Abstract
Objective: To compare the effects of proximal femoral nail antirotation blade (PFNA) and reverse less invasive stabilization system‐distal femur (Liss‐DF) systems in the treatment of proximal femoral fractures.
Methods: Between June 2007 and October 2009, 41 proximal femoral fractures were treated, 22 with PFNA (group A) and 19 with reverse LISS‐DF plates (group B). The time to starting full weight‐bearing, fracture healing time, functional recovery (Parker and Palmer mobility score), neck‐shaft angle discrepancies with the intact contralateral hip, preoperative American Society of Anesthesiologists (ASA) scores, the operation durations and amount of intraoperative bleeding were recorded and compared.
Results: The mean follow‐up period was 11.2 months (range, 10–12 months). Compared with Group A, Group B showed a statistically longer mean time to bear full body weight and heal their fractures, but a smaller neck‐shaft angle discrepancy (all P < 0.05). The groups were similar in ASA score, operation duration, amount of intraoperative bleeding and Parker and Palmer mobility score.
Conclusion: Both PFNA and reverse Liss‐DF were satisfactory for the treatment of proximal femoral fractures, but had different advantages. PFNA allowed earlier weight‐bearing and accelerated fracture healing. Reverse Liss‐DF more effectively avoided coxa vara and may be indicated for patients with very severe osteoporosis.
Keywords: Bone, Comparative study, Femur, Fractures, Treatment outcome
Introduction
With aging of the global population and an increase in high‐energy trauma, the incidence of proximal femoral fractures has been increasing 1 . Early operative intervention can shorten the time confined to bed, reduce the rate of complications (decubitus ulcer, hypostatic pneumonia, deep vein thrombosis or malunion) and decrease deformity and mortality 2 . However, the application of internal fixation is always associated with some complications, such as coxa vara, hardware failure due to “cut‐out” of the femoral head screw, secondary limb shortening after weight bearing and so on 2 , 3 . Therefore there is ongoing development of implants for unstable proximal femoral fractures.
In 2004, proximal femoral nail antirotation (PFNA) was designed to improve rotational and angular stability. This has one helical blade which has been biomechanically demonstrated to result in significantly higher cut‐out resistance in osteoporotic bone, thus making earlier weight‐bearing possible 3 . However, the incidence of postoperative coxa vara is reported to have remained at approximately 2.0%–3.6% 3 , 4 . Recently, the less invasive stabilization system‐distal femur (Liss–DF) was reported to have been used reversed to permit minimal invasion and produce satisfactory results in proximal femoral fracture 2 , 5 . Sidhom et al. reported using a left‐side “upside‐down” Liss‐DF plate to successfully unite a severe osteoporotic right proximal femoral fracture without cut‐out in proximal fragment fracture 6 . However, the time to starting weight‐bearing has to be postponed because this system provides less strength resistance axial loading than does PFNA 2 , 7 . Therefore, the optimum method for treating proximal femoral fracture remains a matter of debate.
The aim of this study was to compare the clinical outcomes of proximal femoral fractures after PFNA and Liss‐DF fixation.
Materials and Methods
Patients' data
Between June 2007 and October 2009, 41 patients with fresh, closed proximal femoral fractures were admitted to our hospital. The patients first underwent tibial tubercle traction and were treated for complications (e.g. shock, hypertension, diabetes). They then underwent surgery 4–10 days (mean, 6 days) after admission. The patients were divided into two groups. Group A (n= 22) underwent internal fixation with the PFNA system (Synthes, Solothurn, Switzerland). This group comprised 9 patients with intertrochanteric fractures (AO classification: 31‐A1 in three patients, 31‐A2 in five and 31‐A3 in one) and 13 patients with subtrochanteric fractures (Seinsheimer classification: type II in seven patients, type III in four and type IV in two). Group B (n= 19) underwent internal fixation with the Liss‐DF system (Synthes). This group comprised 12 patients with subtrochanteric fractures (Seinsheimer classification: type II in two patients, type III in two and type V in eight) and seven patients with intertrochanteric fractures (AO classification: 31‐A2 in three patients and 31‐A3 in four), which included four with coronal plane fractures or comminution of the lateral cortex of the greater trochanter.
Preoperative assessment
The physical status of the patient was evaluated according to the American Society of Anesthesiologists (ASA) grading system 8 , in which a score of I represents normal health, II mild systemic disease, III severe systemic disease, IV severe system disease that is a constant threat to life and V moribund and not expected to survive without surgery.
