Key Points
Question
Are monthly intramuscular injections with extended-release naltrexone hydrochloride as effective as daily oral buprenorphine–naloxone hydrochloride in reducing the use of heroin and other illicit substances in newly detoxified, opioid-dependent individuals?
Findings
In this 12-week, open-label randomized clinical trial including 159 opioid users, treatment with intramuscular extended-release naltrexone was as effective as oral buprenorphine-naloxone in reducing the use of heroin, opioids, and other illicit substances.
Meaning
Maintaining short-term opioid abstinence with extended-release naltrexone should be considered an equal treatment alternative to buprenorphine-naloxone as medication-assisted treatment for opioid-dependent individuals.
Abstract
Importance
To date, extended-release naltrexone hydrochloride has not previously been compared directly with opioid medication treatment (OMT), currently the most commonly prescribed treatment for opioid dependence.
Objective
To determine whether treatment with extended-release naltrexone will be as effective as daily buprenorphine hydrochloride with naloxone hydrochloride in maintaining abstinence from heroin and other illicit substances in newly detoxified individuals.
Design, Setting and Participants
A 12-week, multicenter, outpatient, open-label randomized clinical trial was conducted at 5 urban addiction clinics in Norway between November 1, 2012, and December 23, 2015; the last follow-up was performed on October 23, 2015. A total of 232 adult opioid-dependent (per DSM-IV criteria) individuals were recruited from outpatient addiction clinics and detoxification units and assessed for eligibility. Intention-to-treat analyses of efficacy end points were performed with all randomized participants.
Interventions
Randomization to either daily oral flexible dose buprenorphine-naloxone, 4 to 24 mg/d, or extended-release naltrexone hydrochloride, 380 mg, administered intramuscularly every fourth week for 12 weeks.
Main Outcomes and Measures
Primary end points (protocol) were the randomized clinical trial completion rate, the proportion of opioid-negative urine drug tests, and number of days of use of heroin and other illicit opioids. Secondary end points included number of days of use of other illicit substances. Safety was assessed by adverse event reporting.
Results
Of 159 participants, mean (SD) age was 36 (8.6) years and 44 (27.7%) were women. Eighty individuals were randomized to extended-release naltrexone and 79 to buprenorphine-naloxone; 105 (66.0%) completed the trial. Retention in the extended-release naltrexone group was noninferior to the buprenorphine-naloxone group (difference, −0.1; with 95% CI, −0.2 to 0.1; P = .04), with mean (SD) time of 69.3 (25.9) and 63.7 (29.9) days, correspondingly (P = .33, log-rank test). Treatment with extended-release naltrexone showed noninferiority to buprenorphine-naloxone on group proportion of total number of opioid-negative urine drug tests (mean [SD], 0.9 [0.3] and 0.8 [0.4], respectively, difference, 0.1 with 95% CI, −0.04 to 0.2; P < .001) and use of heroin (mean difference, −3.2 with 95% CI, −4.9 to −1.5; P < .001) and other illicit opioids (mean difference, −2.7 with 95% CI, −4.6 to −0.9; P < .001). Superiority analysis showed significantly lower use of heroin and other illicit opioids in the extended-release naltrexone group. No significant differences were found between the treatment groups regarding most other illicit substance use.
Conclusions and Relevance
Extended-release naltrexone was as effective as buprenorphine-naloxone in maintaining short-term abstinence from heroin and other illicit substances and should be considered as a treatment option for opioid-dependent individuals.
Trial Registration
clinicaltrials.gov Identifier: NCT01717963
This randomized clinical trial compares the effectiveness of extended-release naltrexone with that of buprenorphine-naloxone in maintaining short-term abstinence from heroin and other illicit substances.
Introduction
Substance use disorders involving opioids have a higher risk of death, poly drug use, and blood-borne infections, such as HIV and hepatitis, than other substance use disorders.1,2 Owing to the high risk of relapse and overdose in opioid-dependent individuals, the most commonly prescribed treatment is opioid medication treatment (OMT), in which opioids with longer absorption times and half-lives are prescribed, such as the full opioid agonist methadone3 or partial agonist buprenorphine hydrochloride.4 Because of the injection-deterring potential of naloxone hydrochloride and the better safety profile compared with methadone, daily administration of combined buprenorphine and naloxone (buprenorphine-naloxone) is the first choice of OMT medication in a number of countries. However, the extent to which buprenorphine-naloxone deters injection in practice has been debated.5
Opioid medication treatment is generally found to be effective in reducing illicit opioid use, overdose mortality,6 and associated problems, such as criminal activity7 or injection-related incidents.8 The disadvantages of OMT include continued physical dependence on and diversion of the prescribed opioid. The conventional alternative to OMT is follow-up counseling of drug-free patients after detoxification, which carries an increased risk of relapse to opioid use, especially soon after leaving prison or inpatient treatment programs.9,10 The reduction or loss of opioid tolerance following both short- and long-term abstinence puts the individual at high risk of overdose if opioid use is resumed.11
The opioid agonist naltrexone hydrochloride has been proposed as a third alternative to maintain opioid abstinence, but in oral naltrexone treatment, low adherence, a high drop-out rate, and increased mortality have been described as serious challenges.12,13,14 An alternative to the oral naltrexone product now available is extended-release naltrexone, administered as monthly intramuscular injections. Extended-release naltrexone inhibits the action of heroin and other opioid agonists by a competitive blocking of the opioid receptors. This inhibition has proven effective compared with placebo both in laboratory15 and clinical16,17,18,19 settings, and the effectiveness is in line with previous studies on some implantable naltrexone formulations.17,20 Moreover, contrary to OMT medications, extended-release naltrexone lacks abuse potential and should, in principle, give opioid users a prolonged period of abstinence from opioids with a high level of protection from relapse.
