Key Points
Question
What is the natural progression of symptom change and recovery in children after concussion?
Findings
In this multicenter cohort study that included 2716 children aged 5 to 18 years, symptom improvement primarily occurred in the first 2 weeks after injury in children and in the first 4 weeks after injury in preadolescents and male adolescents. Female adolescents had protracted recovery.
Meaning
It may be that between-sex differences in adolescents should be taken into consideration for recovery management; the derived recovery curves may be useful for evidence-based anticipatory guidance.
This multicenter cohort study describes the natural progression of symptom change by age group (5-7, 8-12, and 13-18 years) and sex and develops centile curves to inform families about children after concussion recovery.
Abstract
Importance
The natural progression of symptom change and recovery remains poorly defined in children after concussion.
Objectives
To describe the natural progression of symptom change by age group (5-7, 8-12, and 13-18 years) and sex, as well as to develop centile curves to inform families about children after injury recovery.
Design, Setting, and Participants
Planned secondary analysis of a prospective multicenter cohort study (Predicting Persistent Postconcussive Problems in Pediatrics). The setting was 9 pediatric emergency departments within the Pediatric Emergency Research Canada (PERC) network. Participants were aged 5 to 18 years with acute concussion, enrolled from August 1, 2013, to May 31, 2015, and data analyses were performed between January 2018 and March 2018.
Exposures
Participants had a concussion consistent with the Zurich Consensus Statement on Concussion in Sport diagnostic criteria and 85% completeness of the Postconcussion Symptom Inventory (PCSI) at each time point.
Main Outcomes and Measures
The primary outcome was symptom change, defined as current rating minus preinjury rating (delta score), at presentation and 1, 2, 4, 8, and 12 weeks after injury, measured using the PCSI. Symptoms were self-rated for ages 8 to 18 years and rated by the child and parent for ages 5 to 7 years. The secondary outcome was recovery, defined as no change in symptoms relative to current preinjury PCSI ratings (delta score = 0). Mixed-effects models incorporated the total score, adjusting for random effects (site and participant variability), fixed-effects indicators (age, sex, time, age by time interaction, and sex by time interaction), and variables associated with recovery. Recovery centile curves by age and sex were computed.
Results
A total of 3063 children (median age, 12.0 years [interquartile range, 9.2-14.6 years]; 60.7% male) completed the primary outcome; 2716 were included in the primary outcome analysis. For the group aged 5 to 7 years, symptom change primarily occurred the first week after injury; by 2 weeks, 75.6% of symptoms had improved (PCSI change between 0 and 2 weeks, −5.3; 95% CI, −5.5 to −5.0). For the groups aged 8 to 12 years and 13 to 18 years, symptom change was prominent the first 2 weeks but flattened between 2 and 4 weeks. By 4 weeks, 83.6% and 86.2% of symptoms, respectively, had improved for the groups aged 8 to 12 years (PCSI change between 0 and 4 weeks, −9.0; 95% CI, −9.6 to −8.4) and 13 to 18 years (PCSI change between 0 and 4 weeks, −28.6; 95% CI, −30.8 to −26.3). Sex by time interaction was significant only for the adolescent group (β = 0.32; 95% CI, 0.21-0.43; P < .001). Most adolescent girls had not recovered by week 12.
Conclusions and Relevance
Symptom improvement primarily occurs in the first 2 weeks after concussion in children and in the first 4 weeks after concussion in preadolescents and male adolescents. Female adolescents appear to have protracted recovery. The derived recovery curves may be useful for evidence-based anticipatory guidance.
Introduction
Concussions are a serious health concern in children because quality-of-life deficits may persist for months.1 In the last decade, rates of pediatric visits to emergency departments (EDs) and primary care providers for concussion and minor head injuries have increased 2- to 4-fold in the United States and in Canada.2,3,4 Although many studies have focused on the rates and factors predictive of persistent postconcussive symptoms, the natural progression of recovery processes remains poorly characterized.
The expected duration for recovery after pediatric concussions is broad, ranging from days to months and even years.5 The 5th International Conference on Concussion in Sport designated prolonged symptoms for pediatric concussion at greater than 4 weeks5,6; however, the definition of recovery is inconsistent throughout the literature7 and is not developmentally specific.5 Previous studies focused on sport-related concussions,8,9,10 used retrospective designs,8,9 enrolled primarily adolescents and young adults,10,11,12 and had limited sample sizes.11,13,14 Other barriers to determining the natural course of recovery include recruitment beyond the acute injury period8,13 and failure to measure outcomes using validated pediatric symptom scoring scales.5,7
While prognosticators for prolonged recovery include older children,8,9,14,15 female sex,10,14,16 number of previous concussions,9,14,17 acute and subacute symptoms,15,18 and symptom burden,19,20,21 recovery progression remains poorly described. Given the extensive brain maturation and significant functional development that occurs throughout childhood and because sex differences exist throughout development, investigating the natural course of recovery from concussion by age and sex is important for effective management decisions and preventive measures. This study examined the natural progression of self-reported symptom recovery from pediatric concussion over the initial 3 months after injury. The objectives were to: (1) describe the natural progression of symptom change by age group (5-7, 8-12, and 13-18 years), (2) examine the natural progression of symptom change by continuous age and sex, and (3) develop centile curves to inform families about children after injury recovery.
Methods
Design and Settings
This study was a planned secondary analysis of the Predicting and Preventing Postconcussive Problems in Pediatrics study,15,22 a prospective multicenter cohort study that recruited participants enrolled from August 1, 2013, to May 31, 2015, at 9 Pediatric Emergency Research Canada (PERC) network EDs. Data analyses were performed between January 2018 and March 2018. The ethics committees of the participating 9 institutions approved the study. All participants provided written informed consent or assent.
Study Population
Participants aged 5 to 18 years who had sustained an acute head injury less than 48 hours from ED presentation and who met the concussion diagnosis criteria according to the Zurich Consensus Statement on Concussion in Sport23 were eligible for inclusion in the study. Exclusion criteria included the following: Glasgow Coma Scale score of 13 or less, any trauma-related abnormality on neuroimaging, multisystem injury requiring hospitalization, severe preexisting neurological developmental delay resulting in communication difficulties, intoxication, absence of trauma as primary event, previous enrollment, language barrier, inability to complete follow-up, or neurosurgical intervention, intubation, or intensive care unit admission.
Study Protocol
The study protocol was previously published.22 Summarized methods are described below.
ED Visit
Research assistants evaluated participants acutely in the ED on enrollment using 3 measures. The first was the Acute Concussion Evaluation (ACE), a 22-item dichotomous symptom inventory evaluating injury characteristics, symptoms, and risk factors for protracted recovery.24 The second was the Child Sports Concussion Assessment Tool version 3 (Child SCAT3),23 a standardized tool for evaluating cognitive assessment and balance (using the Balance Error Scoring System [BESS]).25 The third was the Postconcussion Symptom Inventory (PCSI),26 valid and reliable symptom scales for parents (20 items using a 7-point scale) and developmentally specific self-reported forms for children aged 5 to 7 years (13 items using a 3-point [0, 1, 2] Guttman scale indicating the severity of the symptom; score range, 0-26), aged 8 to 12 years (17 items using a 3-point [0-2] Guttman scale indicating the severity of the symptom; score range, 0-34), and aged 13 to 18 years (20 items using a 7-point [0-7] Guttman scale indicating the severity of the symptoms; score range, 0-120). Child and/or parent provided mechanism of the traumatic event, personal history (eg, migraine), and mental health history.