Surgical treatment
In Group A, each patient was placed in a supine position (Fig. 1A) and the fracture manually reduced under monitoring with a C‐arm. Then a longitudinal incision (length: 4–6 cm) centering on the apex of the greater trochanter was made along the long axis of the femur. A guide wire was inserted from the tip of greater trochanter into the intramedullary cavity to guide a cannulated drill bit into the proximal femur, then reaming rods were inserted into the medullary cavity to the desired insertion depth along the guide wire. The PFNA was manually inserted along the guide wire, which was removed subsequently. Another guide wire was positioned in the center of the femoral neck under the guidance of an aiming arm equipped with the PFNA system. Then a helical PFNA blade was manually inserted over the guide wire, advancing as far as possible into the femoral head. The distal locking bolt was then inserted under the guidance of the aiming arm. Finally, the end cap was inserted into the proximal end of the nail.
Figure 1.
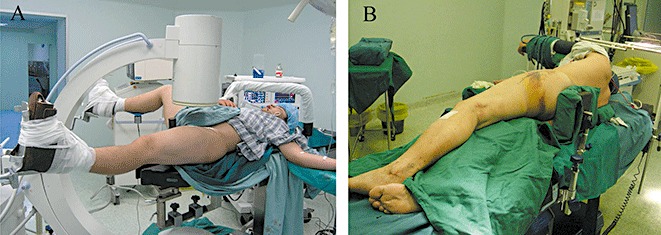
Photographs showing the patient position during surgical treatment of proximal femoral fracture by (A) the PFNA technique and (B) the Liss‐DF technique.
In Group B, each patient was placed on the unaffected side (Fig. 1B). An incision (length: 6–8 cm) was made along the long axis of the femur from the lateral aspect of the greater trochanter. The fascia lata was incised, and the vastus lateralis muscle separated to expose the femur. A space was created for the subsequent placement of a fixation plate under the vastus lateralis muscle. A titanium Liss‐DF plate (Synthes) of an appropriate length was selected; a left plate being selected for right femoral fracture, and vice versa. The plate was attached to the femoral plane in a reverse orientation and adjusted under the monitoring of the C‐arm until the correct position had been achieved. Finally, the proximal and distal fracture segments were fixed with four to five self‐locking screws under the guidance of the aiming arm. The operation duration and volume of blood loss were monitored and compared in both groups.
Postoperative management
In Group A, from 6 hours after surgery, the patients were encouraged to do isometric quadriceps contractions and active flexion and extension of the hip and knee of the affected side without weight‐bearing. One week after surgery, they weres allowed 15% weight‐bearing and then gradually progressed to full weight‐bearing according to the type of fracture and the status of fracture healing.
In Group B, the patients were similarly encouraged to practice joint movements and isometric quadriceps contractions without weight‐bearing from 6 hours after surgery. They started to bear 10%–15% body weight when bridging callus was observed by X‐ray. Then, according to the type of fracture and the status of fracture healing, the patient bore gradually increasing loading until reaching full body weight.
The patient was followed‐up by X‐ray and physical examination. More than 50% visible bridging callus across the fracture on plain radiographs was regarded as the radiographic indicator of fracture healing 2 . Absence of local pain (spontaneous or on percussion) was regarded as the symptomatic indicator of fracture healing. The time of fracture healing was defined as the time point when both indicators were achieved. The difference between the neck‐shaft angle of the unaffected side and that of the operated side recorded at the last follow‐up was defined as the loss of neck‐shaft angle. Additionally, mobility was evaluated at the last follow‐up by the Parker‐Palmer scoring system 9 (Table 1).
Table 1.
Parker and Palmer grading criteria for the assessment of mobility
| Mobility | No difficulty | With an aid | With help from another person | Not at all |
|---|---|---|---|---|
| Able to get about the house | 3 | 2 | 1 | 0 |
| Able to get out of the house | 3 | 2 | 1 | 0 |
| Able to go shopping | 3 | 2 | 1 | 0 |
Statistical analyses
Data were expressed as mean ± standard deviation. The operation durations, times to bearing full body weight and Parker‐Palmers scores were compared by Student's t‐test (Stata 7.0). The ASA scores, bleeding volumes, fracture healing times and losses of neck‐shaft angles were analyzed by rank‐sum tests. A P‐value of less than 0.05 was considered statistically significant.
