Key Points
Question
Is prenatal exposure to air pollution a risk factor for autism spectrum disorder?
Findings
In this population-based cohort study of 132 256 births, maternal exposure to nitric oxide during pregnancy was associated with increased risk of autism spectrum disorder in offspring.
Meaning
Reducing exposures of pregnant women to environmental nitric oxide may be associated with a reduction in autism spectrum disorder incidence in their children.
This large population-based Canadian cohort study evaluates the association between prenatal exposures to airborne pollutants and autism spectrum disorder.
Abstract
Importance
The etiology of autism spectrum disorder (ASD) is poorly understood, but prior studies suggest associations with airborne pollutants.
Objective
To evaluate the association between prenatal exposures to airborne pollutants and ASD in a large population-based cohort.
Design, Setting, and Participants
This population-based cohort encompassed nearly all births in Metro Vancouver, British Columbia, Canada, from 2004 through 2009, with follow-up through 2014. Children were diagnosed with ASD using a standardized assessment with the Autism Diagnostic Interview–Revised and Autism Diagnostic Observation Schedule. Monthly mean exposures to particulate matter with a diameter less than 2.5 µm (PM2.5), nitric oxide (NO), and nitrogen dioxide (NO2) at the maternal residence during pregnancy were estimated with temporally adjusted, high-resolution land use regression models. The association between prenatal air pollution exposures and the odds of developing ASD was evaluated using logistic regression adjusted for child sex, birth month, birth year, maternal age, maternal birthplace, and neighborhood-level urbanicity and income band. Data analysis occurred from June 2016 to May 2018.
Exposures
Mean monthly concentrations of ambient PM2.5, NO, and NO2 at the maternal residence during pregnancy, calculated retrospectively using temporally adjusted, high-resolution land use regression models.
Main Outcomes and Measures
Autism spectrum disorder diagnoses based on standardized assessment of the Autism Diagnostic Interview–Revised and Autism Diagnostic Observation Schedule. The hypothesis being tested was formulated during data collection.
Results
In a cohort of 132 256 births, 1307 children (1.0%) were diagnosed with ASD by the age of 5 years. The final sample size for the PM2.5-adjusted model was 129 439 children, and for NO and NO2, it was 129 436 children; of these, 1276 (1.0%) were diagnosed with ASD. Adjusted odds ratios for ASD per interquartile range (IQR) were not significant for exposure to PM2.5 during pregnancy (1.04 [95% CI, 0.98-1.10] per 1.5 μg/m3 increase [IQR] in PM2.5) or NO2 (1.06 [95% CI, 0.99-1.12] per 4.8 ppb [IQR] increase in NO2) but the odds ratio was significant for NO (1.07 [95% CI, 1.01-1.13] per 10.7 ppb [IQR] increase in NO). Odds ratios for male children were 1.04 (95% CI, 0.98-1.10) for PM2.5; 1.09 (95% CI, 1.02-1.15) for NO; and 1.07 (95% CI, 1.00-1.13) for NO2. For female children, they were for 1.03 (95% CI, 0.90-1.18) for PM2.5; 0.98 (95% CI, 0.83-1.13) for NO; and 1.00 (95% CI, 0.86-1.16) for NO2.
Conclusions and Relevance
In a population-based birth cohort, we detected an association between exposure to NO and ASD but no significant association with PM2.5 and NO2.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by varying degrees of difficulty in social interaction, difficulties in verbal and nonverbal communication, and repetitive behaviors.1 In the United States, the Centers for Disease Control and Prevention estimated a sharp increase in ASD prevalence from 6.7 per 1000 children aged 8 years (or 1 in 150 children) in 2000 to 14.6 per 1000 (or 1 in 68 children) in 2012.2,3 The prevalence in Canada was 15.2 per 1000 children aged 5 to 17 years in 2015 (or 1 in 66 children), and in British Columbia, it was estimated at 14.7 per 1000 (or 1 in 68 children).4
The etiology of ASD is poorly understood.5 Prior research has identified environmental contaminants and air pollution as potential risk factors for ASD.6,7,8,9 Recent systematic reviews have reported that exposure to air pollution is associated with increased risk for ASD.7,8,9 In particular, studies from the United States, Israel, and Taiwan have reported positive associations between particulate matter (PM), nitric oxide (NO), and nitrogen dioxide (NO2) exposures and increased risk for ASD.10,11,12,13,14,15,16 In contrast, 3 studies in Europe observed no associations between ASD17 or autistic traits18,19 with air pollutants. The proportion of children with ASD from these studies ranged from 342 of 49 073 (0.7%)11 to 279 of 524 (53.2%),12 and sample sizes ranged from 443 to 83 385.10,15 Outcome assessments have relied on parent-reported questionnaires, diagnostic codes in health registries, and, in some studies, individual evaluations or validation of all or a subset of children identified with ASD.
