This cohort study compares the use of fetal magnetic resonance imaging and ultrasonography in the identification of brain abnormalities associated with Zika virus.
Key Points
Question
What does magnetic resonance imaging add to ultrasonography for detecting Zika–virus related brain lesions?
Findings
In this cohort study of 82 pregnant women with Zika virus infection, 3 cases of severe fetal brain abnormalities were detected by magnetic resonance imaging; in 2 of these, ultrasonography results were abnormal. In neonates, cranial ultrasonography and brain magnetic resonance imaging revealed new relatively mild findings in 23 of 61 infants; most abnormalities were seen on ultrasonography.
Meaning
Although the combination of prenatal and postnatal ultrasonography and magnetic resonance imaging is needed to capture the full spectrum of Zika–virus related brain injury, ultrasonography detected most abnormal cases.
Abstract
Importance
The evolution of fetal brain injury by Zika virus (ZIKV) infection is not well described.
Objectives
To perform longitudinal neuroimaging of fetuses and infants exposed to in utero maternal ZIKV infection using concomitant magnetic resonance imaging (MRI) and ultrasonography (US), as well as to determine the duration of viremia in pregnant women with ZIKV infection and whether the duration of viremia correlated with fetal and/or infant brain abnormalities.
Design, Setting, and Participants
A cohort of 82 pregnant women with clinical criteria for probable ZIKV infection in Barranquilla, Colombia, and Washington, DC, were enrolled from June 15, 2016, through June 27, 2017, with Colombian women identified by community recruitment and physician referral and travel-related cases of American women recruited from a Congenital Zika Program.
Interventions and Exposures
Women received 1 or more MRI and US examinations during the second and/or third trimesters. Postnatally, infants underwent brain MRI and cranial US. Blood samples were tested for ZIKV.
Main Outcomes and Measures
The neuroimaging studies were evaluated for brain injury and cerebral biometry.
Results
Of the 82 women, 80 were from Colombia and 2 were from the United States. In 3 of 82 cases (4%), fetal MRI demonstrated abnormalities consistent with congenital ZIKV infection. Two cases had heterotopias and malformations in cortical development and 1 case had a parietal encephalocele, Chiari II malformation, and microcephaly. In 1 case, US results remained normal despite fetal abnormalities detected on MRI. Prolonged maternal polymerase chain reaction positivity was present in 1 case. Of the remaining 79 cases with normal results of prenatal imaging, postnatal brain MRI was acquired in 53 infants and demonstrated mild abnormalities in 7 (13%). Fifty-seven infants underwent postnatal cranial US, which detected changes of lenticulostriate vasculopathy, choroid plexus cysts, germinolytic/subependymal cysts, and/or calcification in 21 infants (37%).
Conclusions and Relevance
In a cohort of pregnant women with ZIKV infection, prenatal US examination appeared to detect all but 1 abnormal fetal case. Postnatal neuroimaging in infants who had normal prenatal imaging revealed new mild abnormalities. For most patients, prenatal and postnatal US may identify ZIKV-related brain injury.
Introduction
Almost 70 years after its initial discovery, Zika virus (ZIKV) captured international attention when its devastating effects to the developing human brain became recognized during a pandemic outbreak of congenital microcephaly. Since late 2015, large regions of South and Central America and the Caribbean were affected by1,2 the neurologic phenotype of the congenital ZIKV syndrome and the associated brain imaging findings of neuronal migration abnormalities, callosal and cerebellar malformation, and ventriculomegaly.3,4,5,6,7 The international medical community had to quickly develop an understanding of the infection and provide recommendations for evaluation of exposed and infected pregnant women and their infants.8
Fetal brain injury or malformation owing to congenital infection is usually first suspected during routine antenatal ultrasonography (US). In early 2016, a pregnant woman at 18 weeks’ gestation with laboratory-confirmed ZIKV infection was reported.9 Fetal US studies between 13 and 17 weeks’ gestation demonstrated no abnormalities; by 20 weeks’ gestation, fetal US showed fall-off in fetal head circumference (HC) percentiles.9 Using fetal magnetic resonance imaging (MRI), significant fetal brain malformations were identified. Neuropathologic findings at fetopsy showed striking apoptosis in intermediately differentiated neuronal precursor cells, corresponding with the fetal MRI lesions.10
The primary objective of this study was to perform a longitudinal evaluation of the fetal and neonatal brain of fetuses and infants exposed in utero to maternal ZIKV infection in any trimester, using concomitant MRI and US during the second and third trimesters and in the newborn. We hypothesized that fetal MRI would detect ZIKV-induced brain abnormalities earlier than US and that a broad spectrum of brain abnormalities would be detected among fetuses and infants exposed to maternal ZIKV infection. Secondary objectives were to determine the duration of viremia in pregnant, ZIKV-infected women and whether the duration of viremia correlated with the incidence of fetal and infant brain abnormalities.