However, there is a lack of studies comparing extended-release naltrexone treatment with OMT. Such studies would provide novel information on differences in clinical effectiveness and adverse event profiles between the 2 treatment approaches and allow clinicians to choose the most adequate treatment for a given patient according to the individual’s needs and motivation.
The aim of the present study was to compare the effectiveness of extended-release naltrexone injections administered every fourth week with daily oral buprenorphine-naloxone in reducing the use of heroin and other illicit substances in similarly motivated patients randomized to either treatment after discharge from inpatient treatment or detoxification.
Methods
This randomized clinical trial assigned 159 patients in a clinical setting to treatment with injections of extended-release naltrexone every fourth week vs daily oral buprenorphine-naloxone. The protocol, including all outcome variables, is provided in the Supplement; complete information about the protocol is available in Kunøe et al.21
Inclusion was stopped on July 10, 2015, and the last patient follow-up was performed on October 23, 2015. The study was approved by the Regional Committee for Medical and Health Research Ethics South East Norway, the Norwegian Medicines Agency, and the boards of research ethics at the participating hospitals. Monitoring of the study was conducted by the publicly funded Regional Monitoring Authorities at Oslo University Hospital and Haukeland University Hospital (Innovest) according to Good Clinical Practice standards. Participants provided written informed consent. They were not paid or compensated for taking part in the study, with the exception of reimbursement of travel expenses. A lottery ticket incentive was offered for every urine drug test (UDT) administered (value approximately $2 US).
Participants and Setting
Patients were recruited between November 1, 2012, and July 10, 2015, by study personnel from outpatient clinics and detoxification units at 5 urban addiction clinics in Norway: Oslo University Hospital, Akershus University Hospital, Haukeland University Hospital, Stavanger University Hospital, and Vestfold Hospital Trust. Eligible participants were opioid-dependent (according to DSM-IV criteria) men or women aged 18 to 60 years. Criteria for exclusion were other drug or alcohol dependence or serious somatic or psychiatric illness regarded as contraindications or in need of treatment that would interfere with study participation. Women of childbearing age could not be pregnant or lactating and agreed to use effective birth control. Participants were screened for psychiatric disorders using the Mini-International Neuropsychiatric Interview 6.022 and examined for serious somatic disease. Eligible participants were referred to a detoxification unit following screening and inclusion. The study took place in an outpatient setting, and all participants were discharged from detoxification units, inpatient treatment, or prison. Ethnicity was defined by the participant.
Procedure, Outcomes, and Masking
After detoxification, participants were randomly assigned (1:1) to commence either individually dosed buprenorphine-naloxone, 4 to 24 mg/d (target dose, 16 mg/d) given orally daily in a controlled environment or extended-release naltrexone, 380 mg, given intramuscularly every fourth week for the following 12 weeks. Allocation to treatment group was computerized using a permuted block algorithm provided by the regional monitoring authority and not stratified for site or sex. Following induction into either medication regimen, participants were asked to attend standard drug counseling, but no behavioral interventions could be initiated. At baseline (inclusion) and every 4 weeks thereafter, patients underwent a structured interview using the European version of the Addiction Severity Index covering drug use, physical and mental health, work, education, and criminal activity.23,24,25
Primary outcome variables were comparison of retention in the study, the proportion of total number of UDTs without illicit opioids, and number of days of use of heroin and other illicit opioids. The weekly UDTs were analyzed using specific chromatographic methods and calculated as the number of opioid-negative urine drug screens divided by the total number of attended tests (group proportion) in accordance with recently revised Cochrane guidelines.26 Missing UDTs were considered as testing positive for opioids in all participants. Since a number of participants were abusing illicit opioids other than heroin at the time of inclusion, we discriminated between such use.
Secondary outcome variables were number of days of use of cannabis, amphetamines, cocaine, benzodiazepines, hallucinogens, alcohol, the number of days of injecting (intravenous) drugs, the degree of heroin craving (visual analog scale, 0-10, with 0 indicating none; 10, very strong), thoughts about heroin (visual analog scale, 0-10, with 0 indicating none; 10, constant or very frequent), life satisfaction (Temporal Satisfaction with Life Scale–Present items, 5-35; with 5 indicating very low; 35, very high),27 satisfaction with treatment (visual analog scale, 0-10; with 0 indicating very low; 10, very high), and mental health (Hopkins Symptom Checklist-25 of anxiety and depression, 25-100, with 25 indicating very low; 100, very high).28,29
Data on heroin, other illicit opioids, and substance use were collected every fourth week by an interview using the timeline follow-back technique, where participants reported the number of days of use within the 28 days preceding each interview.30 Retention in treatment was defined as the number of days until dropout from study medication and by the number of patients completing the study at week 12.