Follow-up Questionnaires
Using the age-specific patient versions of the PCSI, symptoms were self-rated for all age groups at presentation and 1, 2, 4, 8, and 12 weeks after injury. Younger children (aged 5-7 years) may have received assistance from their parents in completing their assessment forms. Depending on their choice, participants completed the follow-up questionnaires via a web-based survey or telephone. All data were collected and managed with the Research Electronic Data Capture (REDCap)27 database.
Outcomes
The primary outcome was symptom change, defined as current rating minus preinjury rating (delta score), over time (at presentation and 1, 2, 4, 8, and 12 weeks after injury), measured using the PCSI. The secondary outcome was recovery; children were considered recovered if they had no change in symptoms relative to current preinjury PCSI ratings (delta score = 0).
Statistical Analysis
Natural Progression of Symptom Change
Descriptive statistics were used to summarize patient baseline characteristics for each age group. The primary analysis focused on the natural course of symptom change over 12 weeks, stratified by age group, with adjustment for specific covariates associated with recovery. Based on systematic reviews,5,6,28 a priori selected variables determined to be associated with recovery included the following: age,8,9,14,15 sex,10,14,15,16,17 time, age by time interaction, sex by time interaction, personal migraine history,15 previous concussions with symptoms lasting longer than 1 week,9,14,17 learning disability,15 attention-deficit/hyperactivity disorder,29 anxiety,9 depression,14 sleep disorders,18,30 loss of consciousness and duration,15,18 cognitive scores from the Child SCAT3 (immediate and delayed memory, concentration),8 BESS tandem stance,15 and baseline subscale symptom scores (parent reported).18 For the group aged 5 to 7 years, the variables depression (no participants had a prior depression diagnosis) and BESS tandem stance (this was not validated in that age group) were not predictors. Three linear mixed-effects models (1 model per age group) were fitted, with the total PCSI delta score as the dependent variable (measured at presentation and 1, 2, 4, 8, and 12 weeks). Random effects for model intercept and time were specified under the assumption of a continuous autoregressive (AR) process, namely, AR(1) process for a continuous time covariate, based on a patient nested in site (9-level correlation structure).31,32 To allow for nonlinearity of effects in the mixed models, fractional polynomial transformations were applied to continuous covariates using an initial exploratory modeling procedure that systematically searches for a suitable transformation for each continuous covariate, combined with a backward elimination procedure to limit overfitting.33,34 Because the “shape” of concussion symptom change was of primary interest, a maximum of 4 df was afforded to the transformation of the time covariate, while 2 df was afforded to all other continuous covariates. Graphical assessment of residuals (quantile-quantile plots and plots of standardized residuals against fitted values) revealed neither significant deviations from normality nor strong evidence of heteroscedasticity.
Model contrasts were specified to estimate rate of symptom change over time, continuous age by time interaction, and sex by time interaction and to estimate the PCSI delta score difference between sex and the 75th and 25th quantiles of the continuous age variable. Analyses were performed adjusting covariates at either their median or mode, with the 95% CI adjusted for multiple comparisons by Bonferroni correction. Two-sided P < .05 was considered statistically significant.
Centile Curves of Recovery
In the secondary outcome analysis, the proportion of recovered participants was estimated with associated binomial 95% CIs using the method by Wilson.35 Centile curves (unadjusted) were created with quantile regression of PCSI delta scores, with sex and time as covariates. An initial modeling procedure was generated to determine a suitable fractional polynomial transformation for time (continuous covariate) to allow for nonlinearity. Quantile regressions to estimate quantiles of interest over the study period were fitted.
For all analyses, the continuous PCSI delta score was used, where a 15% cutoff for missing data (nonanswered items) was allowed. To assess any potential bias between missing vs nonmissing time points, a descriptive comparison of selected variables was conducted.
To ensure that multivariable mixed modeling and time point proportion estimations could be achieved for even the smallest cohort, sample size considerations were made. For fitting the mixed models, the smallest cohort was 534 children (aged 5-7 years), translating into an effective sample size of approximately 1451 given 6 measurement time points per patient under the assumptions of an AR(1) correlation structure and r = 0.49 correlation between consecutive time points.36 This enabled a complex regression model to be fitted (ie, 72 df under a 20:1 target of sample size to predictor). Estimating recovered proportions for the smallest subcohort of 151 children (girls aged 5-7 years) allowed estimation of a proportion 95% CI half width of 0.08 (ie, 8% margin of error), assuming a “worst case” hypothetical observed proportion of 0.5. Two-sided P < .05 was considered statistically significant, and all analyses were conducted using a software program (R, version 3.3.2; R Foundation).
Results
A total of 3063 children (60.7% male), with a median age of 12.0 years (interquartile range, 9.2-14.6 years), completed the ED outcome measure (eFigure 1 in the Supplement). Of these participants, 488 aged 5 to 7 years, 1158 aged 8 to 12 years, and 1070 aged 13 to 18 years were included in the mixed-effects models for at least 1 time point. Baseline characteristics by age group are summarized in Table 1. The percentage of patients with missing outcomes (<85% PCSI completeness) for each time point is summarized in eTable 1 in the Supplement. Those missing data vs those not missing data for all time points were similar for both outcomes (eTable 2 and eTable 3 in the Supplement).
Table 1. Participant Characteristics.
