Abstract
Objective
To investigate the relationship between ectonucleotide pyrophosphatase phosphodiesterase‐1(ENPP‐1) expression and transforming growth factor beta 1 (TGF‐β1) of end‐plate chondrocytes after stimulation with intermittent cyclic mechanical tension (ICMT) by using an FX‐4000T Flexercell Tension Plus unit.
Methods
Rat end‐plate chondrocytes were cultured and ICMT (strain at 0.5 Hz sinusoidal curve at 10% elongation) applied for 7 days for 4 h/day and cultured for a further 2 days. End‐plate chondrocytes were also exposed to 10 ng/mL of TGF‐β1. Then, using small interfering RNA technology, small interfering TGF‐β1 (siTGF‐β1) was transfected. Expression of ENPP‐1 and TGF‐β1 was measured by real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR) and western blotting.
Results
Expression of both ENPP‐1 and TGF‐β1 was up‐regulated after ICMT. Both RT‐PCR and western blot showed that ENPP‐1 expression decreases with siRNA TGF‐β1 after 3% elongation 40 min, and cultured for an additional 2 days.
Conclusion
It was found that down‐regulation of ENPP‐1 gene expression induced by ICMT is likely dependent on TGF‐β1 in end‐plate chondrocytes.
Keywords: Ectonucleotide pyrophosphatase phosphodiesterase‐1, End‐plate chondrocytes, Intermittent cyclic mechanical tension, transforming growth factor beta 1
Introduction
In many countries, low back pain is a common health problem, with high medical and disability costs1. Intervertebral disc degeneration is a major cause of this disease2. Research has shown that vertebral endplate calcification occurs prior to intervertebral disc degeneration and that there is a positive correlation between vertebral endplate calcification and the severity of degeneration3. However, the pathophysiology of the intervertebral disc degeneration process remains unclear.
Intervertebral discs are composed of the nucleus pulposus, annulus fibrosus and cartilaginous end plates4. The nucleus pulposus contains type II collagen and high proteoglycan, which attract and hold water within the disc by means of multiple negatively charged glycosaminoglycan side chains. The ability of the matrix to imbibe and release water in relation to the stresses placed on it allows the disc to cushion compressive loads5, 6. The endplates are situated between the intervertebral discs and the vertebral bodies. An intervertebral disc receives its nutritional supply from blood vessels in the vertebral body that terminate at the vertebral endplate. Nutrients must then diffuse from these blood vessels across the endplate and through the matrix to reach the cells of the disc7.
End plate calcification is believed to have an important role in pathogenesis of intervertebral disc degeneration. For example, calcification of the end plate diminishes solute transport through it, altering the ionic composition around the disc cells, which in turn affects the metabolism of the cells and functioning of the disc. Changes in cell metabolism may include decreased synthesis of extracellular matrix molecules, increased degradative enzymes or changes in the cell cycle and thus decrease the viability of the disc8. Previous studies have demonstrated that ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP‐1), an ectonucleoside triphosphate in the cell membrane, hydrolyzes extracellular nucleoside triphosphates into monophosphate and extracellular inorganic pyrophosphate (ePPi)9, 10. The ANK gene is a multipass transmembrane protein that has an important role in making intracellular inorganic pyrophosphate cross the cell membrane. Tissue‐nonspecific alkaline phosphatase (TNAP) hydrolyzes ePPi, reducing the amount of this molecule; this action naturally inhibits PPi crystal formation. The known PPi‐generating proteins include ENPP1, which catalyzes hydrolysis of released adenosine triphosphate (ATP) to produce PPi, and ankyrin repeat (ANK), an intrinsic plasma membrane protein that directly or indirectly mediates efflux of cytosolic PPi. These parallel ATP release/ENPP1 and ANK pathways account for the accumulation of extracellular PPi. They are opposed by the alkaline phosphatase subtype TNAP, an ectopyrophosphatase that clears extracellular PPi. Therefore, calcification regulation is highly dependent on the interactions among ENPP‐1, ANK and TNAP that maintain the amount of ePPi11, 12.