Participants who completed this randomized clinical trial were invited to continue or cross over to either treatment for up to 48 weeks. These data will be described in a subsequent publication.
Statistical Analysis
Minimum sample size was estimated in 2 scenarios. For the noninferiority scenario with a power of 90% and significance level of 5%, we assumed that both groups would retain 70% of their participants at the end of week 12 and set 20% as the noninferiority margin; this yielded a minimum sample size of 58 in each group (116 total).
The superiority scenario assumed extended-release naltrexone participants to have a mean of 7 opioid-negative samples out of the total 12 (0.58) samples, while participants receiving buprenorphine-naloxone would display a mean of 4 opioid-negative samples (0.33). Assuming an SD of 3.0 in both groups and a significance level of 5%, the estimated sample size would be 17 patients per medication arm (34 total) as sufficient to show a significant difference between the arms with a power of 90%. Intention-to-treat analyses of efficacy end points were performed with all randomized participants.
Differences in primary and secondary outcomes were assessed by linear mixed models with fixed effects for time, group, and the interaction between the 2 variables. Random effects for time and site were included in the models. A significant interaction implied differences between the groups’ changes throughout the follow-up. The models were also adjusted for age and sex.
Noninferiority analyses were performed by linear mixed models, where a nonsignificant interaction between time and group was eliminated. Regression coefficients for group variables were combined with the predefined noninferiority margins (8 for heroin, 10 for illicit opioids, and 0.2 for opioid-negative UDTs).
The normality of residuals was assessed by inspecting the histograms. Bootstrap inference based on 1000 replications was generated in the case of skewed residuals; however, differences were negligible and the original results were reported. Adverse events were compared using Fisher exact test. Retention in treatment was assessed by a log-rank test.
The results at P < .05 were considered significant in all superiority analyses. The noninferiority analyses were assessed by 1-sided test at the same significance level. Statistical analyses were conducted by a study-independent statistician blinded to the names of the study medications. The analyses were performed in SPSS, version 24 (SPSS Corp) and SAS, version 9.4 (SAS Institute).
Results
Patient Characteristics
Men and women displayed similar age distributions (mean [SD], 36.2 [8.9] and 35.6 [7.9] years, respectively), years of heavy heroin use (mean, 6.7 [5.5] and 6.9 [5.3], respectively), years of heavy use of other illicit opioids (mean, 2.8 [5.5] and 3.0 [7.6], respectively), age at onset of injection use (mean, 21.2 [7.8] and 21.0 [8.6] years, respectively), and other social characteristics corresponding to data from the national registry on opioid-dependent substance users in Norway. All women and 85.0% of the men were white. Four participants were HIV positive, and 86 (54.1%) tested seropositive for hepatitis C. The mean daily dose of buprenorphine-naloxone during the study was 11.2 mg (range, 6-24 mg). Other characteristics are reported in Table 1.
Table 1. Lifetime and Baseline Clinical Characteristics of Participants Randomized Into Treatment Groupsa.
| Lifetime Characteristic | Extended-Release Naltrexoneb
(n = 80) |
Buprenorphine-Naloxoneb
(n = 79) |
|---|---|---|
| Age, mean (SD), y | 36.4 (8.8) | 35.7 (8.5) |
| Sex, No. (%) | ||
| Male | 61 (76.3) | 54 (68.4) |
| Female | 19 (23.6) | 25 (31.6) |
| White, No. (%) | 72 (90.0) | 70 (88.6) |
| Injecting (intravenous) users, No. (%) | 72 (90.0) | 64 (81.0) |
| HIV positive, No. (%) | 2 (2.5) | 2 (2.5) |
| Hepatitis C seropositive, No. (%) | 44 (55.0) | 42 (53.2) |
| Years of substance use, mean (SD) | ||
| Heavy opioid use | 8.9 (7.8) | 9.6 (10.5) |
| Heroin | 6.9 (5.8) | 6.7 (5.2) |
| Other illicit opioids | 2.4 (5.1) | 3.2 (7.0) |
| Cannabis | 9.0 (7.3) | 10.2 (9.0) |
| Amphetamines | 6.7 (7.3) | 6.3 (6.6) |
| Cocaine | 1.4 (3.1) | 1.7 (2.8) |
| Benzodiazepines | 5.1 (6.0) | 5.9 (8.7) |
| Alcohol for intoxication | 3.5 (4.8) | 2.9 (4.1) |
| Use during past 30 d (baseline), mean (SD) | ||
| Heroin | 7.6 (11.0) | 12.0 (12.9) |
| Other illicit opioids | 8.2 (11.1) | 14.5 (13.2) |
| Cannabis | 8.2 (11.1) | 10.2 (12.6) |
| Amphetamines | 3.4 (7.4) | 5.4 (9.1) |
| Cocaine | 0.2 (0.7) | 1.3 (3.9) |
Intention-to-treat sample, 159.
Naltrexone, naloxone, and buprenorhine were all administered as the hydrochloride form.