Methods
Participants
Between June 15, 2016, and June 27, 2017, we prospectively enrolled 82 pregnant women with ZIKV infection at 2 sites:, Washington, DC (n = 2, travel-related infection), and Barranquilla, Colombia (n = 80). Institutional review board approval was obtained at each site by Children's National Medical Center Institutional Review Board and Comite Institucional De Revision Y Comite Independentiente De Etica en Investigacion. The women provided written informed consent; there was no financial compensation. This study followed the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guideline.
Colombian pregnant women with symptoms meeting Centers for Disease Control and Prevention (CDC) clinical criteria for probable ZIKV infection, with or without confirmatory laboratory testing, were considered eligible. Most women had laboratory confirmation with 1 or more of ZIKV polymerase chain reaction (PCR), IgM, IgG, and plaque-reduction neutralization assay. Women were excluded if they were asymptomatic, with the exception of 1 participant in Colombia with a severely affected (symptomatic) fetus and positive testing. Women in Colombia were identified by direct community recruitment and physician referral and enrolled through an established clinical research facility (BIOMELAB). Travel-related cases of pregnant Washington, DC, residents were referred to the Congenital Zika Program at Children’s National Health System by their obstetricians after ZIKV exposure and met CDC clinical criteria for probable ZIKV infection. Women were excluded if unable to undergo MRI.
Clinical and Laboratory Studies
Clinical data, including timing of symptoms of ZIKV infection, were documented for each participant. Blood samples were collected at the time of the fetal MRIs. Zika virus blood testing for United States–based patients was initially performed at regional health departments (Maryland, Virginia, or Washington, DC) and at the CDC, Atlanta, Georgia. Validated ZIKV testing by PCR, ZIKV envelope, and NS-1 targeted IgM, IgG, and plaque-reduction neutralization assay performed by the CDC’s Arboviral Branch, Fort Collins, Colorado, was possible only for the first 52 participants (2 United States, 50 Colombia). While not a condition of inclusion, all Colombian women (including the 30 without CDC testing) also had a nonvalidated rapid point of care ZIKV IgM and IgG test performed (Health Diagnostics Inc), with positive IgM and/or IgG results in most cases.
Fetal MRI and US Imaging
Women received 1 or more fetal MRI and US examinations during the second and/or third trimesters. For the study, the a priori preferred timing for imaging was 4 and 10 weeks after the onset of ZIKV infection symptoms. Fetal imaging in Colombia was overseen by radiologists (Y.F. and A.M.), after on-site protocol standardization by a radiologist from Children’s National Health System (D.I.B.). The fetal MRI was performed on 1.5-T scanners (General Electric). The US and MRI studies were interpreted by an experienced fetal radiologist (D.I.B.) and neuroradiologist (G.V.).
Magnetic resonance imaging examinations of the fetal brain included single-shot, fast-spin, echo T2-weighted images acquired in the axial, sagittal, and coronal planes; diffusion and T1-weighted images were not routinely acquired. The images were evaluated for structural abnormalities, including malformations, ventriculomegaly, and evidence of acquired brain injury. The fronto-occipital and biparietal cerebral diameters, cerebellar vermis and diameter, and corpus callosum length were measured. Biometric measurements and estimation of fetal cortical maturation were compared with age-expected norms.11 Fetal US was used to evaluate measurements of cerebral HC, biparietal diameter, body biometry, and interval growth between research scans. Fetal HC z scores were calculated from US measurements based on gestational age using the Hadlock method.12
Postnatal MRI and US Imaging
After birth, infants underwent unsedated brain MRI and cranial US scans. The postnatal MRI protocol included T1- and T2-weighted images of the brain, along with axial diffusion and susceptibility-weighted images. During the first postnatal research visit, HC was measured and the z score was calculated using the Fenton growth chart13 and percentile assigned was based on World Health Organization charts.14 Magnetic resonance imaging was not performed when infants were unable to fall asleep naturally or parents declined.
Statistical Analysis
A paired t test was used to determine a difference in HC z score from the first to second fetal US. Findings were considered significant at P < .05. Statistical analysis was conducted with Stata/SE, version 14.2 (StataCorp LLC).
Results
Demographics and Clinical Characteristics
The demographics, clinical characteristics, and timing of imaging of 82 maternal-fetal dyad participants are reported in Table 1. All women, except 1 asymptomatic case, had clinical evidence of ZIKV infection in pregnancy; the first 52 cases all had CDC laboratory test results positive for ZIKV infection (Table 1).