Our study leveraged one of the largest population-based birth cohorts to date with strict diagnostic criteria for all children who were positively identified with ASD, and high-resolution exposure estimates to investigate the association between prenatal exposure to ambient air pollution in Metro Vancouver, British Columbia, Canada.
Methods
Population and Study Design
The cohort included births from 2004 to 2009 to mothers who were registered with the provincial health insurance plan for 275 days or longer and resided in the Greater Vancouver area during the calendar year of their pregnancy.
The study was approved by the University of British Columbia Children’s and Women’s Research Ethics Board and Simon Fraser University. The study used deidentified data, so no consent was sought from individuals.
In British Columbia, the provincial public health insurance program covers nearly all residents.20 We linked birth records in British Columbia from the British Columbia Perinatal Data Registry and British Columbia Vital Statistics with British Columbia Medical Service Plan and hospital discharge data, Statistics Canada data, and British Columbia Autism Assessment Network (BCAAN) data through Population Data British Columbia.21,22,23,24,25,26,27 The British Columbia Perinatal Data Registry contains data abstracted from obstetrical and neonatal medical records on nearly 100% of births in the province from more than 60 acute care facilities, as well as births occurring at home that are attended by registered midwives. This includes data on women who had pregnancies ending in a live birth or stillbirth of at least 20 weeks’ gestation or 500 g birth weight. The registry also collects data on maternal postpartum readmissions up to 42 days postdelivery and baby transfers and readmissions up to 28 days after birth. The estimated gestational age is calculated based on last menstrual period, first ultrasonogram (at <20 weeks’ gestation), clinical estimate from the newborn examination, and documentation from the maternal medical record.28 Health services data from the British Columbia Ministry of Health and British Columbia Vital Statistics Agency includes demographic information, residential location, medical visits paid for on a fee-for-service basis, and data on births, deaths, and hospital discharges. Statistics Canada provides neighborhood-level data on urbanicity and socioeconomic status, measured as income bands, which are 1000 bands of equivalized disposable income for each postal code (1 indicates the lowest income; 1000, the highest). In the urban Vancouver metropolitan area, a postal code represents small geographical areas, such as a high-rise building or 1 side of a city block.29
The study area was based on land use regression (LUR) models used for exposure assessment.30 We obtained a linked file of all single, twin, or triplet births to mothers aged 15 to 49 years in British Columbia. Births with unknown gestational age or missing sex information, stillbirths, and infants who died younger than 1 year of age were not included in the data extract. Using residential postal codes, we excluded births outside of the study area.
To develop exposure estimates for the first birth in 2004, we temporally adjusted LUR models beginning in January 2003. The cohort was followed up until the end of 2014, establishing a 5-year minimum follow-up period for ASD diagnoses.
Case Ascertainment
All primary care practitioners and specialists in British Columbia conduct general developmental surveillance. If a child is suspected of having ASD, they are referred for a BCAAN assessment, which yield person-specific ASD diagnostic data, based on clinical evaluations made by pediatricians, psychiatrists, or psychologists who have completed additional structured training and mentorships. Diagnostic assessment for ASD has been standardized within BCAAN since 2004 (April 1, 2004, to December 31, 2014), using the standardized Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview–Revised (ADI-R) instruments. In addition to detailed clinical history, evaluation of developmental status, and reports from community health practitioners and schools, a clinical diagnostic assessment must include the ADI-R and the ADOS or ADOS-2 as standardized tools.31 Within BCAAN, all clinicians follow the same diagnostic guidelines in case assessment and diagnostic formulation. Clinical diagnostic assessments are provided by BCAAN for children and youth up to 19 years, which is available at no cost because it is covered by the provincial health plan.
Exposure Assessment
We developed temporally adjusted, long-term mean measures of air pollution by combining LUR models at 10 m2 spatial resolution30 with continuous monitoring data (2003-2014) from Metro Vancouver’s Air Quality Monitoring Network (Metro Vancouver Environmental Sampling and Monitoring, written communication, July 8, 2016). These LUR models have previously been evaluated spatially and temporally against ambient pollution measurements,30,32 with model estimates validated with individual-level exposures in pregnant women.33 The use of LUR models provides greater spatial resolution and the ability to capture small-scale, localized variation compared with reliance on air-quality monitoring data alone.30,34 Temporally adjusted LUR models allowed us to incorporate trends and estimate monthly mean air pollution exposures for each pregnant woman at her residential postal code for her entire pregnancy and each trimester. We used predictive mean matching to impute missing air monitoring station data.