Cell growth, differentiation and function can be influenced by appropriate mechanical strain stimulation; excessive mechanical stimulation can damage cell structure. Previous studies have shown that mechanical stress is associated with calcification of the lumbar spine cartilage endplate13, which could affect the metabolism and functioning of the disc, then accelerate the process of intervertebral disc degeneration14. For testing purposes, 1000 μ is probably an appropriate degree of physiological strain, no more than 4000 μ strain occurs during vigorous physiological activity in humans15, 16. Because a 1000 μ strain is equal to 1% elongation strain intermittent cyclic mechanical tension (ICMT) at 0.5 Hz, a sinusoidal curve at 10% elongation, in this study we applied ICMT with 10% elongation strain to end‐plate chondrocytes. We observed that this level of mechanical strain induces calcification and down‐regulation of ank gene expression17. Therefore, we postulated that end‐plate calcification is related to mechanical strain stimulation and down‐regulation of ENPP‐1 expression.
Transforming growth factor beta 1 (TGF‐β1) plays an important role in cell proliferation, differentiation and apoptosis and extracellular matrix synthesis. TGF‐β1 has been shown to be the major growth factor that increases production of ePPi by normal chondrocytes18. Previous studies have shown that there is more ANK mRNA in human chondrocytes exposed to TGF‐β1 than in those without TGF‐β1 exposure (i.e., controls)19, 20. Our previous study showed that when we treated end‐plate chondrocytes with ICMT‐3 (i.e., mechanical strain at 0.5 Hz, a sinusoidal curve with 3% elongation), both ENPP and TGF‐β1 expression increased17. Therefore, we concluded that TGF‐β1 has a very significant role in crystal deposition in end‐plate cartilage. However, the relationship of TGF‐β1 to the mechanism of regulation of calcification gene ENPP‐1 is not clear. Therefore, in this study, we investigated changes in expression of ENPP‐1 in response to cyclic mechanical strain and the relationship between TGF‐β1 and ENPP‐1.
Materials and Methods
Chondrocyte Isolation and Culture
Primary chondrocytes were isolated from lumbar spine end‐plate cartilage of Sprague‐Dawley rats (weight: 160–180 g). Cartilage samples were carefully removed from L1–L5 end plates under the microscope and minced into small pieces (<0.03 mm3). The samples were sequentially digested with 0.25% trypsin (Sigma‐Aldrich, St. Louis, MO, USA) at 37 °C for 20 mins, followed by 0.02% collagenase (Sigma‐Aldrich) at 37 °C for 5 h. The chondrocytes were washed twice with phosphate buffered saline (PBS) and cultured on Petri dishes at 37 °C under a 5% CO2 atmosphere. Monolayer culture was maintained in growth medium comprised of Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA)/F‐12 supplemented with 10% fetal bovine serum (FBS), 50 U/mL penicillin, and 50 mg/mL streptomycin (all from Hyclone, Logan, UT, USA). Cells from the second passage were used in our experiments.
The study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, China). This study protocol was approved by the Medical Laboratory Animals Care and Use Committee of Anhui Province and the Ethics Committee of Yijishan Hospital of Wannan Medical College and in accordance with Chinese guidelines for the ethical care and use of animals.
Application of Intermittent Cyclic Strain
End‐plate chondrocytes (from the second passage) were plated at a density of 1 × 105 cells/cm2 in 2 mL of medium on six‐well flexible silicone rubber BioFlex plates coated with collagen type I (Flexcell International, Hillsborough, NC, USA). The cells were cultured for 48 h to allow them to attach and reach 80%–90% confluency, at which time the growth medium was replaced. Next, intermittent cyclic mechanical strain (ICMT) was applied by using an FX‐4000T Flexercell Tension Plus unit (Flexcell).
For load protocol 1, end‐plate chondrocytes were subjected to 10% elongation for 7 days for 4 h/day and cultured for a further 2 days. For load protocol 2, end‐plate chondrocytes were subjected to 3% elongation for 40 mins.
The cultures were incubated in a humidified atmosphere at 37 °C and 5% CO2 while they were being subjected to ICMT elongation. The cells were harvested immediately after ICMT had been applied.
Alizarin Red Staining
The cartilage mineralization nodules were stained with alizarin red S (AR‐S). The cells were rinsed with PBS and incubated with 40 mmol/L AR‐S (pH 4.2) for 20 mins of rotation. Then they were rinsed with PBS again. The mineralization nodules were then identified and photographed.