Table 1. Demographic and Clinical Characteristics of Maternal-Fetal Participants.
| Characteristic | Value |
|---|---|
| Site of exposure, No. (%) (n = 82) | |
| Central Americaa | 2 (2) |
| Colombia | 80 (98) |
| Maternal age, y | 24.3 (5.3) |
| Symptomatic cases, No. (%) | 81 (99) |
| Reported symptoms, No. (%) (n = 81) | |
| Fever | 77 (95) |
| Rash | 74 (91) |
| Joint pain | 77 (95) |
| Joint swelling | 76 (94) |
| Myalgia | 68 (84) |
| Conjunctivitis or eye pain | 66 (81) |
| Headache | 73 (90) |
| Duration of symptoms, mean (SD), d | 10.5 (8.7) |
| Gestational age at symptom onset, mean (SD), wk | 8.2 (4.4) |
| Timing of clinical symptoms, No. (%) (n = 81) | |
| Preconception | 0 |
| 1st Trimester | 66 (80) |
| 2nd Trimester | 15 (18) |
| 3rd Trimester | 0 |
| Gestational age of fetal MRI and US, mean (SD), wk | |
| Fetal MRI 1 (n = 82) | 25.1 (6.0) |
| Fetal US 1 (n = 82) | 25.1 (6.0) |
| Fetal MRI 2 (n = 51) | 31.0 (4.1) |
| Fetal US 2 (n = 55) | 30.5 (3.8) |
| CDC blood test, mother (n = 52) | |
| Interval from symptoms to first test, mean (SD), wk | 17.4 (7.9) |
| Interval from symptoms to second test, mean (SD), wk | 22.6 (5.2) |
| Positive PCR, No. (%) | 1 (2) |
| Positive IgM, No. (%) | 43 (83) |
| Positive IgG, No. (%) | 52 (100) |
| ZIKV PRNT>10, No. (%) (n = 36) | 34 (94) |
| Pregnancy outcome, No. (%) (n = 82) | |
| Live birth | 80 (98) |
| Elective termination | 1 (1) |
| Fetal death | 1 (1) |
| Gestational age at birth, mean (SD), wk | 38.4 (1.8) |
| Mode of delivery, No. (%) (n = 80) | |
| Vaginal | 35 (44) |
| Cesarean section | 35 (44) |
| Unknown | 10 (13) |
| Infant sex, No. (%) (n = 82) | |
| Male | 33 (40) |
| Female | 40 (49) |
| Unknown | 9 (11) |
| Birthweight, mean (SD), g | 3176 (364) |
| Birth head circumference, mean (SD), cm | 34.3 (1.7) |
| Postnatal neuroimaging, No. (%) (n = 61) | |
| Brain MRI and cranial US | 49 (80) |
| Brain MRI only | 4 (7) |
| Cranial US only | 8 (13) |
| Postnatal neuroimaging age, mean (SD), d | |
| Cranial US (n = 57) | 23.9 (21.4) |
| Brain MRI (n = 53) | 23.9 (21.5) |
Abbreviations: CDC, Centers for Disease Control and Prevention; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PRNT, plaque-reduction neutralization assay; US, ultrasonography; ZIKV, Zika virus.
One case has been previously reported.6
Seventy-five of 80 live-born infants (94%) were born at term (≥37 weeks’ gestation). One pregnancy was terminated at 23 weeks owing to severe fetal brain abnormalities. One case, with normal fetal imaging, resulted in near-term fetal death of an unknown cause. One live-born infant, with significant brain abnormalities shown on fetal imaging, died at age 3 days. One infant required neonatal neurosurgical intervention for an encephalocele.
Fetal MRI vs US Findings
Three of 82 fetuses (4%) had MRI abnormalities potentially consistent with congenital ZIKV infection (Table 2, Figure 1, Figure 2, and Figure 3), 1 of whom had normal fetal US (Table 2). In the 79 other fetuses, brain biometry and sulcal maturation were normal as shown by fetal US and MRI. Excluding the 3 cases with severe fetal brain findings and those with unsure pregnancy dating, the mean (SD) z score of the HC at the first US was 0.27 (1.13) compared with −0.10 (1.03) at the second US among the 44 fetuses who had 2 fetal measures (P = .02).
Table 2. Abnormal Fetal Brain MRI and US Cases Among ZIKV-Exposed Cohort.
| Patient No. | Image 1 | Image 2 | ||||
|---|---|---|---|---|---|---|
| GA, wk | Fetal MRI | Fetal US | GA, wk | Fetal MRI | Fetal US | |
| 1 | 18 6/7 | Abnormal ventricular zone, focal cerebral migration abnormality | Normal | 22 3/7 | Subependymal heterotopias; unexpected cortical indentation in the left parietal region | Normal, slight decrease in HC percentile |
| 2 | 32 1/7 | Abnormal midbrain tectum, cerebellar dysplasia, lateral ventriculomegaly, thin white matter and corpus callosum, heterotopias, cortical dysplasia | Mild ventriculomegaly, small cerebellum, HC 38th percentile | 36 1/7 | Multiple heterotopias, decreased white matter volume, beaked tectum, atrophic cerebral white matter, suspect polymicrogyria | Severe ventriculomegaly, small cerebellum, HC 3.6th percentile |
| 3 | 28 5/7 | Chiari II malformation and parietal encephalocele | Microcephaly (HC <3rd percentile), Chiari II, encephalocele | 37 1/7 | Microcephaly, Chiari II malformation and parietal encephalocele | Microcephaly (HC <3rd percentile), Chiari II, encephalocele |
Abbreviations: GA, gestational age; HC, head circumference; MRI, magnetic resonance imaging; US, ultrasonography; ZIKV, Zika virus.
