMed] [Google Scholar]
- 15.Svaasand L. O., Nelson J. S., “On the physics of laser-induced selective photothermolysis of hair follicles: Influence of wavelength, pulse duration, and epidermal cooling,” J. Biomed. Opt. 9(2), 353–361 (2004). 10.1117/1.1646174 [DOI] [PubMed] [Google Scholar]
- 16.Wanner M., “Laser hair removal,” Dermatol. Ther. 18(3), 209–216 (2005). 10.1111/j.1529-8019.2005.05020.x [DOI] [PubMed] [Google Scholar]
- 17.Haedersdal M., Beerwerth F., Nash J. F., “Laser and intense pulsed light hair removal technologies: from professional to home use,” Br. J. Dermatol. 165(Suppl 3), 31–36 (2011). 10.1111/j.1365-2133.2011.10736.x [DOI] [PubMed] [Google Scholar]
- 18.Fayne R. A., Perper M., Eber A. E., Aldahan A. S., Nouri K., “Laser and light treatments for hair reduction in Fitzpatrick skin types IV-VI: A comprehensive review of the literature,” Am. J. Clin. Dermatol. 19(2), 237–252 (2018). 10.1007/s40257-017-0316-7 [DOI] [PubMed] [Google Scholar]
- 19.G. Jori, and J. D. Spikes, “Photothermal sensitizers: possible use in tumor therapy,” J. Photochem. Photobiol. B: Biol. 6, 93.101 (1990). 10.1016/1011-1344(90)85078-B [DOI] [PubMed]
- 20.Chen W. R., Adams R. L., Higgins A. K., Bartels K. E., Nordquist R. E., “Photothermal effects on murine mammary tumors using indocyanine green and an 808-nm diode laser: an in vivo efficacy study,” Cancer Lett. 98(2), 169–173 (1996). 10.1016/S0304-3835(06)80028-5 [DOI] [PubMed] [Google Scholar]
- 21.Camerin M., Rello S., Villanueva A., Ping X., Kenney M. E., Rodgers M. A., Jori G., “Photothermal sensitisation as a novel therapeutic approach for tumours: studies at the cellular and animal level,” Eur. J. Cancer 41(8), 1203–1212 (2005). 10.1016/j.ejca.2005.02.021 [DOI] [PubMed] [Google Scholar]
- 22.Marshall M. V., Rasmussen J. C., Tan I. C., Aldrich M. B., Adams K. E., Wang X., Fife C. E., Maus E. A., Smith L. A., Sevick-Muraca E. M., “Near-infrared fluorescence imaging in humans with indocyanine green: A review and update,” Open Surg. Oncol. J. 2(2), 12–25 (2010). 10.2174/1876504101002020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan A., Wu J., Tang X., Zhao L., Xu F., Hu Y., “Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies,” J. Pharm. Sci. 102(1), 6–28 (2013). 10.1002/jps.23356 [DOI] [PubMed] [Google Scholar]
- 24.Patel N., Pera P., Joshi P., Dukh M., Tabaczynski W. A., Siters K. E., Kryman M., Cheruku R. R., Durrani F., Missert J. R., Watson R., Ohulchanskyy T. Y., Tracy E. C., Baumann H., Pandey R. K., “Highly effective dual-function near-infrared (NIR) photosensitizer for fluorescence imaging and photodynamic therapy (PDT) of cancer,” J. Med. Chem. 59(21), 9774–9787 (2016). 10.1021/acs.jmedchem.6b00890 [DOI] [PubMed] [Google Scholar]
- 25.Escobedo J. O., Rusin O., Lim S., Strongin R. M., “NIR dyes for bioimaging applications,” Curr. Opin. Chem. Biol. 14(1), 64–70 (2010), doi:. 10.1016/j.cbpa.2009.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong G., Diao S., Chang J., Antaris A. L., Chen C., Zhang B., Zhao S., Atochin D. N., Huang P. L., Andreasson K. I., Kuo C. J., Dai H., “Through-skull fluorescence imaging of the brain in a new near-infrared window,” Nat. Photonics 8(9), 723–730 (2014). 10.1038/nphoton.2014.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kam N. W. S., O’Connell M., Wisdom J. A., Dai H., “Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction,” Proc. Natl. Acad. Sci. U.S.A. 102(33), 11600–11605 (2005). 10.1073/pnas.0502680102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X., El-Sayed I. H., Qian W., El-Sayed M. A., “Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods,” J. Am. Chem. Soc. 128(6), 2115–2120 (2006). 10.1021/ja057254a [DOI] [PubMed] [Google Scholar]