Real‐Time Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. After reverse transcription reaction, real‐time polymerase chain reaction (RT‐PCR) was performed using a Roche LightCycler 480 system using SYBR Premix Ex Taq (Takara, Dalian, China) according to the manufacturer's instructions. The conditions of real‐time PCR were as follows: denaturation at 95 °C for 10 s, 40 cycles at 95 °C for 10 s and 60 °C for 30 s. A dissociation stage was added to the end of the amplification procedure. No non‐specific amplification was detected, as determined by the dissociation curve. Glyceraldehyde‐3‐phosphate dehydrogenase was used as an internal control. Data were analyzed using the delta delta Ct (ΔΔCt, or Ct difference) method, and the results were recorded as fold changes compared to respective controls. Each sample was analyzed in triplicate. The primer sequences of genes are listed in Table 1.
Table 1.
Sequences of primers used in real‐time polymerase chain reaction
| Genes | Forward primer | Reverse primer | Accession number | Product length (bp) |
|---|---|---|---|---|
| TGF‐β1 | 5′‐CATCCATGACATGAACCGACCCTT‐3′ | 5′‐ACAGAAGTTGGCATGGTAGCCCTT‐3′ | NM‐021578.2 | 220 |
| ENPP‐1 | 5′‐TATGCCCAAGAAAGGAATCG‐3′ | 5′‐GCAGCTGGTAAGCACAATGA‐3′ | NM‐006208.2 | 165 |
| GAPDH | 5′‐CTCAACTACATGGTCTACATGTTCCA‐3′ | 5′‐CTTCCCATTCTCAGCCTTGACT‐3′ | NM‐017008.3 | 81 |
Western Blotting
Cells were lysed on ice for 30 mins in lysis buffer containing 50 mM Tris‐HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P‐40, and 0.1% sodium dodecyl sulfate supplemented with protease inhibitors (10 μg/mL leupeptin, 10 μg/mL pepstatin A and 10 μg/mL aprotinin). For western blot analysis, 20 μg of protein sample was resolved on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and electrotransferred onto nitrocellulose membranes (Whatman, Piscataway, NJ, USA). The primary antibody used was anti‐enpp‐1 (Catalog no. 2061, Cell Signaling Technology, Danvers, MA, USA) at a dilution of 1:1000. For normalization of protein loading, β‐actin antibody (Sigma‐Aldrich) was used at a dilution of 1:5000. Infrared‐labeled secondary antibody goat antibody anti‐rabbit IRDye 800 (Li‐Cor Biosciences, Lincoln, NE, USA) was added to bind to the primary antibody. The bound complex was detected using Odyssey Infrared Imaging System (Li‐Cor). The images were analyzed using Odyssey Application Software, version 1.2 (Li‐Cor) to obtain the integrated intensities.
Silencing Experiments with Small Interfering RNA
Small interfering RNA (siRNA) sequences (designed by Shanghai GenePharma, Shanghai, China), TGF‐β1 sense 5′‐CAGCUGUACAUUGACUUUATT‐3′ and antisense 5′‐UAAAGUCAAUGUACAGCUGTT‐3′, were used at final concentrations of 50 nM. Transfection with TGF‐β1 siRNA was performed after ICMT‐3. Briefly, siRNA and lipofectamine 2000 were diluted separately in serum‐free medium, after which diluted lipofectamine was added to the siRNA. After 20 mins incubation at room temperature, the cells were washed with PBS and incubated for 4 h in an siRNA–lipofectamin mixture. Then the mixture was discarded and medium containing 10% FBS added to the culture dish. Two days later, the specificity of siRNA effects was determined using ENPP‐1 mRNA expression as a negative control.
Statistical Analyses
SPSS 11.0 statistical software was used for statistical analyses. Results were recorded as the mean ± standard deviation of at least three independent experiments. Comparisons of overall difference between groups were made by ANOVA and the control and treatment groups were compared using the least significant difference method. A P value of <0.05 was considered significant.
Results
mRNA Expression of Ectonucleotide Pyrophosphatase Phosphodiesterase‐1 and Alizarin Red Staining
There were many endplate chondrocytes in the control and ICMT groups. RT‐PCR showed that mRNA expression of ENPP‐1 decreased (P = 0.0181). Compared with the control group, alizarin red staining revealed increased mineral deposition in the IMCT group (Fig. 1). The endplate chondrocytes stained positively with AR‐S, which directly indicates that ICMT induces their calcification.
Figure 1.