Results
All patients were followed‐up for 10–12 months (mean: 11.2 months). All fractures healed successfully and reached bony union. Groups A and B showed similar ASA scores, operation durations and bleeding volumes (Table 2). At the last follow‐up, both groups also yielded similar Parker‐Palmer scores (Table 2).
Table 2.
A comparison of outcomes in patients treated by PFNA (Group A) and reverse Liss‐DF (Group B) techniques (mean ± standard deviation)
| Group | ASA score | Operation duration (min) | Bleeding volume (mL) | Time to full weight‐bearing (weeks) | Time to fracture healing (weeks) | Parker and Palmer score | Loss of neck‐shaft angle |
|---|---|---|---|---|---|---|---|
| Group A | 2.82 ± 0.85 | 67.36 ± 27.13 | 146.82 ± 87.35 | 9.55 ± 1.77 | 17.41 ± 6.21 | 8.09 ± 0.92 | 8.63 ± 2.80 |
| Group B | 2.68 ± 0.82 | 69.47 ± 17.07 | 150.79 ± 78.20 | 16.16 ± 3.35* | 28.11 ± 14.43* | 8.11 ± 0.81 | 1.57 ± 2.11* |
P < 0.05, compared with group A.
In Group A, 21 patients experienced satisfactory reduction and fixation, and were able to undertake early weight‐bearing. None of them experienced distal peri‐implant fracture or loosening of the PFNA blade (Fig. 2). In the one remaining patient (a 73‐year‐old man), the neck‐shaft angle was about 129.14° one week after surgery (Fig. 3A); after exercise under partial weight‐bearing for five weeks, the patient experienced considerable loss of neck‐shaft angle and developed coxa vara (Fig. 3B). The Parker‐Palmer score on the last follow‐up for this patient was four, indicating an unsatisfactory outcome.
Figure 2.
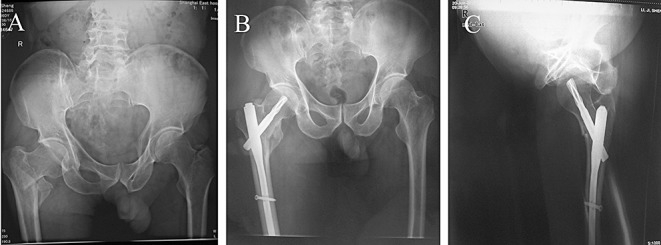
Radiographs from a patient (Group A) with a comminuted intertrochanteric fracture of the right hip treated by the PFNA technique; (A) Before surgery: coxa vara; (B) One week after surgery: the helical blade is located in the center of the femoral neck and the fracture has been satisfactorily reduced as seen in the AP and (C) lateral views.
Figure 3.
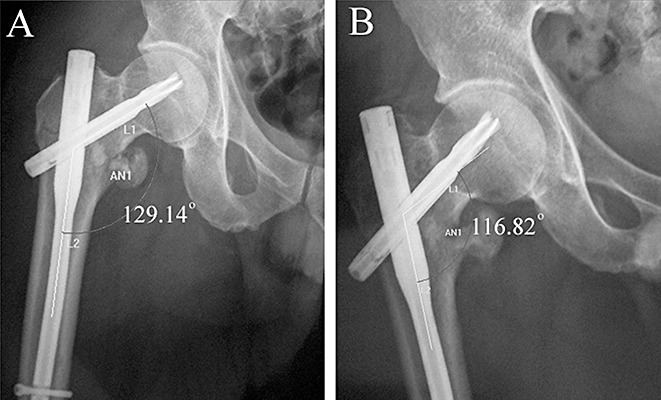
Radiographs of a patient (Group A) with a proximal femoral fracture treated by the PFNA technique; (A) One week after surgery, the neck‐shaft angle is 129.14°; (B) Five weeks after surgery, the neck‐shaft angle has decreased to 116.82°and the hip has developed coxa vara.
In Group B, in most patients the fractures could be spontaneously reduced in the lateral recumbent position and were satisfactorily reduced by gentle traction or rotation (4, 5). The proximal fracture fragment was fixed with four to six locking screws and the distal fragment with at least four bicortical locking screws. The patients were allowed to participate in functional exercises without bearing their body weights. No patient experienced coxa vara or fixation device failure (fracture or bending). Compared with Group A, Group B showed a significantly longer duration to full weight‐bearing and fracture healing (both P < 0.05, Table 2).
Figure 4.