- 29.Camerin M., Jori G., Della Ciana L., Fabbroni S., Bonacchi S., Montalti M., Prodi L., “Photothermal sensitisation and therapeutic properties of a novel far-red absorbing cyanine,” Photochem. Photobiol. Sci. 8(10), 1422–1431 (2009). 10.1039/b908495a [DOI] [PubMed] [Google Scholar]
- 30.Zhang C., Fu Y. Y., Zhang X., Yu C., Zhao Y., Sun S. K., “BSA-directed synthesis of CuS nanoparticles as a biocompatible photothermal agent for tumor ablation in vivo,” Dalton Trans. 44(29), 13112–13118 (2015). 10.1039/C5DT01467K [DOI] [PubMed] [Google Scholar]
- 31.Heidari M., Sattarahmady N., Azarpira N., Heli H., Mehdizadeh A. R., Zare T., “Photothermal cancer therapy by gold-ferrite nanocomposite and near-infrared laser in animal model,” Lasers Med. Sci. 31(2), 221–227 (2016). 10.1007/s10103-015-1847-x [DOI] [PubMed] [Google Scholar]
- 32.McKenzie A. L., “Physics of thermal processes in laser-tissue interaction,” Phys. Med. Biol. 35(9), 1175–1209 (1990). 10.1088/0031-9155/35/9/001 [DOI] [PubMed] [Google Scholar]
- 33.Jaque D., Martínez Maestro L., del Rosal B., Haro-Gonzalez P., Benayas A., Plaza J. L., Martín Rodríguez E., García Solé J., “Nanoparticles for photothermal therapies,” Nanoscale 6(16), 9494–9530 (2014). 10.1039/C4NR00708E [DOI] [PubMed] [Google Scholar]
- 34.Anderson R. R., Parrish J. A., “Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation,” Science 220(4596), 524–527 (1983). 10.1126/science.6836297 [DOI] [PubMed] [Google Scholar]
- 35.Parrish J. A., Anderson R. R., Harrist T., Paul B., Murphy G. F., “Selective thermal effects with pulsed irradiation from lasers: From organ to organelle,” J. Invest. Dermatol. 80(1), 75s–80s (1983). 10.1038/jid.1983.19 [DOI] [PubMed] [Google Scholar]
- 36.Fisher J. W., Sarkar S., Buchanan C. F., Szot C. S., Whitney J., Hatcher H. C., Torti S. V., Rylander C. G., Rylander M. N., “Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation,” Cancer Res. 70(23), 9855–9864 (2010). 10.1158/0008-5472.CAN-10-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blázquez-Castro A., Colombo L. L., Vanzulli S. I., Stockert J. C., “NIR laser pointer for in vivo photothermal therapy of murine LM3 tumor using intratumoral China ink as a photothermal agent,” Lasers Med. Sci. 33(6), 1307–1315 (2018). 10.1007/s10103-018-2483-z [DOI] [PubMed] [Google Scholar]
- 38.Kam N. W. S., O’Connell M., Wisdom J. A., Dai H., “Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction,” Proc. Natl. Acad. Sci. U.S.A. 102(33), 11600–11605 (2005). 10.1073/pnas.0502680102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watt A. A. R., Bothma J. P., Meredith P., “The supramolecular structure of melanin,” Soft Matter 5(19), 3754–3760 (2009). 10.1039/b902507c [DOI] [Google Scholar]
- 40.Jastrzebska M., Mróz I., Barwinski B., Wrzalik R., Boryczka S., “AFM investigations of self-assembled DOPA-melanin nanoaggregates,” J. Mater. Sci. 45(19), 5302–5308 (2010). 10.1007/s10853-010-4575-4 [DOI] [Google Scholar]
- 41.d’Ischia M., Wakamatsu K., Cicoira F., Di Mauro E., Garcia-Borron J. C., Commo S., Galván I., Ghanem G., Kenzo K., Meredith P., Pezzella A., Santato C., Sarna T., Simon J. D., Zecca L., Zucca F. A., Napolitano A., Ito S., “Melanins and melanogenesis: from pigment cells to human health and technological applications,” Pigment Cell Melanoma Res. 28(5), 520–544 (2015). 10.1111/pcmr.12393 [DOI] [PubMed] [Google Scholar]
