Abstract
Objective: We analyzed chondrosarcomas in long bones to assess whether Grade I and II chondrosarcomas should both be grouped as low grade malignant tumors (musculoskeletal Tumor Society Stage I or Stage II), and to explore rational surgical treatment for Grade I and II chondrosarcomas.
Methods: We retrospectively reviewed 66 patients from January 1996 to December 2007 with Grade I and II chondrosarcoma of the extremities without metastases at the Department of Orthopaedics of Shanghai Sixth People's Hospital. Thirty‐eight patients had undergone intralesional or marginal resection, and 28 patients had undergone wide marginal or radical excision based on imaging findings. The mean age of the patients was 43 years (range, 5–85) and the minimum follow‐up was 31 months (mean, 48; range, 5–141). We analyzed grade diagnosis, therapeutic options, and local recurrence rate of the two grades of chondrosarcoma.
Results: Of all patients, 22 experienced local recurrence, making the local recurrence rate 33.3%. A statistically significant difference in outcome between patients with Grade I and Grade II tumors undergoing intralesional resection was identified. No significant difference according to surgical method was found between the two groups in total.
Conclusion: Grade II chondrosarcomas should be grouped as high stage malignant tumors (Stage II) and grade I chondrosarcomas assigned to the low stage malignant tumor group (Stage I). Our experience suggests the surgical method should be related to radiographic margin status and oncologic classification. Wide resection should be considered for Grade II, while intralesional resection is suitable for Grade I.
Keywords: Chondrosarcoma, Neoplasm recurrence, local, Neoplasm staging, Treatment outcome
Introduction
Chondrosarcoma is the second most common primary malignancy of bone after osteosarcoma, and has a slight male predominance 1 . The age distribution of patients with chondrosarcoma shows a gradual age‐related increase, the peak incidence occurring during the fifth and sixth decades of life. The majority of patients are older than 50 years. Chondrosarcoma has a predilection for the trunk and long bones, in particular the humerus and femur. Clinical symptoms are pain and tenderness with or without a mass. Some patients are asymptomatic, as the lesion is sometimes discovered incidentally on radiographs.
Currently, chondrosarcoma is graded histologically on a scale from I to III based on pathological features such as matrix, cellularity and nuclear characteristics 2 . Many researchers have demonstrated that Grade II chondrosarcoma should be classified as a stage II malignant tumor; however there is disagreement about this classification and the corresponding selection of surgical treatment. 3
Material and methods
From January 1996 to December 2007, we treated 66 patients with Grade I and II chondrosarcomas of the extremities at the Department of Orthopaedics of Shanghai Sixth People's Hospital. Inclusion criteria for this study included: (i) a diagnosis, based on conventional criteria, of Grade I or II chondrosarcoma; (ii) available follow‐up data; and (iii) all treatment done in our hospital. Exclusion criteria included: (i) a diagnosis of specific chondrosarcoma, such as clear cell, mesenchymal or extraskeletal myxoid chondrosarcoma; (ii) chondrosarcomas diagnosed as borderline Grade I/II; and (iii) cases with recurrence of chondrosarcoma or a surgical history in another hospital.
All pre‐ and post‐operative radiograms and magnetic resonance imaging (MRI) were reviewed. Radiographic criteria included identification of a destructive lesion, cortical thickening, periosteal reaction, and presence of a soft tissue mass (Grade I chondrosarcoma is showed in Fig. 1 and Grade II chondrosarcoma in Fig. 2). All cases diagnosed radiographically by our hospital imaging department were confirmed by pathological examination. For large tumors, a core biopsy preceded the operation. Excisional biopsy was performed for small tumors. All available histology slides were retrospectively reviewed and an appropriate histologic grade assigned by our hospital's three experienced pathologists (Fig. 3).
Figure 1.

Man aged 45 years. (A) Preoperative radiograph demonstrating a calcified lesion (→) in the left distal femur. Pathology showed the lesion to be a Grade I chondrosarcoma. (B) X‐ray image after curettage with bone graft and internal fixation. During follow‐up for 25 months, no evidence of local recurrence was found. ( ) indicates the site after curettage and allogeneic bone graft.) indicates the site after curettage and allogeneic bone graft.