First, we developed temporal factors as a ratio between the monthly mean of air pollutant concentrations across all monitors for the month of interest over the annual mean of air pollutant concentrations across all monitors for the LUR year.35 Then, we derived minimum concentrations by calculating the mean from the lowest daily mean in a month for all air monitors for each month of the LUR year. To avoid potentially estimating values of zero, LUR models were truncated before temporal adjustment (eFigures 1-3 in the Supplement). ArcGIS software version 10.3 (Esri) and R versions 3.3, 3.4, and 3.5 (The R Project for Statistical Computing), with rgdal and rgeos packages, were used.
Statistical Analyses
To examine the independence of pollutant concentrations, we calculated a Pearson correlation coefficient matrix for all pollutant-time pairs. We selected covariates a priori based on expert knowledge, data availability, past literature, and a list of confounders identified by the Environmental Epidemiology of Autism Research Network.7 We included child sex (categorical), birth month (categorical), birth year (categorical), maternal age (continuous), mother’s birth country (categorical), and neighborhood-level urbanicity (categorical) and income band (continuous). We did not adjust for gestational age or birth weight in our primary analyses, because they may be on the causal pathway between air pollution and ASD.10,16
We examined the association between air pollution exposure and the odds of ASD using single pollutant and single-period models. We calculated odds ratios (ORs) and 95% CIs using logistic regression to estimate increases in odds of ASD per interquartile range (IQR) change in PM2.5, NO, and NO2 levels. We also tested for interactions between sex and exposure for all air pollutants and periods. Additionally, we conducted sex-stratified and sensitivity analyses by individually adding gestational age, status as small for gestational age, birth weight, multiple birth status, maternal parity, and maternal smoking status to our original model; replaced birth month with conception month; and replaced original exposure estimates with estimates using nontruncated, temporally adjusted LUR models. All statistical analyses were performed in R versions 3.3, 3.4, and 3.5.
Results
Of the 132 256 children born in the Vancouver metropolitan area from 2004 through 2009, 1307 (1.0%) were diagnosed with ASD. The median age at assessment for ASD was 4.2 years (IQR, 3.3-5.2 years). Children diagnosed with ASD were more likely to be male (at a ratio of approximately 1 to 5; male children with ASD: 1091 [83.5%]; female children with ASD: 216 [16.5%]), born to slightly older mothers (median [IQR] age: mothers of children with ASD, 32.2 [28.7-35.9] years; mothers of children without ASD: 31.9 [28.2-35.4] years), born to multiparous mothers (mothers of children with ASD: nulliparous, 541 [41.4%]; multiparous, 766 [58.6%]), and residents of lower-income-level neighborhoods (median [IQR] income band: children with ASD, 390 [164-679]; children without ASD: 449 [189-738]; Table 1).
Table 1. Characteristics of Children With and Without Autism Spectrum Disorder.
| Characteristic | Children, No. (%) | |
|---|---|---|
| With ASD (n = 1307) | Without ASD (n = 130 949) | |
| Sex | ||
| Male | 1091 (83.5) | 66 960 (51.1) |
| Female | 216 (16.5) | 63 989 (48.9) |
| Gestational age at birth, median (IQR), wk | 39 (37-40) | 39 (38-40) |
| Maternal age at delivery, median (IQR), y | 32.2 (28.7-35.9) | 31.9 (28.2-35.4) |
| Multiple birth | ||
| Singleton | 1250 (95.6) | 126 942 (96.9) |
| Twins or triplets | 57 (4.4) | 4007 (3.1) |
| Parity | ||
| Nulliparous | 541 (41.4) | 67 834 (51.8) |
| Multiparous | 766 (58.6) | 63 114 (48.2) |
| Mother’s birthplace | ||
| Canada | 563 (43.1) | 66 284 (50.7) |
| India | 83 (6.4) | 12 275 (9.4) |
| China | 128 (9.8) | 11 004 (8.4) |
| Philippines | 127 (9.7) | 6606 (5.1) |
| United Kingdom | 24 (1.8) | 2512 (1.9) |
| United States | 21 (1.6) | 1986 (1.5) |
| Vietnam | 51 (3.9) | 2912 (2.2) |
| Hong Kong | 28 (2.1) | 2045 (1.6) |
| Other countries | 280 (21.5) | 25 102 (19.2) |
| Income band, median (IQR) | 390 (164-679) | 449 (189-738) |
| Urbanicity | ||
| Rural | 6 (0.5) | 456 (0.4) |
| Semiurban | 11 (0.9) | 729 (0.6) |
| Urban | 1277 (98.7) | 129 035 (99.1) |
| Month of birth | ||
| January | 119 (9.1) | 10 423 (8.0) |
| February | 88 (6.7) | 9717 (7.4) |
| March | 116 (8.9) | 10 762 (8.2) |
| April | 109 (8.3) | 10 679 (8.2) |
| May | 111 (8.5) | 11 258 (8.6) |
| June | 114 (8.7) | 11 096 (8.5) |
| July | 92 (7.0) | 11 379 (8.7) |
| August | 109 (8.3) | 11 556 (8.8) |
| September | 109 (8.3) | 11 591 (8.9) |
| October | 109 (8.3) | 11 267 (8.6) |
| November | 129 (9.9) | 10 719 (8.2) |
| December | 102 (7.8) | 10 502 (8.0) |
| Year of birth | ||
| 2004 | 235 (18.0) | 20 700 (15.8) |
| 2005 | 206 (15.8) | 20 759 (15.9) |
| 2006 | 200 (15.3) | 21 465 (16.4) |
| 2007 | 210 (16.1) | 22 302 (17.0) |
| 2008 | 232 (17.8) | 22 516 (17.2) |
| 2009 | 224 (17.1) | 23 207 (17.7) |
Abbreviations: ASD, autism spectrum disorder; IQR, interquartile range.