- 42.d’Ischia M., Napolitano A., Pezzella A., Meredith P., Sarna T., “Chemical and structural diversity in eumelanins: unexplored bio-optoelectronic materials,” Angew. Chem. Int. Ed. Engl. 48(22), 3914–3921 (2009). 10.1002/anie.200803786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panzella L., Gentile G., D’Errico G., Della Vecchia N. F., Errico M. E., Napolitano A., Carfagna C., d’Ischia M., “Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties,” Angew. Chem. Int. Ed. Engl. 52(48), 12684–12687 (2013). 10.1002/anie.201305747 [DOI] [PubMed] [Google Scholar]
- 44.Liebscher J., Mrówczyński R., Scheidt H. A., Filip C., Hădade N. D., Turcu R., Bende A., Beck S., “Structure of polydopamine: a never-ending story?” Langmuir 29(33), 10539–10548 (2013). 10.1021/la4020288 [DOI] [PubMed] [Google Scholar]
- 45.Solano F., “Melanin and melanin-related polymers as materials with biomedical and biotechnological applications—Cuttlefish ink and mussel foot proteins as inspired biomolecules,” Int. J. Mol. Sci. 18(7), 1561 (2017), doi:. 10.3390/ijms18071561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panzella L., Ebato A., Napolitano A., Koike K., “The late stages of melanogenesis: Exploring the chemical facets and the application opportunities,” Int. J. Mol. Sci. 19(6), 1753 (2018), doi:. 10.3390/ijms19061753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meredith P., Riesz J., “Radiative relaxation quantum yields for synthetic eumelanin,” Photochem. Photobiol. 79(2), 211–216 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Busetti A., Soncin M., Reddi E., Rodgers M. A., Kenney M. E., Jori G., “Photothermal sensitization of amelanotic melanoma cells by Ni(II)-octabutoxy-naphthalocyanine,” J. Photochem. Photobiol. B 53(1-3), 103–109 (1999). 10.1016/S1011-1344(99)00132-3 [DOI] [PubMed] [Google Scholar]
- 49.Sethi M., Chakarvarti S. K., “Hyperthermia techniques for cancer treatment: A review,” Int. J. Pharm. Tech. Res. 8(6), 292–299 (2015). [Google Scholar]
- 50.Giraudeau C., Moussaron A., Stallivieri A., Mordon S., Frochot C., “Indocyanine green: photosensitizer or chromophore? Still a debate,” Curr. Med. Chem. 21(16), 1871–1897 (2014). 10.2174/0929867321666131218095802 [DOI] [PubMed] [Google Scholar]
- 51.Liu Y., Ai K., Liu J., Deng M., He Y., Lu L., “Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy,” Adv. Mater. 25(9), 1353–1359 (2013). 10.1002/adma.201204683 [DOI] [PubMed] [Google Scholar]
- 52.Jacques S. L., McAuliffe D. J., “The melanosome: threshold temperature for explosive vaporization and internal absorption coefficient during pulsed laser irradiation,” Photochem. Photobiol. 53(6), 769–775 (1991). 10.1111/j.1751-1097.1991.tb09891.x [DOI] [PubMed] [Google Scholar]
- 53.Procaccini E. M., Riccio G., Bellocci M., Di Martino C., Monfrecola G., “The effects of a diode laser (810 nm) on pigmented guinea-pig skin,” Lasers Med. Sci. 16(3), 171–175 (2001). 10.1007/PL00011351 [DOI] [PubMed] [Google Scholar]
- 54.Padilla-Martinez J. P., Berrospe-Rodriguez C., Aguilar G., Ramirez-San-Juan J. C., Ramos-Garcia R., “Optic cavitation with CW lasers: A review,” Phys. Fluids 26(12), 122007 (2014), doi:. 10.1063/1.4904718 [DOI] [Google Scholar]
- 55. https://www.clinicaltrials.gov/ct2/results?cond=cancer&term=photothermal+therapy&cntry=&state=&city=&dist=
- 56.Rughani M. G., Gupta A., Middleton M. R., “New treatment approaches in melanoma: current research and clinical prospects,” Ther. Adv. Med. Oncol. 5(1), 73–80 (2013). 10.1177/1758834012463260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domingues B., Lopes J. M., Soares P., Pópulo H., “Melanoma treatment in review,” ImmunoTargets Ther. 7, 35–49 (2018). 10.2147/ITT.S134842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shtivelman E., “What’s new in melanoma treatment in 2019?” https://www.cancercommons.org/knowledge-blog/melanoma/whats-new-in-melanoma-treatment-in-2019/