Retention in Treatment
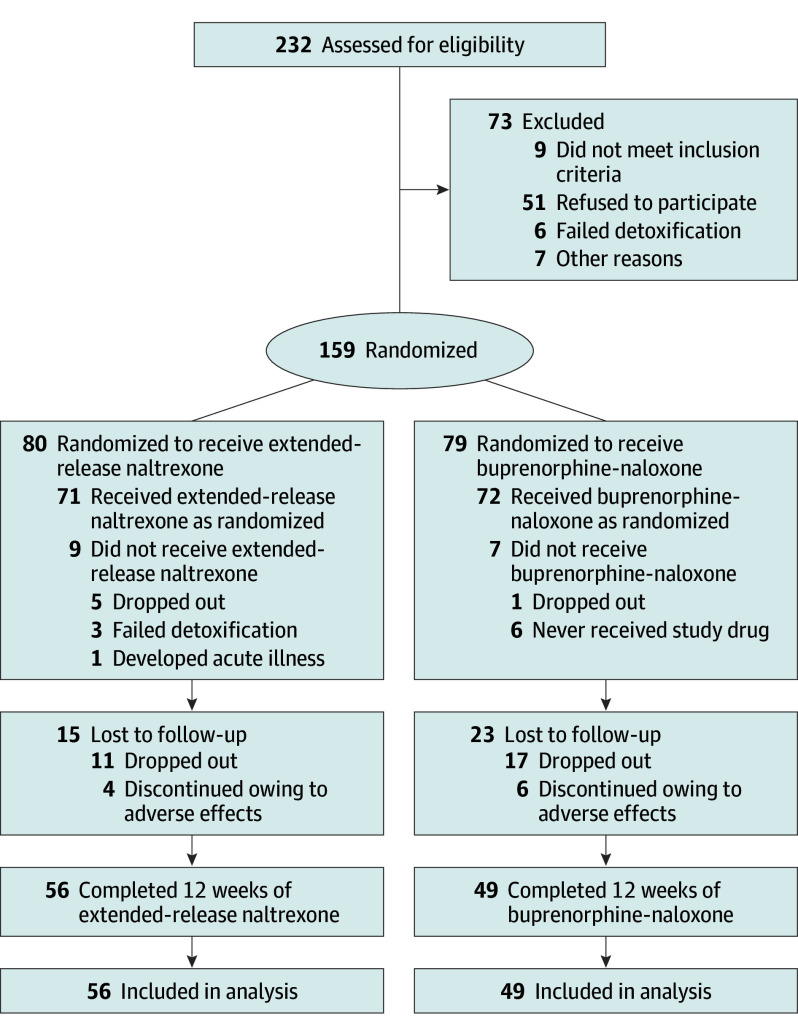
Among the 232 participants assessed for eligibility, 165 were included in the study and 159 were randomized to treatment with extended-release naltrexone (80 [50.3%]) or buprenorphine-naloxone (79 [49.7%]). Reasons for exclusion of 73 individuals were refusal to participate (51 [69.9%]), not meeting inclusion criteria (9 [12.3%]), failed detoxification (6 [8.2%]), and other reasons (7 [9.6%]) (Figure 1). Among the randomized participants, 143 agreed to commence their medication: 71 (49.7%) in the extended-release naltrexone group and 72 (50.3%) in the buprenorphine-naloxone group.
Figure 1. CONSORT Flowchart for Inclusion of Participants.
Screening, randomization, and follow-up. Naltrexone, naloxone, and buprenorhine were all administered as the hydrochloride form.
Participants receiving extended-release naltrexone and buprenorphine-naloxone displayed a similar retention time in the study (mean [SD], 69.3 [25.9] and 63.7 [29.9] days, respectively; P = .33) (Figure 2). The proportion of participants retained in the extended-release naltrexone group was noninferior to the buprenorphine-naloxone group (difference, −0.1; 95% CI, −0.2 to 0.1; P = .04).
Figure 2. Survival Curves for Retention in Treatment and Estimated Mean Number of Days for the Use of Heroin, Other Illicit Opioids, and Major Secondary Outcomes.