| Variable | Total Eligible Sample | Mixed-Effects Model Participants | Centile Curves | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-7 y | 8-12 y | 13-18 y | 5-7 y | 8-12 y | 13-18 y | 5-7 y | 8-12 y | 13-18 y | |
| Total No. | 534 | 1282 | 1247 | 488 | 1158 | 1070 | 528 | 1269 | 1234 |
| Age, median (IQR), y | 6.6 (5.9-7.3) | 10.8 (9.6-11.9) | 15.1 (14.0-16.1) | 6.6 (5.9-7.3) | 10.8 (9.6-11.9) | 15.0 (14.0-16.0) | 6.6 (5.9-7.3) | 10.8 (9.6-11.9) | 15.1 (14.0-16.1) |
| Sex, No. (%) | |||||||||
| Male | 331 (62.0) | 838 (65.4) | 688 (55.2) | 303 (62.1) | 760 (65.6) | 588 (55.0) | 326 (61.7) | 830 (65.4) | 680 (55.1) |
| Female | 203 (38.0) | 444 (34.6) | 558 (44.7) | 185 (37.9) | 398 (34.4) | 482 (45.0) | 202 (38.3) | 439 (34.6) | 554 (44.9) |
| NA | 0 | 0 | 1 (0.1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Time from injury to presentation in the ED, median (IQR), h | 2.6 (1.4-5.6) | 2.7 (1.4-7.4) | 3.2 (1.4-19.8) | 2.7 (1.4-5.6) | 2.8 (1.5-7.3) | 3.3 (1.5-20.1) | 2.6 (1.4-5.6) | 2.7 (1.4-7.3) | 3.2 (1.4-19.9) |
| Relating to Personal History | |||||||||
| Personal migraine history, No. (%) | |||||||||
| No | 503 (94.2) | 1139 (88.8) | 1004 (80.5) | 462 (94.7) | 1041 (89.9) | 877 (82.0) | 500 (94.7) | 1131 (89.1) | 998 (80.9) |
| Yes | 26 (4.9) | 131 (10.2) | 235 (18.8) | 26 (5.3) | 117 (10.1) | 193 (18.0) | 26 (4.9) | 131 (10.3) | 231 (18.7) |
| NA | 5 (0.9) | 12 (0.9) | 8 (0.6) | 0 | 0 | 0 | 2 (0.4) | 7 (0.6) | 5 (0.4) |
| Previous concussions, mean (SD) | 0.1 (0.4) | 0.2 (0.6) | 0.6 (1.0) | 0.1 (0.5) | 0.2 (0.6) | 0.6 (1.0) | 0.1 (0.4) | 0.2 (0.6) | 0.6 (1.0) |
| Symptom duration of prior concussion, No. (%) | |||||||||
| <1 wk | 512 (95.9) | 1180 (92.0) | 955 (76.6) | 473 (96.9) | 1078 (93.1) | 826 (77.2) | 509 (96.4) | 1172 (92.4) | 946 (76.7) |
| ≥1 wk | 16 (3.0) | 93 (7.3) | 281 (22.5) | 15 (3.1) | 80 (6.9) | 244 (22.8) | 16 (3.0) | 92 (7.2) | 280 (22.7) |
| NA | 6 (1.1) | 9 (0.7) | 11 (0.9) | 0 | 0 | 0 | 3 (0.6) | 5 (0.4) | 8 (0.6) |
| Learning disability, No. (%) | |||||||||
| No | 508 (95.1) | 1168 (91.1) | 1120 (89.8) | 468 (95.9) | 1067 (92.1) | 968 (90.5) | 505 (95.6) | 1163 (91.6) | 1111 (90.0) |
| Yes | 22 (4.1) | 103 (8.0) | 118 (9.5) | 20 (4.1) | 91 (7.9) | 102 (9.5) | 22 (4.2) | 100 (7.9) | 117 (9.5) |
| NA | 4 (0.7) | 11 (0.9) | 9 (0.7) | 0 | 0 | 0 | 1 (0.2) | 6 (0.5) | 6 (0.5) |
| Attention-deficit/hyperactivity disorder, No. (%) | |||||||||
| No | 504 (94.4) | 1155 (90.1) | 1109 (88.9) | 465 (95.3) | 1056 (91.2) | 965 (90.2) | 501 (94.9) | 1149 (90.5) | 1101 (89.2) |
| Yes | 26 (4.9) | 113 (8.8) | 129 (10.3) | 23 (4.7) | 102 (8.8) | 105 (9.8) | 26 (4.9) | 111 (8.7) | 127 (10.3) |
| NA | 4 (0.7) | 14 (1.1) | 9 (0.7) | 0 | 0 | 0 | 1 (0.2) | 9 (0.7) | 6 (0.5) |
| Anxiety, No. (%) | |||||||||
| No | 518 (97.0) | 1194 (93.1) | 1096 (87.9) | 476 (97.5) | 1082 (93.4) | 949 (88.7) | 515 (97.5) | 1186 (93.5) | 1088 (88.2) |
| Yes | 13 (2.4) | 81 (6.3) | 143 (11.5) | 12 (2.5) | 76 (6.6) | 121 (11.3) | 13 (2.5) | 81 (6.4) | 141 (11.4) |
| NA | 3 (0.6) | 7 (0.5) | 8 (0.6) | 0 | 0 | 0 | 0 | 2 (0.2) | 5 (0.4) |
| Depression, No. (%) | |||||||||
| No | 531 (99.4) | 1260 (98.3) | 1169 (93.7) | 488 (100) | 1144 (98.8) | 1018 (95.1) | 528 (100) | 1252 (98.7) | 1159 (93.9) |
| Yes | 0 | 14 (1.1) | 73 (5.9) | 0 | 14 (1.2) | 52 (4.9) | 0 | 14 (1.1) | 73 (5.9) |
| NA | 3 (0.6) | 8 (0.6) | 5 (0.4) | 0 | 0 | 0 | 0 | 3 (0.2) | 2 (0.2) |
| Sleep disorders, No. (%) | |||||||||
| No | 524 (98.1) | 1256 (98.0) | 1198 (96.1) | 483 (99.0) | 1143 (98.7) | 1036 (96.8) | 521 (98.7) | 1248 (98.3) | 1188 (96.3) |
| Yes | 5 (0.9) | 16 (1.2) | 41 (3.3) | 5 (1.0) | 15 (1.3) | 34 (3.2) | 5 (0.9) | 16 (1.3) | 41 (3.3) |
| NA | 5 (0.9) | 10 (0.8) | 8 (0.6) | 0 | 0 | 0 | 2 (0.4) | 5 (0.4) | 5 (0.4) |
| Mechanism of injury, No. (%) | |||||||||
| Sports and recreational play | 240 (44.9) | 862 (67.2) | 969 (77.7) | 226 (46.3) | 784 (67.7) | 837 (78.2) | 239 (45.3) | 857 (67.5) | 963 (78.0) |
| Non–sport-related injury or fall | 247 (46.3) | 334 (26.1) | 160 (12.8) | 224 (45.9) | 299 (25.8) | 139 (13.0) | 246 (46.6) | 332 (26.2) | 157 (12.7) |
| Motor vehicle crash | 5 (0.9) | 13 (1.0) | 37 (3.0) | 4 (0.8) | 12 (1.0) | 26 (2.4) | 4 (0.8) | 13 (1.0) | 37 (3.0) |
| Assault | 2 (0.4) | 13 (1.0) | 24 (1.9) | 2 (0.4) | 13 (1.1) | 21 (2.0) | 2 (0.4) | 13 (1.0) | 23 (1.9) |
| Other | 36 (6.7) | 53 (4.1) | 54 (4.3) | 32 (6.6) | 50 (4.3) | 47 (4.4) | 36 (6.8) | 53 (4.2) | 54 (4.4) |
| NA | 4 (0.7) | 7 (0.5) | 3 (0.2) | 0 | 0 | 0 | 1 (0.2) | 1 (0.1) | 0 |
| Helmet use in sports, No./total No. (%)a | |||||||||
| No | 214/240 (89.2) | 525/862 (60.9) | 553/969 (57.1) | 201/226 (88.9) | 485/784 (61.9) | 465/837 (55.6) | 213/239 (89.1) | 520/857 (60.7) | 549/963 (57.0) |
| Yes | 26/240 (10.8) | 337/862 (39.1) | 416/969 (42.9) | 25/226 (11.1) | 299/784 (38.1) | 372/837 (44.4) | 26/239 (10.9) | 337/857 (39.3) | 414/963 (43.0) |
| Mouth guard use in sports, No./total No. (%)a | |||||||||