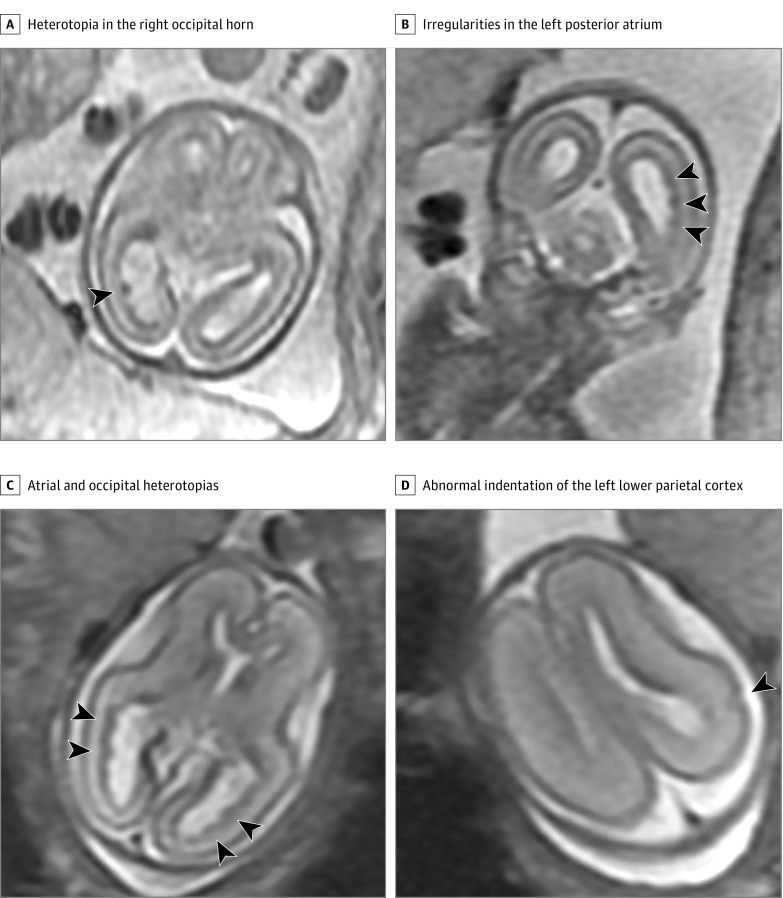
Figure 1. Fetal Magnetic Resonance Imaging Performed at 18 and 22 Weeks’ Gestational Age.
A, Axial image at 18 weeks’ gestational age demonstrates a prominent heterotopia in the right occipital horn (arrowhead). B, Coronal image at 18 weeks’ gestational age shows multiple small irregularities in the left posterior atrium consistent with heterotopias (arrowheads). C, Axial image at 22 weeks’ gestational age shows bilateral atrial and occipital heterotopias (arrowheads). D, Axial image at 22 weeks’ gestational age at a higher level shows an abnormal indentation of the left lower parietal cortex (arrowhead) consistent with a developing cortical dysplasia.
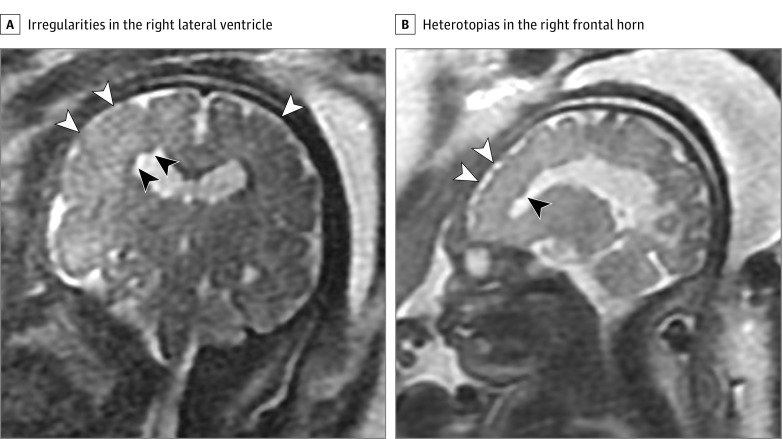
Figure 2. Fetal Magnetic Resonance Imaging Performed at 32 Weeks’ Gestational Age.