) indicates the site after curettage and allogeneic bone graft.) indicates the site after curettage and allogeneic bone graft.
Figure 2.
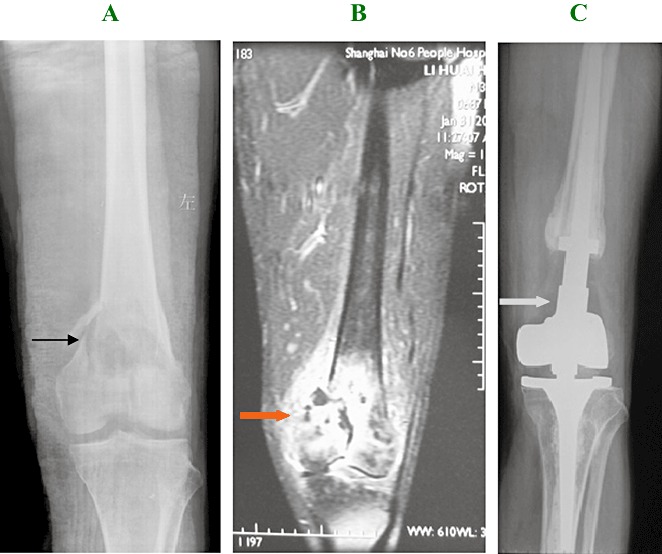
Man aged 31 years. (A) Radiograph demonstrating an osteolytic lesion and cortical disruption with pathological fracture of the medial aspect of the distal femur. Grade II chondrosarcoma was confirmed by pathology preoperatively. (→) indicates the lesion before surgery. (B) MRI showing the lesion involves articular cartilage and surrounding soft tissue. ( ) indicates the lesion before surgery. (C) A 25‐month postoperative X‐ray image showing the appearance after treatment by wide marginal excision and artificial prosthetic replacement. There is no evidence of local recurrence. (
) indicates the lesion before surgery. (C) A 25‐month postoperative X‐ray image showing the appearance after treatment by wide marginal excision and artificial prosthetic replacement. There is no evidence of local recurrence. ( ) indicates the prosthetic replacement.
) indicates the prosthetic replacement.
Figure 3.

(A) Grade I chondrosarcoma showing low cellularity with a chondroid matrix and absent mitoses. (B) Mitoses are seen in cells of a grade II chondrosarcoma.
There were 22 patients with Grade I and 44 with Grade II chondrosarcoma in long bones. The sex distribution was comparable, with men predominating in both groups. The mean age of patients was 45 years (range, 10–79) for Grade I and 47 years (range, 28–72) for Grade II chondrosarcomas. All patients were followed up for an average period of 48 months (range, 5–67). Mean follow‐up times were similar for Grade I at 24.8 months (range, 4–131) and Grade II chondrosarcomas at 31.1 months (range, 3–150). Follow‐up information consisted of residual symptoms, resection margin status, tumor site and grade, and any recurrence of the lesion.
Resection margins were defined as radical, wide, marginal or intralesional, using standard criteria established by the Musculoskeletal Tumor Society (MSTS) staging system. Based on available patient records and histologic material it was not easy to establish whether intralesional or marginal resection had occurred, so we grouped the intralesional and marginal resections together for analysis. Thirty‐eight patients had intralesional and marginal resections, 18 wide resections and 10 amputations.
For statistical analysis, χ2 analyses were used to assess significant differences in outcome by gender, anatomic site, tumor stage, and recurrence rate of the two grades of lesions (Table 1). The 66 patients consisted of 53 males and 13 females ranging in age from 10 to 79 years (mean, 48). All patients were treated only by surgery without adjuvant radiation or chemotherapy. There were 13 patients with tumors in the femur, 15 in the tibia, 12 in the humerus and 2 in a metacarpal bone. Of the patients with Grade I, 6 cases recurred and the remainder were alive without recurrence. Of the patients with Grade II, 28 cases were alive with no evidence of disease, and 16 cases had recurred with unrelated causes.
Table 1.