| No | 230/240 (95.8) | 707/859 (82.3) | 681/967 (70.4) | 217/226 (96.0) | 644/781 (82.5) | 581/835 (69.6) | 229/239 (95.8) | 702/854 (82.2) | 676/961 (70.3) |
| Yes | 10/240 (4.2) | 152/859 (17.7) | 286/967 (29.6) | 9/226 (4.0) | 137/781 (17.5) | 254/835 (30.4) | 10/239 (4.2) | 152/854 (17.8) | 285/961 (29.7) |
| Relating to Symptoms After Injury | |||||||||
| Loss of consciousness, No. (%) | |||||||||
| No | 419 (78.5) | 991 (77.3) | 907 (72.7) | 384 (78.7) | 898 (77.5) | 786 (73.5) | 417 (79.0) | 985 (77.6) | 899 (72.9) |
| Yes | 37 (6.9) | 159 (12.4) | 199 (16.0) | 32 (6.6) | 143 (12.3) | 173 (16.2) | 36 (6.8) | 158 (12.5) | 198 (16.0) |
| Unknown | 74 (13.9) | 126 (9.8) | 137 (11.0) | 72 (14.8) | 117 (10.1) | 111 (10.4) | 74 (14.0) | 125 (9.9) | 136 (11.0) |
| NA | 4 (0.7) | 6 (0.5) | 4 (0.3) | 0 | 0 | 0 | 1 (0.2) | 1 (0.1) | 1 (0.1) |
| Duration of loss of consciousness, mean (SD), min | 0.1 (0.5) | 0.1 (0.7) | 0.2 (0.9) | 0.1 (0.5) | 0.1 (0.7) | 0.2 (0.9) | 0.1 (0.5) | 0.1 (0.7) | 0.2 (0.9) |
| Seizure, No. (%) | |||||||||
| No | 520 (97.4) | 1252 (97.7) | 1212 (97.2) | 478 (98.0) | 1138 (98.3) | 1044 (97.6) | 517 (97.9) | 1245 (98.1) | 1202 (97.4) |
| Yes | 10 (1.9) | 23 (1.8) | 24 (1.9) | 10 (2.0) | 20 (1.7) | 22 (2.1) | 10 (1.9) | 23 (1.8) | 24 (1.9) |
| NA | 4 (0.7) | 7 (0.5) | 11 (0.9) | 0 | 0 | 4 (0.4) | 1 (0.2) | 1 (0.1) | 8 (0.6) |
| Child SCAT3 cognitive testing, mean (SD) | |||||||||
| Orientation | 2.2 (1.5) | 3.6 (0.7) | 3.7 (0.5) | 2.2 (1.5) | 3.6 (0.7) | 3.7 (0.6) | 2.2 (1.5) | 3.6 (0.7) | 3.7 (0.5) |
| Concentration | 2.2 (1.4) | 3.9 (1.2) | 4.5 (1.2) | 2.2 (1.4) | 3.9 (1.2) | 4.5 (1.2) | 2.2 (1.4) | 3.9 (1.2) | 4.5 (1.2) |
| Immediate memory | 11.0 (3.2) | 13.4 (1.8) | 13.8 (1.5) | 11.0 (3.2) | 13.4 (1.8) | 13.8 (1.5) | 11.0 (3.2) | 13.4 (1.8) | 13.8 (1.5) |
| Delayed memory | 3.3 (1.7) | 4.0 (1.2) | 4.0 (1.2) | 3.3 (1.8) | 4.0 (1.2) | 4.0 (1.2) | 3.3 (1.7) | 4.0 (1.2) | 4.0 (1.2) |
| ED PCSI delta score, mean (SD) | 7.0 (4.4) | 11.4 (6.1) | 36.5 (21.8) | 7.0 (4.4) | 11.2 (6.0) | 36.1 (21.8) | 7.0 (4.4) | 11.4 (6.1) | 36.5 (21.8) |
Abbreviations: ED, emergency department; IQR, interquartile range; NA, not answered; PCSI, Postconcussion Symptom Inventory; Child SCAT3, Child Sports Concussion Assessment Tool version 3.
Denominators for the variables Helmet use in sports and Mouth guard use in sports relate to the total number of participants with a sport concussion who had answered the question.
Association Between Predicted Symptom Change and Time, Stratified by Age Group
All age groups demonstrated a nonlinear association of time; symptoms significantly decreased at each time point, with the greatest association in the first week after injury (Table 2). Although symptom change was significant for all time points, when examining the rate effect size, symptom change primarily occurred in the first week after injury for the group aged 5 to 7 years, plateauing between weeks 1 and 2. By 2 weeks, 75.6% of symptoms had improved (PCSI change between 0 and 2 weeks, −5.3; 95% CI, −5.5 to −5.0). For the groups aged 8 to 12 years and 13 to 18 years, symptom change was prominent the first 2 weeks but flattened between 2 and 4 weeks. By 4 weeks, 83.6% and 86.2% of symptoms, respectively, had resolved for the groups aged 8 to 12 years (PCSI change between 0 and 4 weeks, −9.0; 95% CI, −9.6 to −8.4) and 13 to 18 years (PCSI change between 0 and 4 weeks, −28.6; 95% CI, −30.8 to −26.3).
Table 2. Rates of Symptom Change Across Time Pointsa.
| Time, wk | PCSI Change (95% CI) | PCSI Change, % | Symptom Change, % | PCSI Change (95% CI) per wkb |
|---|---|---|---|---|
| 5-7 y | ||||
| 0-1 | −4.4 (−4.7 to −4.2) | −17.1 | 63.8 | −4.4 (−4.7 to −4.2) |
| 1-2 | −0.8 (−0.9 to −0.8) | −3.2 | 11.8 | −0.8 (−0.9 to −0.8) |
| 2-4 | −0.4 (−0.4 to −0.4) | −1.6 | 6.0 | −0.2 (−0.2 to −0.2) |
| 4-8 | −0.2 (−0.2 to −0.2) | −0.6 | 2.3 | −0.0 (−0.0 to −0.0) |
| 8-12 | −0.0 (−0.0 to −0.0) | −0.1 | 0.6 | −0.0 (−0.0 to −0.0) |
| 0-12 | −5.9 (−6.2 to −5.6) | −22.6 | 84.5 | −0.5 (−0.5 to −0.5) |
| 8-12 y | ||||
| 0-1 | −6.2 (−6.5 to −5.8) | −18.1 | 56.9 | −6.2 (−6.5 to −5.8) |
| 1-2 | −1.8 (−1.9 to −1.6) | −5.2 | 16.2 | −1.8 (−1.9 to −1.6) |
| 2-4 | −1.1 (−1.3 to −1.0) | −3.3 | 10.5 | −0.6 (−0.6 to −0.5) |
| 4-8 | −0.6 (−0.6 to −0.5) | −1.6 | 5.1 | −0.1 (−0.2 to −0.1) |
| 8-12 | −0.2 (−0.2 to −0.1) | −0.4 | 1.4 | −0.0 (−0.0 to −0.0) |
| 0-12 | −9.7 (−10.1 to −9.4) | −28.6 | 90.1 | −0.8 (−0.8 to −0.8) |
| 13-18 y | ||||
| 0-1 | −18.8 (−19.8 to 17.7) | −15.6 | 56.6 | −18.8 (−19.8 to −17.7) |
| 1-2 | −5.9 (−6.5 to −5.3) | −4.9 | 17.7 | −5.9 (−6.5 to −5.3) |
| 2-4 | −3.9 (−4.5 to −3.4) | −3.3 | 11.8 | −2.0 (−2.3 to −1.7) |
| 4-8 | −2.0 (−2.3 to −1.6) | −1.6 | 5.9 | −0.5 (−0.6 to −0.4) |
| 8-12 | −0.6 (−0.7 to −0.4) | −0.5 | 1.7 | −0.1 (−0.2 to −0.1) |
| 0-12 | −31.1 (−32.6 to −29.5) | −25.9 | 93.7 | −2.6 (−2.7 to −2.5) |
Abbreviation: PCSI, Postconcussion Symptom Inventory.