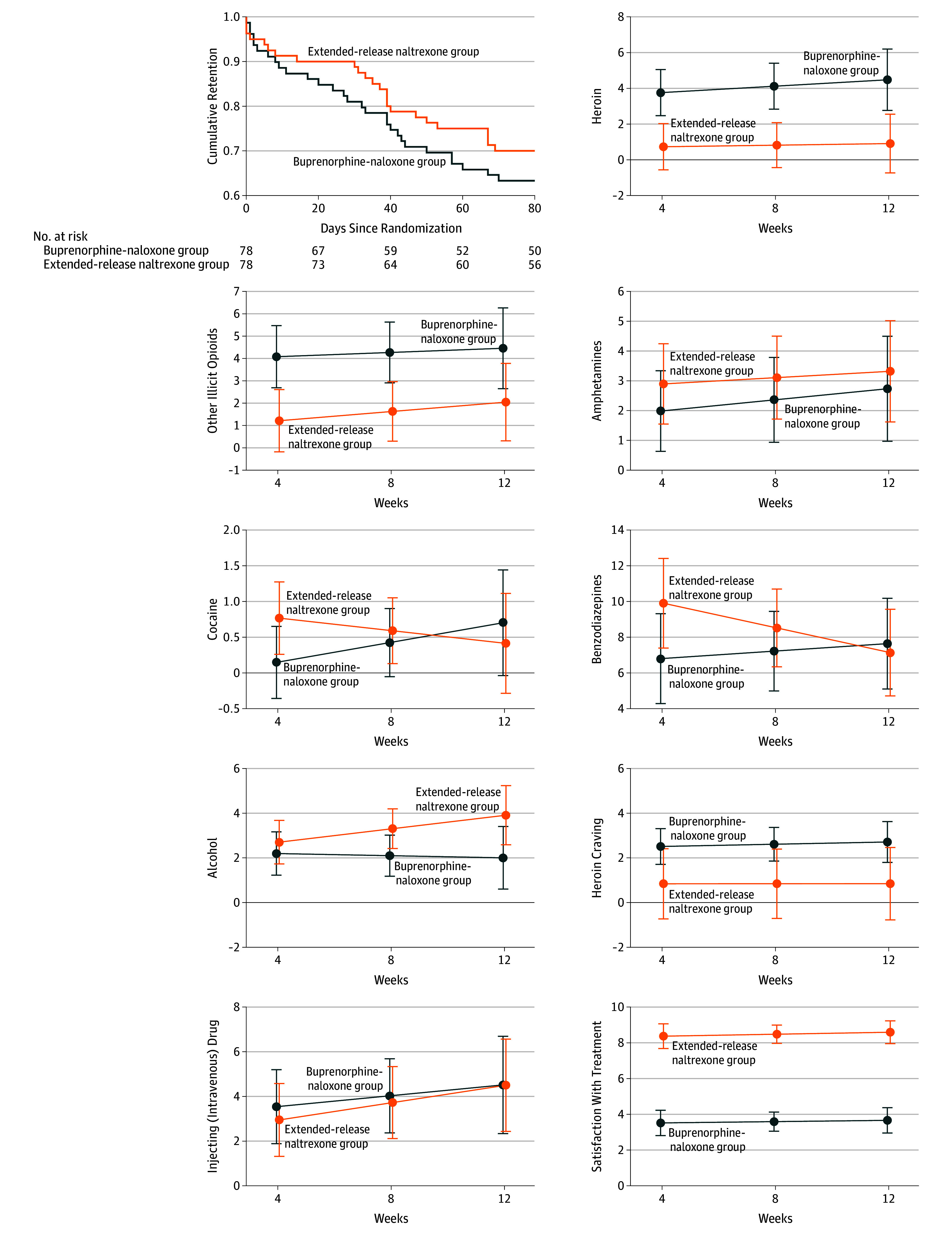
Visual analog scales were used to assess heroin craving (0-10, with 0 indicating none; 10, very strong) and satisfaction with treatment (0-10, with 0 indicating very low; 10, very high). Naltrexone, naloxone, and buprenorhine were all administered as the hydrochloride form. Error bars indicate 95% CIs.
After 12 weeks (84 days), 105 (66.0%) participants had attended all scheduled follow-up appointments and taken their medication as prescribed. Fifty-three participants dropped out: 24 in the extended-release naltrexone group and 29 in the buprenorphine-naloxone group.
Primary Outcomes
Treatment with extended-release naltrexone was noninferior to buprenorphine-naloxone regarding the group proportion of the total number of opioid-negative UDTs (mean [SD], 0.9 [0.3] and 0.8 [0.4], respectively; mean difference, 0.1 with 95% CI, −0.04 to 0.2; P < .001). Regarding days of use of heroin (mean difference, −3.2 with 95% CI, −4.9 to −1.5; P < .001) and other illicit opioids (mean difference, −2.7 with 95% CI, −4.6 to −0.9; P < .001), extended-release naltrexone treatment showed noninferiority to buprenorphine-naloxone under the predefined conditions. Assessing superiority of 1 treatment over the other showed no significant differences between the treatment groups in the proportion of negative UDTs (P = .18). However, extended-release naltrexone participants used significantly less heroin at all time points and less other illicit opioids at weeks 4 and 8, even though the pattern of use was not significantly different between groups (P = .64 for heroin, P = .71 for illicit opioids).
Secondary Outcomes
There were no significant differences between the treatment groups in the pattern of use of amphetamine (P = .73), cocaine (P = .13), alcohol (P = .21), cannabis (P = .78), or injecting drugs (P = .68) (Figure 2). However, participants receiving extended-release naltrexone had a significant reduction in days of benzodiazepine use (P = .04), while the buprenorphine-naloxone group remained stable. There were no significant differences between groups at different time points. Hallucinogens were used once or twice by 5 participants receiving extended-release naltrexone and 4 receiving buprenorphine-naloxone (Table 2).