Summary of the demographic data, treatment, and outcome
| Variable | Total | Grade I | Grade II | P‐value |
|---|---|---|---|---|
| Grade | 66 | 22 | 44 | — |
| Gender | P= 0.913 | |||
| Female | 13 | 5 | 8 | |
| Male | 53 | 17 | 36 | |
| Site | P= 0.095 | |||
| Femur | 37 | 10 | 27 | |
| Tibia | 15 | 4 | 11 | |
| Humerus | 12 | 6 | 6 | |
| Metacarpal | 2 | 2 | 0 | |
| Treatment | P= 0.018 | |||
| M or I | 38 | 18 | 20 | |
| Wide margin | 18 | 3 | 15 | |
| Radical | 10 | 1 | 9 | |
| Outcome (LR) | 22 | 6 | 16 | — |
LR, local recurrence; M or I, marginal or intralesional.
The log‐rank test was applied to tumor recurrence outcome according to resection type, marginal status and tumor grade. All statistical tests were two‐sided and P < 0.05 was considered significant.
Results
Grade I local recurrence‐free survival rate was 60%, as compared to 15% for patients with Grade II chondrosarcomas, who had undergone a range of surgical treatments. Log‐rank analysis showed no significant difference in outcome between Grade I and II chondrosarcomas when grouped together regardless of surgical method (Fig. 4, P > 0.05).
Figure 4.

Curves showing the probability of recurrence‐free survival for patients with Grade I and II chondrosarcomas. The difference between patients with Grade I (60%) and Grade II (40%) chondrosarcoma in recurrence‐free survival at almost 140 months was not statistically significant (P= 0.0638).
Of the 38 cases treated by intralesional curettage, the Grade I local recurrence‐free survival rate was 60%, in contrast to 15% of Grade II chondrosarcomas (P= 0.0216, Fig. 5). Grade II was associated with worse outcomes when managed by intralesional resection.
Figure 5.

Curves showing local recurrence‐free survival with intralesional excision. The local recurrence‐free survival at almost 140 months was significantly less for Grade II than for Grade I tumors (P= 0.0261). Recurrence‐free survival with intralesional excision is 60% for Grade I and 15% for Grade II.
Discussion
Radiological features are essential to the diagnosis and treatment of chondrosarcoma. However, it is difficult to distinguish between Grade I and II chondrosarcomas by radiology 4 , 5 . A diameter of the lesion >5 cm has been shown to be the most reliable predictor of malignancy 6 .
Computed tomography (CT) and MRI are indispensable adjunct tools for assessing the characteristics of chondrosarcoma 7 . CT is especially recommended for the flat bones, because it can be difficult to discern patterns of bone destruction and the presence of matrix mineralization in flat bones. MRI is useful for delineating the extent of bone and soft tissue involvement. Dynamic contrast‐enhanced MRI has greater sensitivity, yet an absolute distinction between benign and malignant cannot be made on radiological grounds alone 8 . A large demineralized soft tissue mass associated with a bone lesion are the radiological features which should raise the level of suspicion for higher grade chondrosarcoma 9 .
Fiorenzaet al. 10 have reported their research on conventional chondrosarcoma, and shown that Grade I and II chondrosarcomas behave differently. Moreover, they grouped Grade II and III chondrosarcomas as MSTS Stage II tumors and classified Grade I as MSTS Stage I. Lee et al. 11 reported a statistical analysis of the data in their series, which showed a significant difference in survival between Grade I and Grade II chondrosarcomas (P= 0.01), but no difference in survival between Grade II and Grade III chondrosarcomas. However, Reith et al. 3 have reported that their χ2 analysis of grade versus outcome showed no significant difference in outcome between Grade I and Grade II chondrosarcomas; they grouped Grade I and Grade II chondrosarcomas as MSTS Stage I tumors. From our experience, Grade I and Grade II chondrosarcomas have very different recurrence rates, so we consider that Grade II chondrosarcomas should be classified as MSTS stage II tumors and Grade I as MSTS stage I.
Bauer et al. 12 reviewed 23 patients with Grade I chondrosarcoma who had been treated by intralesional curettage. Two patients had local recurrence, both Stage IA lesions, one of the proximal tibia and the other the calcaneus. None had metastases or died of tumor‐related causes during a minimum follow‐up of 2 years (mean, 7 years; range, 2–25 years). In contrast, some studies have reported poor outcomes after intralesional resection. Tsuchiya et al. 13 treated six cases of borderline chondrosarcoma. Two patients with Grade I chondrosarcoma had local recurrence.