The PCSI change is the predicted estimated change of the PCSI delta score (current symptoms minus the preinjury symptom score) between specific time points based on the predicted PCSI delta score of 1 individual per age group, adjusting to the mean or mode of all other covariates. The predicted PCSI score is representative of the mean predicted PCSI delta score. Predicted baseline PCSI delta scores are 6.96 for the group aged 5 to 7 years, 10.80 for the group aged 8 to 12 years, and 33.14 for the group aged 13 to 18 years. The PCSI change (percentage) is the PCSI change divided by the total score range. The symptom change (percentage) is the PCSI change divided by the predicted PCSI delta score baseline value. Maximum PCSI scores for each age group are 26 for the group aged 5 to 7 years, 34 for the group aged 8 to 12 years, and 120 for the group aged 13 to 18 years.
P < .001 for all.
Association Between Predicted Symptom Change, Continuous Age by Time Interaction, and Sex by Time Interaction Within Age Groups
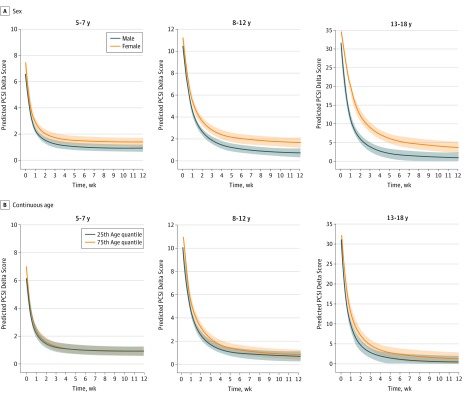
Female sex was associated with higher PCSI delta scores in all age groups at each time point (eTable 4 in the Supplement). Although statistically significant, the estimated PCSI delta score differences between boys and girls were small for the younger age groups (5-7 and 8-12 years). Sex by time interaction was significant only for the adolescent group (β = 0.32; 95% CI, 0.21-0.43; P < .001) (Figure 1A). Comparisons of expected symptom change rates (Table 3) for the group aged 13 to 18 years indicated that girls initially (week 1 after the ED visit) improved at a slower rate than boys (also shown by the slopes for the group aged 13-18 years in Figure 1A). However, between weeks 1 and 2, girls demonstrated faster symptom improvement compared with boys. From weeks 2 to 12, symptom change rates of girls returned to being significantly slower than those of boys (female slope is less slanted in Figure 1A, and estimates are listed in Table 3). Predicted symptom change for boys occurred in the first 2 weeks and flattened between weeks 2 and 4. By 4 weeks, 91.8% of symptoms had improved in boys (PCSI change, −29.13; 95% CI, −32.3 to −25.97), and 79.9% of symptoms had improved in girls (PCSI change, −27.89; 95% CI, −31.32 to −24.44), with female symptom change plateauing between weeks 4 and 8.
Figure 1. Association of Sex and Continuous Age With Symptom Change Score Over Time (Measured in Weeks After Emergency Department Visit).
The symptom change score is the Postconcussion Symptom Inventory (PCSI) delta score over time. The shaded areas represent the 95% CIs. A, The curves are the predicted trajectory of 1 girl and 1 boy, adjusting for the mean or mode of all other covariates, with age fixed to its median for all age groups. B, The curves reflect the predicted trajectory, contrasting a patient at the 75th quantile with a patient at the 25th quantile at the various time points, while all other covariates are constant, with sex fixed to its mode value of male. Covariates are personal migraine history, previous concussions with symptoms lasting longer than 1 week, learning disability, attention-deficit/hyperactivity disorder, anxiety, depression, sleep disorders, loss of consciousness and duration, cognitive scores from the Child Sports Concussion Assessment Tool version 3 (Child SCAT3) (immediate and delayed memory, concentration), Balance Error Scoring System (BESS) tandem stance, and baseline subscale symptom scores (parent reported). For the group aged 5 to 7 years, the depression variable (no participants had prior depression diagnosis) and the BESS tandem stance (not validated in that age group) were not added as predictors.
Table 3. Comparing the Rate of Recovery Between Girls and Boys for the Group Aged 13 to 18 Yearsa.
| Time, wk | Girls 13-18 y | Boys 13-18 y | PCSI Change Difference, Girls Minus Boysb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PCSI Change (95% CI) | PCSI Change, % | Symptom Change, % | PCSI Change (95% CI) per Week | PCSI Change (95% CI) | PCSI Change, % | Symptom Change, % | PCSI Change (95% CI) per Week | ||
| 0-1 | −16.1 (−17.8 to −14.3) | −13.4 | 46.0 | −16.1 (−17.8 to −14.3) | −21.0 (−22.6 to −19.4) | −17.5 | 66.1 | −21.0 (−22.6 to −19.4) | 4.9 (2.9 to 6.9) |
| 1-2 | −6.9 (−7.7 to −6.0) | −5.7 | 19.7 | −6.9 (−7.7 to −6.0) | −5.1 (−5.9 to −4.3) | −4.2 | 16.1 | −5.1 (−5.9 to −4.3) | −1.8 (−2.6 to −0.9) |
| 2-4 | −5.0 (−5.8 to −4.1) | −4.1 | 14.3 | −2.5 (−2.9 to −2.1) | −3.1 (−3.8 to −2.3) | −2.6 | 9.7 | −1.5 (−1.9 to −1.1) | −1.9 (−2.7 to −1.1) |
| 4-8 | −2.6 (−3.1 to −2.1) | −2.2 | 7.5 | −0.7 (−0.8 to −0.5) | −1.4 (−1.9 to −0.9) | −1.2 | 4.4 | −0.4 (−0.5 to −0.2) | −1.2 (−1.7 to −0.7) |
| 8-12 | −0.8 (−0.9 to −0.6) | −0.6 | 2.2 | −0.2 (−0.2 to −0.2) | −0.4 (−0.5 to −0.2) | −0.3 | 1.2 | −0.1 (−0.1 to −0.1) | −0.4 (−0.6 to −0.2) |
| 0-12 | −31.3 (−33.5 to −29.1) | −26.1 | 89.6 | −2.6 (−2.8 to −2.4) | −30.9 (−33.0 to −28.9) | −25.8 | 97.4 | −2.6 (−2.8 to −2.4) | NA |
Abbreviations: NA, not applicable; PCSI, Postconcussion Symptom Inventory.
The PCSI change is the predicted estimated change of the PCSI delta score (current symptoms minus the preinjury symptom score) between specific time points based on the predicted PCSI delta score of 1 individual per age group, adjusting to the mean or mode of all other covariates. The predicted PCSI score is representative of the mean predicted PCSI delta score (34.89 for girls and 31.73 for boys). The PCSI change (%) is the PCSI change divided by the total score range. The symptom change (%) is the PCSI change divided by the predicted PCSI delta score baseline value.
P < .001 for all.