A, Coronal image at 32 weeks’ gestational age reveals small irregularities in the body of the right lateral ventricle (black arrowheads) consistent with heterotopias. There is a relative lack of sulcation of the posterior frontal lobes (white arrowheads) consistent with cortical dysplasia or polymicrogyria. B, Off midline sagittal image shows additional heterotopias in the right frontal horn (black arrowhead) and abnormal sulcation of the anterior right frontal cortex (white arrowheads) consistent with additional areas of cortical dysplasia or polymicrogyria.
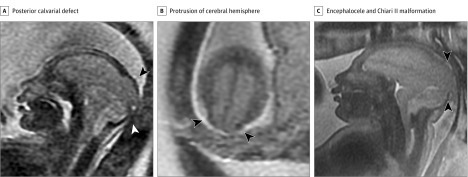
Figure 3. Fetal Magnetic Resonance Imaging Performed at 28 and 37 Weeks’ Gestational Age.
A, Sagittal image at 28 weeks’ gestational age shows a broad posterior calvarial defect through which the posterior cerebral hemisphere herniates (black arrowhead), consistent with an encephalocele. Extracerebral and posterior fossa subarachnoid fluid spaces are absent; the fourth ventricle is not visualized, typical of stigmata of a Chiari II malformation (white arrowhead). Severe microcephaly is evident. B, Axial image at 28 weeks’ gestational age near the vertex shows the posterior protrusion of cerebral hemisphere through the calvarial defect (arrowheads) and lack of cerebrospinal fluid around the cerebral hemispheres. C, Sagittal midline image at 37 weeks’ gestational age demonstrates similar findings of a posterior encephalocele (arrowheads) and stigmata of a Chiari II malformation as evident in panel A.
Additional Changes on Postnatal Imaging
Birth HC mean (SD) z score was 0.07 (0.96) and of normal percentile for gestational age (Fenton chart, mean 50th percentile [range, 5th to 100th percentile]), except in the infant with encephalocele (HC, 31 cm [0.3 percentile]). Birth HC was not reported for case 2. Because the 3 cases of severe fetal MRI abnormalities had no postnatal MRI or US, all infants with postnatal imaging had normal results of fetal studies. Postnatal brain MRIs (n = 53) were acquired at a mean (SD) age of 23.9 (21.5) days and cranial US (n = 57) at 23.9 (21.4) days (Table 1).
Excluding choroid plexus cysts, 23 of 61 infants (38%) with postnatal imaging had findings on brain MRI and/or cranial US. Magnetic resonance imaging and US identified different findings. Seven of 53 infants (13%) had abnormal MRIs (eTable in the Supplement). After normal findings on serial fetal imaging, 1 infant had a chronic parietal infarct shown on brain MRI at 16 days; the infarction was considered a potential complication of ZIKV.6 Seventeen infants had postnatal MRIs of limited quality owing to excessive patient motion or incomplete studies. Cranial US in 21 of 57 infants (37%) had 1 or more findings (eTable in the Supplement); all were poorly characterized by MRI. Eight cases (14%) of choroid plexus cysts have unclear prognostic significance.
Limited Correlation of Viremia With Fetal Brain Injury
Of the 3 cases with severe fetal brain findings, 2 (cases 1 and 2, Table 2) had negative maternal ZIKV PCR results at 9 and 12 weeks after symptom onset, and the mother of fetus 3 was asymptomatic. In these 3 fetuses, however, maternal ZIKV IgM was positive in 2 and equivocal in 1; all had positive ZIKV IgG and plaque-reduction neutralization assay (>10). Only the fetus with chronic parietal infarction identified by postnatal brain MRI6 had maternal ZIKV genome detectable by PCR longer than 3 weeks from symptom onset. In this case, maternal ZIKV PCR became negative by 10 weeks after the onset of symptoms. The ZIKV PCR result was negative in all other maternal samples; however, many samples were acquired more than 3 weeks from symptom onset. All newborns had negative ZIKV PCR and IgM serologic test results.
Discussion
To our knowledge, this is the first longitudinal study using serial fetal and neonatal brain imaging (concomitant MRI and US) in infants with intrauterine ZIKV exposure. Among our predominantly Colombian cohort, we found that 96% of fetuses had normal MRI and US results during pregnancies complicated by ZIKV infection. Fetal brain abnormalities identified by MRI in 4% of the infants were significant and not well recognized on US. Normal fetal brain MRI findings remained normal on subsequent fetal studies. Despite normal fetal MRI study findings, we found a small but significant decrease in fetal HC by serial US measurements, the clinical significance of which is as yet unclear. In contrast to the sensitivity of fetal US, neonatal cranial US detected changes in 37% of the infants, which were not well recognized on postnatal MRI. Postnatal brain MRI found mild changes that were not detected during the fetal period. Overall, MRI and US were complementary and in combination provided a more complete evaluation of the fetal and neonatal brain after in utero ZIKV exposure.