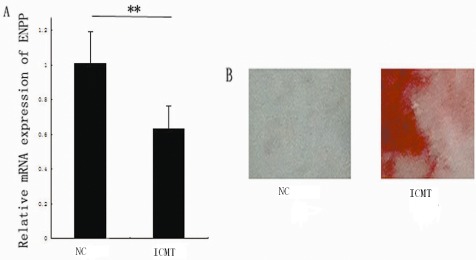
The effect of intermittent cyclic mechanical tension on mRNA expression of ectonucleotide pyrophosphatase phosphodiesterase‐1and end‐plate calcification. (A) Down‐regulation of ENPP‐1 expression in the ICMT‐10 group as assessed by RT‐PCR. The columns represent the mean ± SE *P < 0.05 vs. NC. (B) Alizarin red staining revealed more mineral deposition in the IMCT than in the NC group (×100). NC, normal control.
Expression of Ectonucleotide Pyrophosphatase Phosphodiesterase‐1 and Transforming Growth Factor Beta 1 with Intermittent Cyclic Mechanical Tension‐3
Real‐time PCR showed that the mRNA expression of ENPP‐1increased (P = 0.0058). mRNA expression of TGF‐β1 was increased by ICMT‐3 (P = 0.0127, Fig. 2). These results indicate that calcification gene ENPP‐1 is involved in TGF‐β1 regulation.
Figure 2.
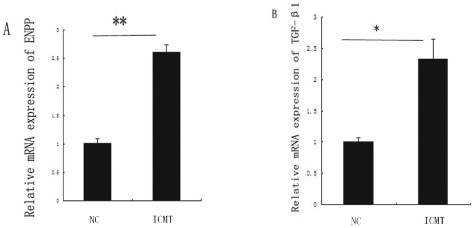
Expression of ectonucleotide pyrophosphatase phosphodiesterase‐1and transforming growth factor beta 1 with intermittent cyclic mechanical tension‐3. (A) Up‐regulation of ENPP‐1 expression with ICMT‐3 as assessed by real‐time RT‐PCR. (B) Up‐regulation of TGF‐β1 expression in the ICMT‐3 group as assessed by RT‐PCR. The columns represent the mean ± SE. *, P < 0.05, **, P < 0.01 vs. NC. NC, normal control.
Contribution of Transforming Growth Factor Beta 1 to Regulation of Expression of Ectonucleotide Pyrophosphatase Phosphodiesterase‐1 with Intermittent Cyclic Mechanical Tension
Both RT‐PCR and western blotting revealed that TGF‐β1 induced ENPP‐1 expression after ICMT‐3 (Fig. 3A, B). siRNA technology was used to determine the contributions to ENPP‐1 expression induced by TGF‐β1 (Fig. 3C, D). Our experiments showed that siRNAs are efficient. According to both RT‐PCR and western blot analysis, they reduced ENPP‐1 expression. These results indicate that the expression of ENPP‐1 is likely caused by endogenous TGF‐β1.
Figure 3.
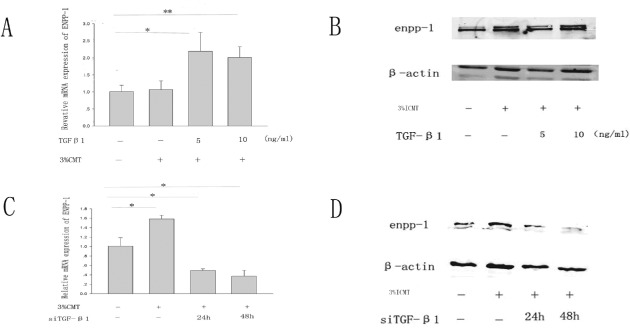
Contribution of transforming growth factor beta 1 to regulation of expression of ectonucleotide pyrophosphatase phosphodiesterase‐1with cyclic mechanical strain. (A, B) Total mRNA and proteins were extracted from rat end‐plate exposed to (5, 10) ng/mL of TGF‐β1 for 24 h after ICMT‐3. (C, D) Effect of siRNA on TGF‐β1 induced expression of ENPP‐1. Total mRNA and proteins were extracted from rat end‐plate stimulated with ICMT‐3, in the presence of siRNA on TGF‐β1 for 24 or 48 h. The columns represent the mean ± SE. *, P < 0.05, **, P < 0..01 vs. NC. NC, normal control.