Table 2. Days of Use of Heroin and Other Illegal Substances Assessed at Weeks 4, 8, and 12a.
| Time Point | Extended-Release Naltrexone | Buprenorphine-Naloxone | Extended-Release Naltrexone vs Buprenorphine-Naloxone | |||
|---|---|---|---|---|---|---|
| No. of Participants | Mean (SD)b | No. of Participants | Mean (SD)b | Mean Difference (95% CI)c | P Valuec | |
| Heroin Use | ||||||
| Week 4 | 63 | 0.8 (1.5) | 65 | 3.7 (7.4) | −3.0 (−4.9 to −1.2) | .001 |
| Week 8 | 59 | 0.8 (1.9) | 55 | 4.4 (9.1) | −3.3 (−5.1 to −1.5) | <.001 |
| Week 12 | 57 | 1.1 (2.3) | 50 | 4.1 (8.4) | −3.6 (−6.0 to −1.2) | .003 |
| Other Illicit Opioids Use | ||||||
| Week 4 | 63 | 1.2 (2.2) | 65 | 4.2 (7.9) | −2.9 (−4.8 to −0.9) | .004 |
| Week 8 | 59 | 1.8 (4.7) | 55 | 4.0 (8.5) | −2.6 (−4.6 to −0.7) | .007 |
| Week 12 | 57 | 2.0 (5.0) | 50 | 4.4 (8.7) | −2.4 (−4.9 to 0.1) | .06 |
| Cannabis Use | ||||||
| Week 4 | 63 | 6.7 (9.8) | 65 | 5.3 (9.4) | 1.4 (−1.8 to 4.7) | .38 |
| Week 8 | 59 | 6.4 (8.9) | 55 | 4.8 (8.5) | 1.6 (−1.3 to 4.6) | .28 |
| Week 12 | 57 | 7.5 (9.7) | 50 | 5.1 (9.6) | 1.8 (−1.5 to 5.1) | .27 |
| Amphetamine Use | ||||||
| Week 4 | 63 | 2.9 (6.0) | 65 | 2.0 (5.3) | 9 (−1.0 to 2.8) | .35 |
| Week 8 | 59 | 3.4 (7.0) | 55 | 1.9 (5.4) | 8 (−1.2 to 2.7) | .46 |
| Week 12 | 57 | 3.4 (7.5) | 50 | 2.1 (5.7) | 0.6 (−1.9 to 3.0) | .64 |
| Cocaine Use | ||||||
| Week 4 | 63 | 0.8 (3.2) | 65 | 0.1 (0.3) | 0.6 (−0.1 to 1.3) | .09 |
| Week 8 | 59 | 0.5 (1.8) | 55 | 0.7 (3.4) | 0.2 (−0.5 to 0.8) | .62 |
| Week 12 | 57 | 0.5 (1.8) | 50 | 0.6 (2.9) | −0.3 (−1.3 to 0.7) | .58 |
| Benzodiazepine Use | ||||||
| Week 4 | 63 | 1.1 (11.2) | 65 | 6.9 (1.3) | 3.1 (−0.5 to 6.7) | .09 |
| Week 8 | 59 | 8.0 (11.3) | 55 | 6.6 (9.4) | 1.3 (−1.8 to 4.4) | .41 |
| Week 12 | 57 | 6.7 (9.5) | 50 | 7.3 (1.4) | −0.5 (−4.0 to 3.0) | .78 |
| Alcohol Use for Intoxication | ||||||
| Week 4 | 63 | 3.0 (4.4) | 65 | 2.3 (3.8) | 0.5 (−0.9 to 1.9) | .47 |
| Week 8 | 59 | 2.9 (4.6) | 55 | 1.9 (3.1) | 1.2 (−0.1 to 2.5) | .06 |
| Week 12 | 57 | 4.4 (7.3) | 50 | 2.1 (3.6) | 1.9 (−0.02 to 3.8) | .05 |
Hallucinogens were used once or twice by 5 participants receiving extended-release naltrexone hydrochloride and 4 receiving buprenorphine–naloxone hydrochloride.
Means and SDs are descriptive numbers, not adjusted for repeated measurements or for site effects.
Results of linear mixed model for difference between groups; adjusted for repeated measurements and site effect; random effect for time included.
At all time points, participants receiving extended-release naltrexone reported significantly less heroin craving and thoughts about heroin (Table 2) than did buprenorphine-naloxone participants. Satisfaction with treatment was significantly higher among extended-release naltrexone participants and they would also recommend their treatment to others to a higher extent compared with buprenorphine-naloxone participants. Life satisfaction was significantly higher among extended-release naltrexone participants at weeks 4 and 8, but not at week 12. The Hopkins Symptom Checklist-25 scores showed no significant differences between the groups. Correcting the analyses for sex and age did not change the results.
Adverse Events
More adverse events were reported by extended-release naltrexone than buprenorphine-naloxone participants (49 [69.0%] vs 25 [34.7%]; P < .001), but only 10 participants discontinued treatment owing to adverse events: 4 in the extended-release naltrexone group and 6 in the buprenorphine-naloxone group. A number of events were related to induced or experienced withdrawal symptoms, such as nausea, chills, shivering, diarrhea, and sneezing, and were more frequent among the extended-release naltrexone participants (28 [39.4%] vs 10 [13.9%] events).
There were no deaths, but 6 (8.5%) extended-release naltrexone and 3 (4.2%) buprenorphine-naloxone participants reported a serious adverse event (Table 3). All recovered completely and maintained their study medication.