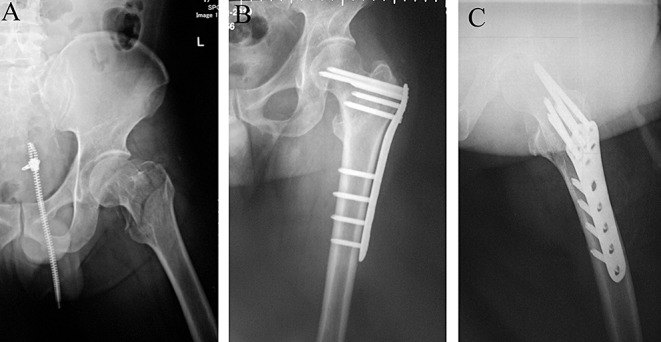
Radiographs of a patient (Group B) with a comminuted intertrochanteric fracture of the left hip combined with a fracture of the greater trochanter treated by the reverse Liss‐DF technique; (A) before surgery; (B) One week after surgery: satisfactory reduction and stable fixation can be seen in the AP and (C) lateral views.
Figure 5.
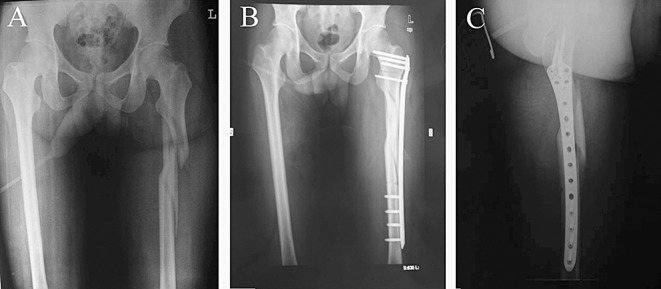
Radiographs of a patient (Group B) with a comminuted subtrochanteric fracture treated by the reverse Liss‐DF technique; (A) Before surgery; (B) One week after surgery: satisfactory fracture reduction and stable fixation can be seen in the AP and (C) lateral views.
Discussion
Proximal femoral fracture occurs primarily in elderly persons. Early operative treatment reduces both mortality and morbidity, giving the best chance of early independence and reducing the risks of prolonged bed rest 10 , 11 . However, surgical treatment may pose high risks to elderly patients because osteoporosis decreases the bone purchase of internal fixation devices and various associated chronic diseases, such as chronic bronchitis and coronary heart disease, can decrease tolerance of surgical invasion. In recent years, PFNA and reverse Liss‐DF have been increasingly used in the treatment of proximal femoral fractures, both reportedly involving minimal surgical invasion and producing satisfactory results 2 , 3 . However, the respective advantages or disadvantages of the two systems have not been intensively investigated. In the present study, we compared the two techniques in regard to the risk of coxa vara, biomechanical strength against axial loading and degree of surgical invasion.
Coxa vara is a major cause of pain after proximal femoral fracture surgery and hence leads to a decrease in the quality of the patient's life. Although the helical PFNA blade was designed to enhance bone purchase to prevent cut‐out 3 , 12 , in this study one patient still developed coxa vara and some patients treated with PFNA experienced loss of neck‐shaft angle. These undesirable consequences might have resulted from several factors. Firstly, the proximal fragment fracture was typically abducted and/or externally rotated, thus extensive abduction of the distal fracture fragment was required to reduce the fracture. In some cases, the femur might be have been abducted insufficiently in order to more conveniently plan the point for inserting and advancing the PFNA nail, thus leading to the subsequent development of coxa vara. Secondly, in some cases the affected limb might be have been excessively adducted during insertion of the intramedullary nail, which could have further weakened the support from the posterior‐lateral femur and increased the stress on the PFNA blade 3 , 4 , 12 . Thirdly, the PFNA blade might have disrupted the blood supply to the proximal femur, resulting in subsequent bone necrosis, loss of holding strength, and then cutting by the loosened blade during loading bearing 13 . Finally, because these elderly patients typically had various degrees of osteoporosis, the PFNA blade was under a relatively high stress but provided poor purchase; thus, the blade could easily have cut the osteoporotic femoral head/neck and, in some cases, even penetrated through the femoral head/neck, resulting in coxa vara 12 .