Discussion
End‐plate calcification correlates with the process of vertebral disc degeneration, which can cause or promote the process of intervertebral disc disease7, 21. Mechanical strain is one of the causes of calcification13. In this study, chondrocytes stained positively with AR‐S at pH 4.2, showing that mechanical strain can induce calcification in endplate chondrocytes. In addition, after ICMT, expression of calcification gene ANK decreases and the differentiated phenotype of end‐plate chondrocytes is lost, with concomitant cessation of expression of type II collagen, aggrecan, and sox‐917. Shi et al. demonstrated that ICMT inhibits chondrogenesis in mesenchymal stem cells22. These data led us to postulate that mechanical strain can lead to calcification and change the expression of ENPP‐1. Our data shows that expression of ENPP‐1 in rat lumbar end‐plate chondrocytes decreases with 10% mechanical strain stimulation. Therefore, the ENPP‐1 gene has an effect on the process of calcification.
Early studies demonstrated that mesangial cells treated with mechanical strain can produce collagenous proteins and fibronectin23, 24; this production correlates with increased expression of TGF‐β125, 26. Other studies have shown that amounts of ANK mRNA and protein increase with TGF‐β1 stimulation19, 27. The present study demonstrated that expression of TGF‐β1 and ENPP‐1 increases with ICMT‐3 (i.e., 3% elongation strain for 40 mins) and that ENPP‐1 expression is greater in end‐plate chondrocytes exposed to TGF‐β1 than in controls. Both ENPP‐1 and TGF‐β1 expression are up‐regulated by ICMT‐3. We postulate that ENPP‐1 gene expression depends mainly on endogenous TGF‐β1 regulation.
Previous studies have suggested that the ANK gene plays an important role in the process of calcification stimulated by mechanical strain. However, the effect of ENPP‐1 remained unclear. Our study demonstrated that calcification of end‐plate chondrocytes is associated with expression of ENPP‐1 and TGF‐β1 and that TGF‐β1 regulates ENPP‐1 expression with mechanical strain stimulation.
The current study has several limitations. ENPP‐1 expression decreased with 10% elongation strain stimulation; however, the process of endplate chondrocyte calcification did not reflect this. Whether other factors can also promote endplate chondrocyte calcification remains unknown. Further studies are necessary to verify our results.
In conclusion, our data demonstrate that down‐regulation of ENPP‐1 gene expression by ICMT may be caused by endogenous TGF‐β1. This indicates that mechanical strain controls endplate chondrocyte function.
Disclosure: This study was supported by the Chinese National Natural Sciences Fund project (Grant No.30973025 and No.81272048) and by the Chinese Anhui Province Education Department Key Fund project (Grant No. KJ2010A320).
References
- 1. Wenig CM, Schmidt CO, Kohlmann T, Schweikert B. Costs of back pain in Germany. Eur J Pain, 2009, 13: 280–286. [DOI] [PubMed] [Google Scholar]
- 2. Raj PP. Intervertebral disc: anatomy‐physiology‐pathophysiology‐treatment. Pain Pract, 2008, 8: 18–44. [DOI] [PubMed] [Google Scholar]
- 3. Xu HG, Chen XW, Wang H, Lu LM, Liu P, Xia LZ. Correlation between chondrocyte apoptosis of vertebral cartilage endplate and degeneration of intervertebral disc. Zhong Hua Yi Xue Za Zhi, 2008, 88: 194–197. (In Chinese). [PubMed] [Google Scholar]
- 4. Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol, 2001, 20: 107–121. [DOI] [PubMed] [Google Scholar]
- 5. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine, 2006, 31: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem, 1998, 124: 686–693. [DOI] [PubMed] [Google Scholar]
- 7. Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine, 2004, 29: 2700–2709. [DOI] [PubMed] [Google Scholar]
- 8. Gruber HE, Norton HJ, Sun Y, Hanley EN Jr. Crystal deposits in the human intervertebral disc: implications for disc degeneration. Spine J, 2007, 7: 444–450. [DOI] [PubMed] [Google Scholar]
- 9. Howell DS, Martel‐Pelletier J, Pelletier JP, Morales S, Muniz O. NTP pyrophosphohydrolase in human chondrocalcinotic and osteoarthritic cartilage. II. Further studies on histologic and subcellular distribution. Arthritis Rheum, 1984, 27: 193–199. [DOI] [PubMed] [Google Scholar]