Fetal MRI study findings were either strikingly abnormal or normal and did not show the spectrum of anomalies that we had anticipated. The fetal brain anomalies included cerebellar hypoplasia, abnormal cortical folding, ventriculomegaly, brainstem dysplasia, and encephalocele with Chiari II malformation and have been described in other reports of congenital ZIKV infection.9,10,15 Conversely, it remains unclear whether encephalocele and other neural tube defects have causal associations with ZIKV infection. The finding of heterotopias has been described at autopsy in a microcephalic fetus with ZIKV.16 Heterotopias are a form of neuronal migration defect. In the nodular periventricular form seen in our cases, neuronal clusters abut the ventricular wall in a region transiently occupied during development by the germinal matrix, a zone rich in the progenitor cells that are a known tropic target for ZIKV.10,17 The heterotopias were not detected by US.
Based on previous reports of congenital ZIKV syndrome,18,19 we anticipated a higher rate of fetal brain abnormalities because most women enrolled in our study had symptomatic ZIKV infection in the first trimester. Early pregnancy is a period of higher risk based on other cohort studies18 and is supported by neuropathologic analyses of ZIKV tropism in the developing brain.10 In our cohort, fetal brain abnormalities were shown in only 1 of 66 women (2%) with symptomatic infection in the first trimester, which is lower than 12.7% reported among women in the French Territories of the Americas.18 Conversely, 1 of our cases (case 2) with significant fetal brain anomalies had maternal symptoms of ZIKV in the second trimester. The reason for this difference between our cohort and others is likely multifactorial, with other, yet unknown, genetic and environmental influences that affect the natural history of congenital ZIKV. Differences in case definition used in different parts of Brazil also contribute to varying rates of microcephaly from 3% in Rio de Janeiro19 to 41% in Recife.20 Furthermore, dizygotic twins infected with ZIKV can have different clinical outcomes.21
Compared with the fetal brain abnormalities shown on MRI, the postnatal brain MRI abnormalities were mild. Germinolytic cysts, seen on MRI in 2 cases, have been reported in other ZIKV cohorts,22 but the other brain MRI findings are nonspecific and of unknown association with ZIKV exposure. One of the infants in our cohort had evidence of a cerebral infarct detected by postnatal brain MRI.6 Based on the brain MRI features, the injury preceded birth. It is unclear whether this lesion is the result of a direct destructive viral insult or owing to vaso-occlusive ischemia. Cerebral vasculopathy has been described in ZIKV infection23 and was evident on postnatal US in the form of lenticulostriate vasculopathy in our study and others.22 Potential embolic sources include placental thromboembolism secondary to placental inflammation, a mechanism noted in cerebral infarctions following congenital infections.24 In the appropriate epidemiologic context, congenital ZIKV infection may be associated with fetal and neonatal cerebral infarction.23,25
The high prevalence in our postnatal cranial US studies of germinolytic and subependymal cysts, as well as lenticulostriate vasculopathy, was unexpected. Cranial US findings of germinolytic or subependymal cysts and lenticulostriate vasculopathy may be associated in some cases with congenital infection.7,26,27 In a report of ZIKV-related birth outcomes from Brazil, 7 of 38 infants (18%) who underwent cranial US had features of lenticulostriate vasculopathy, subependymal cysts, choroid plexus cyst, or cranial hemorrhage.22 These postnatal US changes were less evident on brain MRI, possibly because the signal of the cysts is similar to that of cerebrospinal fluid and the cyst walls are thin, making US more sensitive for detecting these changes.
We hypothesized that prolonged maternal viremia would be associated with fetal brain injury owing to ZIKV infection; however, this was not observed in our study. In a previously reported case of prolonged maternal viremia associated with severe fetal brain damage, there was resolution of maternal PCR only after delivery of the fetus.9 In our 3 cases of severe fetal brain abnormalities, maternal PCR studies were performed outside the usual window of viremia, a feature of other studies of congenital ZIKV syndrome.28 Zika viremia was prolonged in only 1 of our cases, who subsequently developed cerebral infarction.
For most of our cases, fetal MRI did not add value beyond US. Most of our cases had normal fetal MRI findings and, for pregnant women and their families, MRI provided additional reassurance. Because only the infants with normal fetal MRI findings had postnatal imaging, we cannot report the predictive associations of fetal MRI. Postnatal imaging findings, however, were relatively mild among the 23 cases who had a finding on brain MRI and/or cranial US. Therefore, normal fetal imaging had predictive associations with normal postnatal imaging or mild postnatal imaging findings unlikely to be of significant clinical consequence. Fetal MRI at 18 weeks in one instance provided a more timely and accurate diagnosis of significant brain abnormalities; informed decisions about management of the pregnancy would not have been possible at that gestational age with use of fetal US alone. Early identification of ZIKV-related fetal brain damage may be important for optimal coordination of multidisciplinary care and intervention at birth and beyond. Because prolonged maternal viremia was not associated with abnormal brain findings in our cases, maternal ZIKV testing cannot direct the need for advanced fetal imaging. Fetal MRI may be most useful when it is performed early in situations where termination of pregnancy is a personal and legal option. Besides this scenario, we found US to be adequate in most cases for detecting ZIKV-related brain anomalies and monitoring fetal HC.