From our experience, an appropriate surgical technique which observes surgical oncologic principles is the most important element in the avoidance of local recurrence. If the lesion has not involved soft tissue, Grade I tumors near joints should be treated by intralesional curettage and filled with a bone graft or cement.
For Grade I chondrosarcomas, the choice of surgical technique is influenced by the aggressiveness of the tumor and its relationship to weight‐bearing articulating structures. If weight bearing is less of a problem, we prefer intralesional curettage and filling of the bone defect with an allogeneic or autologous bone graft. For large lesions invading the whole bone, we prefer internal fixation for the cavity remaining after tumor removal.
In our study no fracture was seen after implantation. The technique of curettage and subsequent bone graft plugging has several advantages. Firstly, both the affected bone and the functional capacity of the joint are preserved, allowing for a quick return to active movement and partial weight bearing. Secondly, radiographic follow‐up of the affected site is easy since recurrence or secondary changes are visible early and relatively simple to localize. Thirdly, in case of a recurrence, curettage and bone cement plugging can be repeated. If desired, autogenic bone, which is only available in limited amounts, can be saved for subsequent operations.
The surgical possibilities for Grade II chondrosarcomas are limited and dependent on the location of the chondrosarcoma and the degree of bone destruction. Only a small proportion of cases can be treated by intralesional resection. Wide en bloc resections are indicated, especially for Grade II chondrosarcomas. In our study, 20% of the patients with Grade II chondrosarcomas already had pathological fractures at presentation, and many of the tumors had invaded soft tissue around the lesion, so intralesional resection is not suitable for Grade II chondrosarcomas. The functional results after intralesional resection with salvage of the articulating structures were always better, but most patients treated in this way experienced recurrence within several months of surgery, and the recurrence sometimes necessitated amputation.
References
- 1. Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist, 2008, 13: 320–329. [DOI] [PubMed] [Google Scholar]
- 2. Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: A clinicopathologic analysis with emphasis on histologic grading. Cancer, 1977, 40: 818–831. [DOI] [PubMed] [Google Scholar]
- 3. Reith JD, Horodyski MB, Scarborough MT. Grade 2 chondrosarcoma: stage I or stage II tumor? Clin Orthop Relat Res, 2003, 415: 45–51. [DOI] [PubMed] [Google Scholar]
- 4. Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group . Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am, 2007, 89: 2113–2123. [DOI] [PubMed] [Google Scholar]
- 5. Sanerkin NG. The diagnosis and grading of chondrosarcoma of bone: a combined cytologic and histologic approach. Cancer, 1980, 45: 582–594. [DOI] [PubMed] [Google Scholar]
- 6. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. AJR Am J Roentgenol, 1997, 169: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 7. Littrell LA, Wenger DE, Wold LE, et al. Radiographic, CT, and MR imaging features of dedifferentiated chondrosarcomas: A retrospective review of 174 de novo cases. Radiographics, 2004, 24: 1397–1409. [DOI] [PubMed] [Google Scholar]
- 8. Geirnaerdt MJ, Hogendoorn PC, Bloem JL, et al. Cartilaginous tumors: fast contrast‐enhanced MR imaging. Radiology, 2000, 214: 539–546. [DOI] [PubMed] [Google Scholar]
- 9. Bovée JVMG, Cleton‐Jansen AM, Taminiau AHM, et al. Emerging pathways in the development of chondrosarcoma of bone and the implications for targeted treatment. Lancet Oncol, 2005, 6: 599–607. [DOI] [PubMed] [Google Scholar]
- 10. Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br, 2002, 84: 93–99. [DOI] [PubMed] [Google Scholar]
- 11. Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am, 1999, 81: 326–338. [DOI] [PubMed] [Google Scholar]
- 12. Bauer HC, Brosjö O, Kreicbergs A, et al. Low risk of recurrence of enchondroma and low‐grade chondrosarcoma in extremities. 80 patients followed for 2–25 years. Acta Orthop Scand, 1995, 66: 283–288. [DOI] [PubMed] [Google Scholar]
- 13. Tsuchiya H, Ueda Y, Morishita H, et al. Borderline chondrosarcoma of long and flat bones. J Cancer Res Clin Oncol, 1993, 119: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]