Table 3. Reported AEs Among 143 Participants Taking at Least 1 Dose of Study Medicationa.
| Outcome | No. (%) | P Valueb | |
|---|---|---|---|
| Extended-Release Naltrexone (n = 71) | Buprenorphine-Naloxone (n = 72) | ||
| Deaths | 0 | 0 | |
| Nonserious AE | 43 (60.6) | 22 (30.6) | <.001 |
| Serious AEc | 6 (8.5) | 3 (4.2) | .33 |
| Pneumonia-related | 2 (2.8) | 0 | |
| Withdrawal-related | 3 (4.2) | 0 | |
| Acute pain | 1 (1.4) | 1 (1.4) | |
| Opioid overdose | 0 | 1 (1.4) | |
| Planned surgery | 0 | 1 (1.4) | |
| Insomnia | 8 (11.3) | 3 (4.2) | .13 |
| Anxiety and depression symptoms | 12 (16.9) | 6 (8.3) | .14 |
| Injection site problems | 4 (5.6) | 0 | |
| Withdrawal-related AEd | 28 (39.4) | 10 (13.9) | <.001 |
Abbreviation: AE, adverse event.
Naltrexone, naloxone, and buprenorhine were all administered as the hydrochloride form.
Determined with Fisher exact test; empty cells indicate not applicable.
Two participants reported 2 serious AEs each.
Thirty-seven participants reported 2 or more withdrawal-related events.
Discussion
To our knowledge, this is the first study comparing the effectiveness of extended-release naltrexone injections with that of daily oral buprenorphine-naloxone, the standard OMT in Norway and other countries. Treatment with extended-release naltrexone was as effective as buprenorphine-naloxone in maintaining retention in treatment and reducing the use of heroin, other illicit opioids, and the use of other illicit substances except cannabis; injecting behavior; and craving for opioids. The main clinical implication of these findings is that extended-release naltrexone seems to be as safe and effective as buprenorphine-naloxone treatment for maintaining short-term abstinence from heroin, opioids, and other illicit substances in opioid-dependent individuals newly detoxified and/or discharged from inpatient treatment or prison. Since we discriminated between heroin and other illicit opioids, mainly oral formulations, our data also seem to be clinically relevant for the growing number of individuals who are addicted to prescribed opioids.
Induction into extended-release naltrexone treatment required full detoxification to a greater extent than into the buprenorphine-naloxone treatment. The Norwegian guidelines for detoxification of opioid users turned out to be insufficient for study detoxification and frequently produced adverse effects related to withdrawal symptoms on induction of extended-release naltrexone and, to some extent, buprenorphine-naloxone. We therefore changed our detoxification strategy during the first year of the study in accordance with the most recent literature at the time of our study,31,32,33 which reduced the number of new adverse events related to induction of treatment. Serious adverse events were equally distributed between the groups and were not directly related to the given treatment, which explains why there were no dropouts among participants reporting a serious adverse event.
Satisfaction with treatment and willingness to recommend their treatment to others were significantly higher among extended-release naltrexone participants. This finding may be due to the perception of being protected against relapse of opioid use and possible overdose and better opportunities to return to work or educational activities when not having to meet daily or every second day for supervised intake of an opioid agonist. However, the high availability of OMT in Norway34 makes it likely that the majority of participants were mainly motivated to receive the novel extended-release naltrexone treatment and not buprenorphine-naloxone. As treatment preference has been shown to be important for treatment satisfaction and adherence in other settings,35,36 it is difficult to know whether extended-release naltrexone would be equally effective in individuals with lower motivation for opioid abstinence.
There was only 1 reported overdose in the study, which is much lower than most reports on the first 12 weeks after discharge from treatment or prison.9,37,38 This low rate may reflect the high motivation for treatment and good response to regular follow-up by the same study worker in this group of participants.
The rather low reported mean use of opioids the last 30 days before inclusion is probably due to the fact that a number of participants included in the study had already completed detoxification or had sustained abstinence for varying periods of time (prison or inpatient treatment), while others were still actively using opioids at study enrollment.
The doses of buprenorphine-naloxone used in the study were adjusted to community-based practice representing treatment as usual. Our mean daily dose of 11.2 mg therefore corresponded fairly well with the 2016 National OMT Report mean dose of 13 mg/d.39
Limitations
One limitation of the present study is the lack of blinding. However, previous blinded placebo-controlled studies in clinical16,17 and laboratory15 settings seem sufficient to prove efficacy for the extended-release naltrexone medication. Owing to an increased risk of overdose in newly detoxified opioid users, the use of placebo and/or masking of medications were considered unethical. In addition to substantial practical challenges in managing 4 different medication arms, we regard most patients as capable of demasking or recognizing their respective treatments quickly, given their long experience with opioid use. Since we wanted to perform the study in a naturalistic setting, attempts to demask the treatment could easily be a disturbing element interfering with a true-effectiveness assessment. We therefore question the value of such a scheme in clinical trials for opioid dependence.