Limitations
This study is limited by variation in timing of maternal infection and symptoms, ZIKV testing, MRI, and US; fetal MRI technique; and incomplete postnatal imaging. We anticipated higher enrollment of travel-related cases in Washington, DC. The 1 enrolled asymptomatic woman came to the attention of the Colombian research team owing to an identified fetal abnormality and positive ZIKV testing. Abnormal cases were identified at both sites.
Our fetal MRI protocol included only T2-weighted sequences, a widely used approach, because the resolution and quality of T1-, diffusion-, and susceptibility-weighted sequences in fetal MRI are limited compared with postnatal MRI, owing to fetal motion artifact and the intrauterine fetal location precluding use of a dedicated head coil. Postnatal brain MRI, which includes these additional sequences, would therefore be expected to detect more abnormalities than fetal MRI. In some cases, postnatal MRI was limited owing to movement artifact. It is possible that small cysts may have been present, but not recognized, because of infant movement. Even with considerable efforts to acquire postnatal imaging in all infants, some were lost to follow-up, parents declined appointments, or owing to neonatal death or a medical condition, did not have postnatal neuroimaging. Despite the technical challenges of fetal MRI, radiologists in Colombia with limited prior experience became rapidly proficient at acquiring high-quality fetal MRI studies, supporting the value and potential for multinational collaborative research partnerships.29
The limitations of ZIKV laboratory testing have become widely recognized, especially in areas with circulating ZIKV and other circulating Flaviviruses where multiple opportunities for exposure are possible. Although laboratory confirmation was not a requirement for inclusion, validated ZIKV testing was performed by the CDC for the first 52 participants. Only 1 woman had positive PCR identification. Although PCR is the most specific mode of ZIKV confirmation, its diagnostic utility in this clinically defined population was limited because most women were enrolled more than 12 weeks from symptom onset; thus, negative test results do not exclude infection when performed beyond 3 weeks of symptom onset. Likewise, the utility of IgM testing was limited because most women did not have testing performed within the 2- to 12-week window after symptom onset, when this test is known to be most sensitive.
Conclusions
This study of prospectively enrolled women with pregnancies complicated by ZIKV infection found that sequential fetal neuroimaging was normal in most fetuses, and in the small number of positive studies, the findings were severely abnormal. Absence of prolonged maternal viremia did not have predictive associations with normal fetal or neonatal brain imaging. Postnatal imaging can detect changes not seen on fetal imaging, supporting the current CDC recommendation for postnatal cranial US.8 There is a need for long-term follow-up to assess the neurodevelopmental significance of these early neuroimaging findings, both normal and abnormal; such studies are in progress.
eTable. Postnatal Neuroimaging Findings on Brain MRI and Cranial US
References
- 1.Messina JP, Kraemer MU, Brady OJ, et al. . Mapping global environmental suitability for Zika virus. eLife. 2016;5:e15272. doi: 10.7554/eLife.15272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552-1563. Medline: doi: 10.1056/NEJMra1602113 [DOI] [PubMed] [Google Scholar]
- 3.Cuevas EL, Tong VT, Rozo N, et al. . Preliminary report of microcephaly potentially associated with Zika virus infection during pregnancy—Colombia, January-November 2016. MMWR Morb Mortal Wkly Rep. 2016;65(49):1409-1413. doi: 10.15585/mmwr.mm6549e1 [DOI] [PubMed] [Google Scholar]
- 4.Del Campo M, Feitosa IM, Ribeiro EM, et al. ; Zika Embryopathy Task Force–Brazilian Society of Medical Genetics ZETF-SBGM . The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet A. 2017;173(4):841-857. doi: 10.1002/ajmg.a.38170 [DOI] [PubMed] [Google Scholar]
- 5.Melo ASO, Chimelli L, Tanuri A. Congenital Zika virus infection: beyond neonatal microcephaly—reply. JAMA Neurol. 2017;74(5):610-611. doi: 10.1001/jamaneurol.2017.0051 [DOI] [PubMed] [Google Scholar]
- 6.Mulkey SB, Vezina G, Bulas DI, et al. . Neuroimaging findings in normocephalic newborns with intrauterine Zika virus exposure. Pediatr Neurol. 2018;78:75-78. doi: 10.1016/j.pediatrneurol.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 7.Levine D, Jani JC, Castro-Aragon I, Cannie M. How does imaging of congenital Zika compare with imaging of other TORCH infections? Radiology. 2017;285(3):744-761. doi: 10.1148/radiol.2017171238 [DOI] [PubMed] [Google Scholar]
- 8.Adebanjo T, Godfred-Cato S, Viens L, et al. . Contributors. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection—United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089-1099. doi: 10.15585/mmwr.mm6641a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driggers RW, Ho CY, Korhonen EM, et al. . Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142-2151. doi: 10.1056/NEJMoa1601824 [DOI] [PubMed] [Google Scholar]
- 10.Ho CY, Ames HM, Tipton A, et al. . Differential neuronal susceptibility and apoptosis in congenital Zika virus infection. Ann Neurol. 2017;82(1):121-127. doi: 10.1002/ana.24968 [DOI] [PubMed] [Google Scholar]
- 11.Kline-Fath BM, Bulas DI, Bahado-Singh R, eds. Fundamental and Advanced Fetal Imaging: Ultrasound and MRI. Philadelphia, PA: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 12.Hadlock FP, Deter RL, Harrist RB, Park SK. Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology. 1984;152(2):497-501. doi: 10.1148/radiology.152.2.6739822 [DOI] [PubMed] [Google Scholar]
- 13.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention WHO growth standards are recommended for use in the US for infants and children 0 to 2 years of age. 2010; https://www.cdc.gov/growthcharts/who_charts.htm. Accessed August 15, 2017.