The 75th quantile of age (older children within each age group) was associated with higher symptom scores at different time points over time (Figure 1B and eTable 5 in the Supplement). The interaction between continuous age and time was significant in each age group. Although statistically significant, the estimated PCSI delta score difference between the 75th and 25th quantiles (eTable 5 in the Supplement) and the effect sizes of the difference in PCSI change rates between the 75th and 25th quantiles over time were small (eTable 6 in the Supplement).
Proportion of Recovered Participants and Unadjusted Recovery Centile Curves
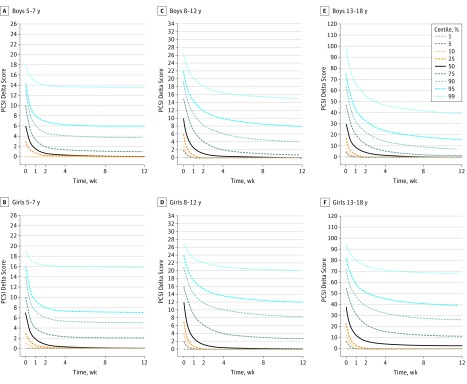
The proportion of recovered participants overall and by sex, stratified by age group, is shown in eFigure 2 in the Supplement. Significant differences were observed between the sexes in the proportion of recovered participants for all age groups, with a greater proportion of boys recovered at 2, 4, and 12 weeks for the group aged 5 to 7 years, at 2, 4, 8, and 12 weeks for the group aged 8 to 12 years, and at all time points for the group aged 13 to 18 years.
Unadjusted recovery centile curves by age group and sex (Figure 2) show the range of scores to be expected in a cohort followed up over time. The 50th percentile for recovered participants was between 2 and 4 weeks for boys (all age groups) and for girls in the 2 younger age groups and was beyond the 12-week time point for adolescent girls.
Figure 2. Unadjusted Recovery Centile Curves at Different Time Points, Stratified by Age Group and Sex.
The curves show the range of scores to be expected in a cohort followed up over time. To enhance clarity, the 95% CIs are omitted from the graphs. PCSI indicates Postconcussion Symptom Inventory.
Discussion
Across all pediatric age groups, pronounced symptom improvement occurred in the first week after concussion. Symptom change primarily occurred within 2 weeks after injury for all groups except adolescent girls. Symptom change plateaued between the first and second weeks for younger children and between the second and fourth weeks for older children and adolescent boys. Adolescent girls had a protracted symptom change trajectory; symptom improvement occurred predominantly in the first 4 weeks and plateaued between weeks 4 and 8, with less than half of them reaching full recovery by 12 weeks after injury.
Consistent with the literature, younger age is not associated with protracted recovery,9,29 whereas adolescence is a risk factor for longer recovery trajectories.5,18,28 Given that parents may have helped younger children complete the PCSI, faster symptom improvement herein might be explained by differential symptom perception by parents. Current guidelines indicate that the expected symptom duration for pediatric concussion (age range, 5-18 years of age), with identification of prolonged symptoms, is 4 weeks.5,6 We observed that expected symptom change trajectories varied across developmental age groups and for older adolescents by sex. Based on the rate of symptom change over time, our results suggested that those at risk of prolonged symptoms can be identified at 2 weeks for younger children, at 4 weeks for older children and male adolescents, and at 4 weeks for female adolescents (despite protracted recovery).
Our findings are consistent with the literature acknowledging female sex as an apparent modifier of prolonged recovery.10,14,16,17,18 In our study, girls across age groups were more symptomatic than boys, and adolescent girls’ symptom change was slower than that of adolescent boys. Sex differences are thought to be multifactorial and might be explained by physiological differences, such as neck strength37,38 and pubertal stage and hormonal differences.39,40,41 Psychosocial differences between the sexes may contribute to differential perception, influencing symptom reporting, given that female athletes have a higher injury rate42 and report more symptoms with greater severity.43
The prospective nature of our study resulted in good ecological validity for participants’ personal characteristics, and the findings are representative of the common differences in mechanism of injury (eg, concussions related to both sport and nonsport are included). The sample size was adequate to investigate symptom change over time, while discriminating between age and sex. The mixed model generated was robust for controlling the association between sex and the covariates imputed in the model. Although recovery in terms of duration and symptom course has been reported in previous studies,5 this is the first large representative study, to our knowledge, investigating symptom change trajectories from different injury mechanisms and across the school-age spectrum (range, 5-18 years) and adjusting for variables associated with a longer recovery period.
Limitations and Future Directions
Our study has limitations. While the PCSI is a valid, reliable, and developmentally adapted scale, preserving the psychometric properties per age group (by not standardizing) limited our ability to directly compare age groups. Although recovery proportions were similar between the groups aged 8 to 12 years and 13 to 18 years, this cannot be confirmed. The inclusion criteria required 85% completeness of the PCSI at each time point; however, this rule does not discriminate between participants who actually dropped out entirely vs those who had missing data at a given time point vs those who participated without completing enough PCSI items. The mixed models are robust to missing-at-random mechanisms, especially when variables predicting missingness have been included as model covariates.44 As such, a list-wise deletion for dealing with greater than 15% incomplete PCSI was deemed acceptable. However, it is unknown to what extent the presence of missing data affected the results. The prevalence of symptoms in our study was similar to what is reported in the literature.5 Finally, clinically significant differences for the PCSI and other concussion scales are poorly defined, limiting our ability to define clinically significant effect sizes in this study. Future research should define the clinical significance of the PCSI.
Our population was sampled from a population with acute concussion who were initially seen in pediatric EDs; therefore, our results may not be generalizable to those having delayed symptoms (>48 hours), those seeking care outside of an ED (eg, family medicine clinic or sports clinic), those receiving care on the sideline by a certified professional (eg, athletic trainer), or those not obtaining any care. This may have biased our sample to children with higher initial symptom burden or more severe mechanisms of injury. However, the participants were sampled from a heterogeneous population across Canada. We could not control for socioeconomic status, which has demonstrated associations with delayed postinjury recovery.45 Similarly, we could not prove that symptoms were specific to concussion vs other disorders with similar symptoms (eg, vestibular issues), possibly contributing to delayed symptom improvement. Motivation and/or pressure to return to sport, pubertal status, and hormonal levels may influence the natural recovery trajectory. These variables were not adjusted for in our model but might have contributed to the adolescent sex differences observed. Future research should investigate the association between additional variables and sex-specific symptom change trajectory. Finally, the lack of a control group did not allow us to definitively attribute ongoing symptoms to the injury. Concussion symptoms can be nonspecific; a study46 on long-term neuropsychological recovery demonstrated no differences between concussion and orthopedic injuries over time. However, other evidence suggests that manifestation of symptoms is more frequent and severe after a concussion47 compared with orthopedic injuries.48,49,50
Recovery is a multifaceted construct that includes symptom, neurophysiological, and neuropsychological recovery. Each individual may have different recovery trajectories over time, and neurophysiological abnormalities may persist beyond the clinical symptom recovery.51 Given this difference, understanding the association between symptom change and time was deemed more informative than between recovery and time. This study only included self-rated clinical symptom recovery; therefore, it is not representative of neurophysiological and neuropsychological recovery. Although self-reported clinical symptom assessments are the most frequently used outcome measure for concussion,7 they are subjective and can be a source of bias. There is no ideal score to measure symptom change and recovery over time, but the PCSI is a valid and reliable scale and is a tool of the National Institute of Neurological Disorders and Stroke and Department of Defense Sport-Related Concussion Common Data Elements.52 Future research should include validated objectives (eg, neuroimaging and neuropsychological assessments) to investigate symptom change and recovery.