Conclusions
Maintaining short-term abstinence from illicit opioids and other substances with extended-release naltrexone was as effective and safe as buprenorphine-naloxone. Extended-release naltrexone should be an available treatment option for opioid-dependent individuals
Clinical Trial Protocol and Statistical Analysis Plan
References
- 1.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. [DOI] [PubMed] [Google Scholar]
- 2.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695. [DOI] [PubMed] [Google Scholar]
- 3.Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction: a clinical trial with methadone hydrochloride. JAMA. 1965;193:646-650. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 5.Duke AN, Correia CJ, Walsh SL, Bigelow GE, Strain EC. Acute effects of intramuscular and sublingual buprenorphine and buprenorphine/naloxone in non-dependent opioid abusers. Psychopharmacology (Berl). 2010;211(3):303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2(2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukten A, Røislien J, Skurtveit S, Waal H, Gossop M, Clausen T. A day-by-day investigation of changes in criminal convictions before and after entering and leaving opioid maintenance treatment: a national cohort study. BMC Psychiatry. 2013;13:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skeie I, Brekke M, Gossop M, et al. Changes in somatic disease incidents during opioid maintenance treatment: results from a Norwegian cohort study. BMJ Open. 2011;1(1):e000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukten A, Stavseth MR, Skurtveit S, Tverdal A, Strang J, Clausen T. High risk of overdose death following release from prison: variations in mortality during a 15-year observation period. Addiction. 2017;112(8):1432-1439. [DOI] [PubMed] [Google Scholar]
- 10.Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three-, and six-month outcomes after brief inpatient opioid detoxification. Am J Drug Alcohol Abuse. 2001;27(1):19-44. [DOI] [PubMed] [Google Scholar]
- 11.Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degenhardt L, Larney S, Kimber J, Farrell M, Hall W. Excess mortality among opioid-using patients treated with oral naltrexone in Australia. Drug Alcohol Rev. 2015;34(1):90-96. [DOI] [PubMed] [Google Scholar]
- 13.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roozen HG, de Waart R, van den Brink W. Efficacy and tolerability of naltrexone in the treatment of alcohol dependence: oral versus injectable delivery. Eur Addict Res. 2007;13(4):201-206.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17851241&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology (Berl). 2006;189(1):37-46.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16972105&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 16.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506-1513.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21529928&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69(9):973-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JD, Friedmann PD, Kinlock TW, et al. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med. 2016;374(13):1232-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JD, McDonald R, Grossman E, et al. Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction. 2015;110(6):1008-1014. [DOI] [PubMed] [Google Scholar]
- 20.Hulse GK, Morris N, Arnold-Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66(10):1108-1115. [DOI] [PubMed] [Google Scholar]
- 21.Kunøe N, Opheim A, Solli KK, et al. Design of a randomized controlled trial of extended-release naltrexone versus daily buprenorphine-naloxone for opioid dependence in Norway (NTX-SBX). BMC Pharmacol Toxicol. 2016;17(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. [PubMed] [Google Scholar]
- 23.McLellan AT, Luborsky L, Cacciola J, et al. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173(7):412-423. [DOI] [PubMed] [Google Scholar]
- 24.Kokkevi A, Hargers C. EUROPASI: European adaptation of a multidimensional assessment instrument for drug and alcohol dependence. Eur Addict Res. 1995;1(4):208-210. [Google Scholar]
- 25.Kokkevi A. Psychosocial assessment in substance abuse and dependence. Curr Opin Psychiatry. 2001;14(3):167-172. [Google Scholar]
- 26.Cochrane Drugs and Alcohol. CDAG resources for authors http://cda.cochrane.org/cdag-resources-authors. Accessed January 7, 2016.
- 27.Pavot W, Diener E, Suh E. The Temporal Satisfaction With Life Scale. J Pers Assess. 1998;70(2):340-354. [Google Scholar]
- 28.Joukamaa M, Lehtinen V, Karlsson H, Rouhe E. SCL-25 and recognition of mental disorders reported by primary health care physicians. Acta Psychiatr Scand. 1994;89(5):320-323. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1-15. [DOI] [PubMed] [Google Scholar]
- 30.Sobell LC, Sobell MB, Litten RZ, Allen JP. Timeline Follow-Back: a Technique for Assessing Self-Reported Alcohol Consumption. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- 31.Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174(5):459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse. 2012;38(3):187-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannelli P, Wu LT, Peindl KS, Swartz MS, Woody GE. Extended release naltrexone injection is performed in the majority of opioid dependent patients receiving outpatient induction: a very low dose naltrexone and buprenorphine open label trial. Drug Alcohol Depend. 2014;138:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.EMCDDA . European Drug Report: Trend and Developments. Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction; 2015. [Google Scholar]
- 35.Sullivan MA, Rothenberg JL, Vosburg SK, et al. Predictors of retention in naltrexone maintenance for opioid dependence: analysis of a stage I trial. Am J Addict. 2006;15(2):150-159. [DOI] [PubMed] [Google Scholar]
- 36.Nunes EV, Krupitsky E, Ling W, et al. Treating opioid dependence with injectable extended-release naltrexone (XR-NTX): who will respond? J Addict Med. 2015;9(3):238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend. 2009;105(1-2):9-15. [DOI] [PubMed] [Google Scholar]
- 38.UNODC . World Drug Report 2015. Vienna: United Nations Office on Drug and Crime; 2015. [Google Scholar]
- 39.Waal H, Bussesund K, Clausen T, Skeie I, Håseth A, Lillevold P. The annual OMT status survey for 2016. Statusrapport 2016-Er kvalitetsforbedring nå viktigere enn kapasitetsutvikling? Oslo, Norway: Norwegian Centre for Addiction Research; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Trial Protocol and Statistical Analysis Plan