In this study, fixation by the reverse Liss‐DF technique produced better results in regard to maintaining the neck‐shaft angle and preventing coxa vara compared with the PFNA, most likely due to the following factors. Firstly, the screws were locked with the plate to form a rigid structure, which effectively prevented screw loosening and loss of fracture reduction. This rigid structure provided a good purchase, even in osteoporotic bone, for preventing the screws from sliding and pull–out 14 . The proximal screws interlock with the relatively wide Liss‐DF plate to form a stable frame that uniformly distributes the loading (shear, rotation and compression) to each screw; this helps to maintain the neck‐shaft angle and prevent coxa vara, screw pull‐out and fracture of the plate or screws 2 , 15 . Secondly, the Liss‐DF plate could accept four to six screws at the proximal fragment fracture and thus provided a high pull‐out resistance. In other studies on fixation of proximal fractures of severely osteoporotic femurs, the results of Liss‐DF plates were also found to be satisfactory, although they provide less purchase in these patients 6 . Multiple screws may increase the fixation strength of the proximal fragment, especially for fractures affecting the greater trochanter or combined with severely comminuted subtrochanteric fractures 2 . Thirdly, fixation with a Liss‐DF plate in the lateral recumbent position decreases the tension on the iliopsoas and adductor, which facilitates spontaneous reduction of the posterior‐medial fragment, reduces the stress on the proximal screws and therefore prevents development of coxa vara. If surgery is performed in the regular supine position, the affected limb needs to be abducted because the proximal fragment tends to protrude anteriorly and laterally; this would increase the tension on the adductor and iliopsoas and impair reduction of the posterior‐medial fragment. Meanwhile, because the screws cannot provide compression in the supine position, the plate cannot produce satisfactory reduction of the posterior‐medial fragment. These adverse factors would cause lack of support of the medial cortical buttress, which could eventually lead to coxa vara 16 , 17 . Finally, the Liss‐DF system provides sufficient mechanical strength to stabilize the proximal fracture end, thus maintaining the neck‐shaft angle and reduction 7 . As described, no screw loosening/fracture or coxa vara occurred in any patients even though they actively participated in exercise without weight‐bearing immediately after surgery.
Despite the advantages of the Liss‐DF technique, intramedullary fixation remains the treatment of choice for proximal femoral fracture, because of its biomechanical superiorities in regard to axial loading 7 . The PFNA system not only acts as an internal splint, but can also bear a large axial loading because it produces a small bending moment. Additionally, the helical blade in the PFNA system compacts the cancellous bone, enhancing its bone purchase in the femoral neck‐head; the blade also locks with the intramedullary nail preventing rotation or collapse of the proximal fragment. These factors allow the patient to bear partial weight at an early stage after surgery 3 , 4 , 12 , 18 . In this study, we encouraged the PFNA‐treated patients to participate in joint movement exercises as early as possible after surgery. The patients were permitted to bear partial body weight from one week after surgery, and all fractures eventually healed satisfactorily. Compared with PFNA, the Liss‐DF has been found to provide lower fixation strength; therefore, weight‐bearing too early might induce stress concentration and, potentially, loosening of the locking screws 7 . Consequently, several studies have suggested that patients treated by the Liss‐DF technique should start exercise with partial weight‐bearing only when callus has bridged the fracture ends, and then gradually progress to full weight‐bearing 2 , 15 . In this study, we instructed the Liss‐DF‐treated patients to exercise without weight‐bearing, and no negative effect on the function of the operated limb was observed. This delayed weight‐bearing, however, may have resulted in decreased loading of the fracture ends and thus slower healing, as evidenced by the longer fracture duration in Group B. Nevertheless, the Liss‐DF technique is favorable in that it does not require stripping of the periosteum and therefore can best maintain blood supply to the fracture ends and avoid non‐union 2 , 15 . In this study, all fractures treated by the Liss‐DF technique healed to bony unions.
Additionally, the minimal surgical invasion is another major advocated advantage of the PFNA technique 3 , 10 . However, the Liss‐DF technique is favorable in that, in the lateral position, the greater trochanter is clearly exposed to accurately locate the surgerical incision; the fractures are easily (sometimes spontaneously) reduced under the effect of gravity, thus reducing the operation time. Moreover, the minimally invasive procedure can reduce trauma to the patient. In this study, the operation durations and bleeding volume were similar in the two groups. Therefore, the technique of reverse Liss‐DF in a lateral position is also a suitable treatment for proximal femoral fractures.
In conclusion, both PFNA and Liss‐DF techniques can provide minimal invasion and satisfactory outcomes. The PFNA technique allows patients to bear body weight earlier and promotes faster healing of the fracture, but involves a higher risk of coxa vara. In comparison, the Liss‐DF technique may be the best choice for severely osteoporotic patients because it reduces the risk of coxa vara.