- 10. Ryan LM, Wortmann RL, Karas B, Lynch MP, McCarty DJ. Pyrophosphohydrolase activity and inorganic pyrophosphate content of cultured human skin fibroblasts. Elevated levels in some patients with calcium pyrophosphate dihydrate deposition disease. J Clin Invest, 1986, 77: 1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hessle L, Johnson KA, Anderson HC, et al Tissue‐nonspecific alkaline phosphatase and plasma cell membrane glycoprotein‐1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA, 2002, 99: 9445–9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson HC, Harmey D, Camacho NP, et al Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia‐related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double‐deficient mice. Am J Pathol, 2005, 166: 1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bian Q, Liang QQ, Wan C, et al Prolonged upright posture induces calcified hypertrophy in the cartilage end plate in rat lumbar spine. Spine, 2011, 36: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 14. Peng B, Hou S, Shi Q, Jia L. The relationship between cartilage end‐plate calcification and disc degeneration: an experimental study. Chin Med J (Engl), 2001, 114: 308–312. [PubMed] [Google Scholar]
- 15. Fermor B, Gundle R, Evans M, Emerton M, Pocock A, Murray D. Primary human osteoblast proliferation and prostaglandin E2 release in response to mechanical strain in vitro . Bone, 1998, 22: 637–643. [DOI] [PubMed] [Google Scholar]
- 16. Burr DB, Milgrom C, Fyhrie D, et al In vivo measurement of human tibial strains during vigorous activity. Bone, 1996, 18: 405–410. [DOI] [PubMed] [Google Scholar]
- 17. Xu HG, Zhang XH, Wang H, et al Intermittent cyclic mechanical tension‐induced calcification and downregulation of ankh gene expression of end plate chondrocytes. Spine, 2012, 37: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 18. Rosenthal AK, McCarty BA, Cheung HS, Ryan LM. A comparison of the effect of transforming growth factor beta 1 on pyrophosphate elaboration from various articular tissues. Arthritis Rheum, 1993, 36: 539–542. [DOI] [PubMed] [Google Scholar]
- 19. Cailotto F, Bianchi A, Sebillaud S, et al Inorganic pyrophosphate generation by transforming growth factor‐beta‐1 is mainly dependent on ANK induction by Ras/Raf‐1/extracellular signal‐regulated kinase pathways in chondrocytes. Arthritis Res Ther, 2007, 9: R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sohn P, Crowley M, Slattery E, Serra R. Developmental and TGF‐beta‐mediated regulation of Ank mRNA expression in cartilage and bone. Osteoarthritis Cartilage, 2002, 10: 482–490. [DOI] [PubMed] [Google Scholar]
- 21. Melrose J, Burkhardt D, Taylor TK, et al Calcification in the ovine intervertebral disc: a model of hydroxyapatite deposition disease. Eur Spine J, 2009, 18: 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi Y, Li H, Zhang X, et al Continuous cyclic mechanical tension inhibited Runx2 expression in mesenchymal stem cells through RhoA‐ERK1/2 pathway. J Cell Physiol, 2011, 226: 2159–2169. [DOI] [PubMed] [Google Scholar]
- 23. Harris RC, Haralson MA, Badr KF. Continuous stretch‐relaxation in culture alters rat mesangial cell morphology, growth characteristics, and metabolic activity. Lab Invest, 1992, 66: 548–554. [PubMed] [Google Scholar]
- 24. Hirakata M, Kaname S, Chung UG, et al Tyrosine kinase dependent expression of TGF‐beta induced by stretch in mesangial cells. Kidney Int, 1997, 51: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 25. Yasuda T, Kondo S, Homma T, Harris RC. Regulation of extracellular matrix by mechanical stress in rat glomerular mesangial cells. J Clin Invest, 1996, 98: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riser BL, Cortes P, Heilig C, et al Cyclic stretching force selectively up‐regulates transforming growth factor‐beta isoforms in cultured rat mesangial cells. Am J Pathol, 1996, 148: 1915–1923. [PMC free article] [PubMed] [Google Scholar]
- 27. Hirose J, Ryan LM, Masuda I. Up‐regulated expression of cartilage intermediate‐layer protein and ANK in articular hyaline cartilage from patients with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum, 2002, 46: 3218–3229. [DOI] [PubMed] [Google Scholar]