The clinical implications of identifying symptom change trajectories by age and sex are important for effective management decisions and preventive measures. Identifying at-risk children at the right time and referring them for expedited treatment may prevent persistent symptoms and promote faster recovery. Between-sex differences in adolescents should be taken into consideration for recovery definitions and management. Adolescent girls may potentially benefit from individualized management protocols, with the goal of promoting faster recovery. The centile curves herein provide an overview for primary care providers to compare how other children of the same age group and sex have recovered, providing a better perspective to families and children on recovery status.
Conclusions
Results of this study demonstrated that symptom improvement primarily occurred in the first 2 weeks after injury in children and in the first 4 weeks in preadolescents and male adolescents. Female adolescent have protracted recovery. The derived recovery curves could be used for evidence-based anticipatory guidance.
eFigure 1. Flow Diagram of Exclusion Criteria at Recruitment
eFigure 2. Overall and by Sex Proportion of Recovered Participants Stratified by Age Groups
eTable 1. Proportion of Missing Data, Based on >15% Missing Items on the PCSI or Did Not Complete the PCSI
eTable 2. Comparing Those With Complete Time Points to Those With Incomplete Time Points—Mixed-Effects Models
eTable 3. Comparing Those With Complete Time Points to Those With Incomplete Time Points—Centile Curves
eTable 4. Comparing Female and Male PCSI Delta Score at Each Time Point
eTable 5. Comparing 75th and 25th PCSI Delta Score at Each Time Point
eTable 6. Comparing the Rate of Recovery Between the 75th and 25th Quantile Continuous Age
References
- 1.Novak Z, Aglipay M, Barrowman N, et al. ; Pediatric Emergency Research Canada Predicting Persistent Postconcussive Problems in Pediatrics (PERC 5P) Concussion Team . Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatr. 2016;170(12):. doi: 10.1001/jamapediatrics.2016.2900 [DOI] [PubMed] [Google Scholar]
- 2.Zemek RL, Grool AM, Rodriguez Duque D, et al. Annual and seasonal trends in ambulatory visits for pediatric concussion in Ontario between 2003 and 2013. J Pediatr. 2017;181:222-. doi: 10.1016/j.jpeds.2016.10.067 [DOI] [PubMed] [Google Scholar]
- 3.Taylor AM, Nigrovic LE, Saillant ML, et al. Trends in ambulatory care for children with concussion and minor head injury from eastern Massachusetts between 2007 and 2013. J Pediatr. 2015;167(3):738-744. doi: 10.1016/j.jpeds.2015.05.036 [DOI] [PubMed] [Google Scholar]
- 4.Macpherson A, Fridman L, Scolnik M, Corallo A, Guttmann A. A population-based study of paediatric emergency department and office visits for concussions from 2003 to 2010. Paediatr Child Health. 2014;19(10):543-546. doi: 10.1093/pch/19.10.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GA, Anderson V, Babl FE, et al. What is the difference in concussion management in children as compared with adults? a systematic review. Br J Sports Med. 2017;51(12):949-957. doi: 10.1136/bjsports-2016-097415 [DOI] [PubMed] [Google Scholar]
- 6.McCrory P, Meeuwisse W, Dvořák J, et al. Consensus Statement on Concussion in Sport: the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 7.Haider MN, Leddy JL, Pavlesen S, et al. A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med. 2017;0:1-14. doi: 10.1136/bjsports-2016-096551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DJ, Coxe K, Li H, et al. Length of recovery from sports-related concussions in pediatric patients treated at concussion clinics. Clin J Sport Med. 2018;28(1):56-63. doi: 10.1097/JSM.0000000000000413 [DOI] [PubMed] [Google Scholar]
- 9.Corwin DJ, Zonfrillo MR, Master CL, et al. Characteristics of prolonged concussion recovery in a pediatric subspecialty referral population. J Pediatr. 2014;165(6):1207-1215. doi: 10.1016/j.jpeds.2014.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312. doi: 10.1177/0363546512444554 [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg MA, Meehan WP III, Mannix R. Duration and course of post-concussive symptoms. Pediatrics. 2014;133(6):999-1006. doi: 10.1542/peds.2014-0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makdissi M, Darby D, Maruff P, Ugoni A, Brukner P, McCrory PR. Natural history of concussion in sport: markers of severity and implications for management. Am J Sports Med. 2010;38(3):464-471. doi: 10.1177/0363546509349491 [DOI] [PubMed] [Google Scholar]
- 13.Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374-e381. doi: 10.1542/peds.2009-0925 [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013;132(1):8-17. doi: 10.1542/peds.2013-0432 [DOI] [PubMed] [Google Scholar]
- 15.Zemek R, Barrowman N, Freedman SB, et al. ; Pediatric Emergency Research Canada (PERC) Concussion Team . Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED [published correction appears in JAMA. 2016;315(23):2624]. JAMA. 2016;315(10):1014-1025. doi: 10.1001/jama.2016.1203 [DOI] [PubMed] [Google Scholar]
- 16.Kostyun RO, Hafeez I. Protracted recovery from a concussion: a focus on gender and treatment interventions in an adolescent population. Sports Health. 2015;7(1):52-57. doi: 10.1177/1941738114555075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colvin AC, Mullen J, Lovell MR, West RV, Collins MW, Groh M. The role of concussion history and gender in recovery from soccer-related concussion. Am J Sports Med. 2009;37(9):1699-1704. doi: 10.1177/0363546509332497 [DOI] [PubMed] [Google Scholar]
- 18.Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941-948. doi: 10.1136/bjsports-2017-097729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCrea M, Guskiewicz K, Randolph C, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc. 2013;19(1):22-33. doi: 10.1017/S1355617712000872 [DOI] [PubMed] [Google Scholar]
- 20.Meehan WP III, Mannix RC, Stracciolini A, Elbin RJ, Collins MW. Symptom severity predicts prolonged recovery after sport-related concussion, but age and amnesia do not. J Pediatr. 2013;163(3):721-725. doi: 10.1016/j.jpeds.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meehan WP III, Mannix R, Monuteaux MC, Stein CJ, Bachur RG. Early symptom burden predicts recovery after sport-related concussion. Neurology. 2014;83(24):2204-2210. doi: 10.1212/WNL.0000000000001073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zemek R, Osmond MH, Barrowman N; Pediatric Emergency Research Canada (PERC) Concussion Team . Predicting and Preventing Postconcussive Problems in Paediatrics (5P) study: protocol for a prospective multicentre clinical prediction rule derivation study in children with concussion. BMJ Open. 2013;3(8):e003550. doi: 10.1136/bmjopen-2013-003550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus Statement on Concussion in Sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258. doi: 10.1136/bjsports-2013-092313 [DOI] [PubMed] [Google Scholar]