Disclosure
No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (No. 30772197).
References
- 1. Saarenpää I, Heikkinen T, Ristiniemi J, et al Functional comparison of the dynamic hip screw and the Gamma locking nail in trochanteric hip fractures: a matched‐pair study of 268 patients. Int Orthop, 2009, 33: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma CH, Tu YK, Yu SW, et al Reverse LISS plates for unstable proximal femoral fractures. Injury, 2010, 41: 827–833. [DOI] [PubMed] [Google Scholar]
- 3. Mereddy P, Kamath S, Ramakrishnan M, et al The AO/ASIF proximal femoral nail antirotation (PFNA): a new design for the treatment of unstable proximal femoral fractures. Injury, 2009, 40: 428–432. [DOI] [PubMed] [Google Scholar]
- 4. Sahin S, Ertürer E, Oztürk I, et al Radiographic and functional results of osteosynthesis using the proximal femoral nail antirotation (PFNA) in the treatment of unstable intertrochanteric femoral fractures. Acta Orthop Traumatol Turc, 2010, 44: 127–134. [DOI] [PubMed] [Google Scholar]
- 5. Pryce Lewis JR, Ashcroft GP. Reverse LISS plating for proximal segmental femoral fractures in the polytrauma patient: a case report. Injury, 2007, 38: 235–239. [DOI] [PubMed] [Google Scholar]
- 6. Sidhom SA, Pinder R, Shaw DL. Reverse LISS plate stabilisation of a subtrochanteric fracture of the femur in a patient with osteopetrosis: is this the answer? Injury, 2006, 37: 113–115. [Google Scholar]
- 7. Kim JW, Oh CW, Byun YS, et al A biomechanical analysis of locking plate fixation with minimally invasive plate osteosynthesis in a subtrochanteric fracture model. J Trauma, 2011, 70: E19–23. [DOI] [PubMed] [Google Scholar]
- 8. Tang X, Liu L, Yang TF, et al Preliminary effect of proximal femoral nail antirotation on emergency treatment of senile patients with intertrochanteric fracture. Chin J Traumatol, 2010, 13: 212–216. [PubMed] [Google Scholar]
- 9. Parker MJ, Palmer CR. A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br, 1993, 75: 797–798. [DOI] [PubMed] [Google Scholar]
- 10. Strauss E, Frank J, Lee J, et al Helical blade versus sliding hip screw for treatment of unstable intertrochanteric hip fractures: a biomechanical evaluation. Injury, 2006, 37: 984–989. [DOI] [PubMed] [Google Scholar]
- 11. Moran CG, Wenn RT, Sikand M, et al Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am, 2005, 87: 483–489. [DOI] [PubMed] [Google Scholar]
- 12. Simmermacher RK, Ljungqvist J, Bail H, et al The new proximal femoral nail antirotation (PFNA) in daily practice: results of a multicentre clinical study. Injury, 2008, 39: 932–939. [DOI] [PubMed] [Google Scholar]
- 13. Baixauli EJ, Baixauli F Jr, Baixauli F, et al Avascular necrosis of the femoral head after intertrochanteric fractures. J Orthop Trauma, 1999, 13: 9–12. [DOI] [PubMed] [Google Scholar]
- 14. Szypryt P, Forward D. The use and abuse of locking plates. Orthop Trauma, 2009, 23: 281–290. [Google Scholar]
- 15. Oh CW, Kim JJ, Byun YS, et al Minimally invasive plate osteosynthesis of subtrochanteric femur fractures with a locking plate: a prospective series of 20 fractures. Arch Orthop Trauma Surg, 2009, 129: 1659–1665. [DOI] [PubMed] [Google Scholar]
- 16. Verettas DA, Galanis B, Kazakos K, et al Fractures of the proximal part of the femur in patients under 50 years of age. Injury, 2002, 33: 41–45. [DOI] [PubMed] [Google Scholar]
- 17. Kyle RF, Cabanela ME, Russell TA, et al Fractures of the proximal part of the femur. Instr Course Lect, 1995, 44: 227–253. [PubMed] [Google Scholar]
- 18. Sommers MB, Roth C, Hall H, et al A laboratory model to evaluate cutout resistance of implants for pertrochanteric fracture fixation. J Orthop Trauma, 2004, 18: 361–368. [DOI] [PubMed] [Google Scholar]