- 15.Moore CA, Staples JE, Dobyns WB, et al. . Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171(3):288-295. doi: 10.1001/jamapediatrics.2016.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Štrafela P, Vizjak A, Mraz J, et al. . Zika virus–associated microencephaly: a thorough description of neuropathologic findings in the fetal central nervous system. Arch Pathol Lab Med. 2017;141(1):73-81. doi: 10.5858/arpa.2016-0341-SA [DOI] [PubMed] [Google Scholar]
- 17.Chimelli L, Avvad-Portari E. Congenital Zika virus infection: a neuropathological review. Childs Nerv Syst. 2018;34(1):95-99. Medline: doi: 10.1007/s00381-017-3651-3 [DOI] [PubMed] [Google Scholar]
- 18.Hoen B, Schaub B, Funk AL, et al. . Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994. doi: 10.1056/NEJMoa1709481 [DOI] [PubMed] [Google Scholar]
- 19.Pacheco O, Beltrán M, Nelson CA, et al. . Zika virus disease in Colombia: preliminary report [published online June 15, 2016]. N Engl J Med. doi: 10.1056/NEJMoa1604037 [DOI] [PubMed] [Google Scholar]
- 20.de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, et al. ; Investigators from the Microcephaly Epidemic Research Group . Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16(12):1356-1363. [DOI] [PubMed] [Google Scholar]
- 21.Linden VV, Linden HVJ, Leal MC, et al. . Discordant clinical outcomes of congenital Zika virus infection in twin pregnancies. Arq Neuropsiquiatr. 2017;75(6):381-386. doi: 10.1590/0004-282x20170066 [DOI] [PubMed] [Google Scholar]
- 22.Nogueira ML, Nery Júnior NRR, Estofolete CF, et al. . Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin Microbiol Infect. 2018;24(6):646-652. doi: 10.1016/j.cmi.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 23.Landais A, Césaire A, Fernandez M, et al. . ZIKA vasculitis: a new cause of stroke in children? J Neurol Sci. 2017;383:211-213. Medline: doi: 10.1016/j.jns.2017.10.045 [DOI] [PubMed] [Google Scholar]
- 24.Chabrier S, Husson B, Dinomais M, Landrieu P. Nguyen The Tich S. New insights (and new interrogations) in perinatal arterial ischemic stroke. Thromb Res. 2011;127(1):13-22. doi: 10.1016/j.thromres.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Mulkey SB, DeBiasi RL, du Plessis AJ. Cerebral infarction due to Zika virus. J Neurol Sci. 2018;387:109-110. Medline: doi: 10.1016/j.jns.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 26.Soares de Souza A, Moraes Dias C, Braga FD, et al. . Fetal infection by Zika virus in the third trimester: report of 2 cases. Clin Infect Dis. 2016;63(12):1622-1625. doi: 10.1093/cid/ciw613 [DOI] [PubMed] [Google Scholar]
- 27.de Vries LS, Gunardi H, Barth PG, Bok LA, Verboon-Maciolek MA, Groenendaal F. The spectrum of cranial ultrasound and magnetic resonance imaging abnormalities in congenital cytomegalovirus infection. Neuropediatrics. 2004;35(2):113-119. doi: 10.1055/s-2004-815833 [DOI] [PubMed] [Google Scholar]
- 28.van der Linden V, Pessoa A, Dobyns W, et al. . Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth—Brazil. MMWR Morb Mortal Wkly Rep. 2016;65(47):1343-1348. doi: 10.15585/mmwr.mm6547e2 [DOI] [PubMed] [Google Scholar]
- 29.Heymann DL, Liu J, Lillywhite L. Partnerships, not parachutists, for Zika research. N Engl J Med. 2016;374(16):1504-1505. doi: 10.1056/NEJMp1602278 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Postnatal Neuroimaging Findings on Brain MRI and Cranial US