- 24.Gioia GA, Collins M, Isquith PK. Improving identification and diagnosis of mild traumatic brain injury with evidence: psychometric support for the Acute Concussion Evaluation. J Head Trauma Rehabil. 2008;23(4):230-242. doi: 10.1097/01.HTR.0000327255.38881.ca [DOI] [PubMed] [Google Scholar]
- 25.Guskiewicz KM. Assessment of postural stability following sport-related concussion. Curr Sports Med Rep. 2003;2(1):24-30. doi: 10.1249/00149619-200302000-00006 [DOI] [PubMed] [Google Scholar]
- 26.Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the Postconcussion Symptom Inventory in children and adolescents. Arch Clin Neuropsychol. 2014;29(4):348-363. doi: 10.1093/arclin/acu014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zemek RL, Farion KJ, Sampson M, McGahern C. Prognosticators of persistent symptoms following pediatric concussion: a systematic review. JAMA Pediatr. 2013;167(3):259-265. doi: 10.1001/2013.jamapediatrics.216 [DOI] [PubMed] [Google Scholar]
- 29.Miller JH, Gill C, Kuhn EN, et al. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J Neurosurg Pediatr. 2016;17(4):491-496. doi: 10.3171/2015.8.PEDS14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murdaugh DL, Ono KE, Reisner A, Burns TG. Assessment of sleep quantity and sleep disturbances during recovery from sports-related concussion in youth athletes. Arch Phys Med Rehabil. 2018;99(5):960-966. doi: 10.1016/j.apmr.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 31.Box GEP, Jenkins GM, Reinsel GC. Time Series Analysis: Forecasting and Control 3rd ed. Englewood Cliffs, NJ: Prentice Hall; 1994. [Google Scholar]
- 32.Jones RH. Longitudinal Data with Serial Correlation: A State-Space Approach. Boca Raton, FL: Chapman & Hall; 1993. doi: 10.1007/978-1-4899-4489-4 [DOI] [Google Scholar]
- 33.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Stat. 1994;43(3):429. doi: 10.2307/2986270 [DOI] [Google Scholar]
- 34.Sauerbrei W, Royston P. Building multivariable prognostic and diagnostic models: transformation of the predictors by using fractional polynomials. J R Stat Soc [Ser A]. 1999;162(1):71-94. doi: 10.1111/1467-985X.00122 [DOI] [Google Scholar]
- 35.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209-212. doi: 10.1080/01621459.1927.10502953 [DOI] [Google Scholar]
- 36.Faes C, Molenberghs G, Aerts M, Verbeke G, Kenward MG. The effective sample size and an alternative small-sample degrees-of-freedom method. Am Stat. 2009;63(4):389-399. doi: 10.1198/tast.2009.08196 [DOI] [Google Scholar]
- 37.Eckner JT, Oh YK, Joshi MS, Richardson JK, Ashton-Miller JA. Effect of neck muscle strength and anticipatory cervical muscle activation on the kinematic response of the head to impulsive loads. Am J Sports Med. 2014;42(3):566-576. doi: 10.1177/0363546513517869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tierney RT, Sitler MR, Swanik CB, Swanik KA, Higgins M, Torg J. Gender differences in head-neck segment dynamic stabilization during head acceleration. Med Sci Sports Exerc. 2005;37(2):272-279. doi: 10.1249/01.MSS.0000152734.47516.AA [DOI] [PubMed] [Google Scholar]
- 39.Decloe MD, Meeuwisse WH, Hagel BE, Emery CA. Injury rates, types, mechanisms and risk factors in female youth ice hockey. Br J Sports Med. 2014;48(1):51-56. doi: 10.1136/bjsports-2012-091653 [DOI] [PubMed] [Google Scholar]
- 40.Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma. 2010;27(3):527-539. doi: 10.1089/neu.2009.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emerson CS, Headrick JP, Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res. 1993;608(1):95-100. doi: 10.1016/0006-8993(93)90778-L [DOI] [PubMed] [Google Scholar]
- 42.Dick RW. Is there a gender difference in concussion incidence and outcomes? Br J Sports Med. 2009;43(suppl 1):i46-i50. doi: 10.1136/bjsm.2009.058172 [DOI] [PubMed] [Google Scholar]
- 43.Brown DA, Elsass JA, Miller AJ, Reed LE, Reneker JC. Differences in symptom reporting between males and females at baseline and after a sports-related concussion: a systematic review and meta-analysis. Sports Med. 2015;45(7):1027-1040. doi: 10.1007/s40279-015-0335-6 [DOI] [PubMed] [Google Scholar]
- 44.Verbeke G, Molenberghs G. How ignorable is missing at random? In:Linear Mixed Models for Longitudinal Data. New York, NY: Springer; 2009:375-386. doi: 10.1007/978-1-4419-0300-6_21 [DOI] [Google Scholar]
- 45.Bernard CO, Ponsford JA, McKinlay A, McKenzie D, Krieser D. Predictors of post-concussive symptoms in young children: injury versus non-injury related factors. J Int Neuropsychol Soc. 2016;22(8):793-803. doi: 10.1017/S1355617716000709 [DOI] [PubMed] [Google Scholar]
- 46.Babikian T, Satz P, Zaucha K, Light R, Lewis RS, Asarnow RF. The UCLA Longitudinal Study of Neurocognitive Outcomes Following Mild Pediatric Traumatic Brain Injury. J Int Neuropsychol Soc. 2011;17(5):886-895. doi: 10.1017/S1355617711000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor HG, Dietrich A, Nuss K, et al. Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology. 2010;24(2):148-159. doi: 10.1037/a0018112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittenberg W, Wittner MS, Miller LJ. Postconcussion syndrome occurs in children. Neuropsychology. 1997;11(3):447-452. doi: 10.1037/0894-4105.11.3.447 [DOI] [PubMed] [Google Scholar]
- 49.Yeates KO, Taylor HG, Rusin J, et al. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics. 2009;123(3):735-743. doi: 10.1542/peds.2008-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeates K, Taylor H, Barry C, Drotar D, Wade S, Stancin T. Neurobehavioral symptoms in childhood closed-head injuries: changes in prevalence and correlates during the first year postinjury. J Pediatr Psychol. 2001;26(2):79-91. doi: 10.1093/jpepsy/26.2.79 [DOI] [PubMed] [Google Scholar]
- 51.Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? a systematic review. Br J Sports Med. 2017;51(12):935-940. doi: 10.1136/bjsports-2016-097464 [DOI] [PubMed] [Google Scholar]
- 52.Broglio SP, Kontos AP, Levin H, et al. National Institute of Neurological Disorders and Stroke and Department of Defense Sport-Related Concussion Common Data Elements Version 1.0 Recommendations. [published online July 23, 2018]. J Neurotrauma. 2018. doi: 10.1089/neu.2018.5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram of Exclusion Criteria at Recruitment
eFigure 2. Overall and by Sex Proportion of Recovered Participants Stratified by Age Groups
eTable 1. Proportion of Missing Data, Based on >15% Missing Items on the PCSI or Did Not Complete the PCSI
eTable 2. Comparing Those With Complete Time Points to Those With Incomplete Time Points—Mixed-Effects Models
eTable 3. Comparing Those With Complete Time Points to Those With Incomplete Time Points—Centile Curves
eTable 4. Comparing Female and Male PCSI Delta Score at Each Time Point
eTable 5. Comparing 75th and 25th PCSI Delta Score at Each Time Point
eTable 6. Comparing the Rate of Recovery Between the 75th and 25th Quantile Continuous Age