Air pollution concentrations, which exhibited a seasonal pattern, decreased over the 2003 to 2009 study period, from an annual mean of 5.7 to 5.0 μg/m3 for PM2.5, 16.0 to 11.0 ppb for NO, and 16.0 to 13.5 ppb for NO2 (eFigure 4 in the Supplement). Concentrations of PM2.5 were higher during summer because of secondary PM2.5 formation and episodic smoke from forest fire activity.36 In contrast, NO and NO2 concentrations were higher in winter.
For mothers, the median monthly mean exposure during pregnancy of PM2.5 was 3.5 μg/m3 (IQR, 2.7-4.2 μg/m3); of NO, 18.3 ppb (IQR, 14.0-24.7 ppb); and of NO2, 14.3 ppb (IQR, 12.2-17.0 ppb). Values were similar across the full pregnancy and trimesters (eTable 1 in the Supplement). Correlation across periods (eFigure 5 in the Supplement) was high for both PM2.5 (r = 0.8-1.0) and NO2 (r = 0.6-1.0), suggesting a limited ability to differentiate between exposures across trimesters.
In unadjusted logistic regression models, we estimated an increase in odds of ASD with increased air pollution exposure for the full pregnancy (PM2.5: OR, 1.08 [95% CI, 1.03-1.14] per 1.5-μg/m3 [IQR] increase; NO: 1.09 [95% CI, 1.03-1.14] per 10.7-ppb [IQR] increase; NO2: 1.11 [95% CI, 1.05-1.17] per 4.8-ppb [IQR] increase; Table 2) and for each trimester. The associations remained after adjusting for child sex, birth month, birth year, maternal age, maternal birthplace, urbanicity, and income band for exposure to NO (OR, 1.07 [95% CI, 1.01-1.13]), but the association was not statistically significant for PM2.5 (OR, 1.04 [95% CI, 0.98-1.10]) or NO2 (OR, 1.06 [95% CI, 0.99-1.12]) for the same IQR increase in each pollutant (Table 2). In the comparison of odds for male vs female children in full pregnancy models, the ORs in male children were 1.04 (95% CI, 0.98-1.10) for PM2.5; 1.09 (95% CI, 1.02-1.15) for NO; and 1.07 (95% CI, 1.00-1.13) for NO2. For female children, they were 1.03 (95% CI, 0.90-1.18) for PM2.5; 0.98 (95% CI, 0.83-1.13) for NO; and 1.00 (95% CI, 0.86-1.16) for NO2 (Table 2; eFigure 6 in the Supplement). Tests of interactions between sex and exposure for all air pollutants and periods were not statistically significant.
Table 2. Odds of Autism Spectrum Disorder for Prenatal Exposure to Air Pollutants.
| Modela | Children, No. | Children With Autism Spectrum Disorder, No. | Odds Ratio (95% CI) |
|---|---|---|---|
| Per interquartile range (1.5 μg/m3) of 2.5-µm particulate matter | |||
| Unadjusted full pregnancy model | 131 440 | 1300 | 1.08 (1.03-1.14) |
| Adjusted full pregnancy model | 129 439 | 1276 | 1.04 (0.98-1.10) |
| Adjusted full pregnancy model: male children | 66 612 | 1064 | 1.04 (0.98-1.10) |
| Adjusted full pregnancy model: female children | 62 827 | 212 | 1.03 (0.90-1.18) |
| Per interquartile range (10.7 ppb) of nitric oxide | |||
| Unadjusted full pregnancy model | 131 437 | 1300 | 1.09 (1.03-1.14) |
| Adjusted full pregnancy model | 129 436 | 1276 | 1.07 (1.01-1.13) |
| Adjusted full pregnancy model: male children | 66 611 | 1064 | 1.09 (1.02-1.15) |
| Adjusted full pregnancy model: female children | 62 825 | 212 | 0.98 (0.83-1.13) |
| Per interquartile range (4.8 ppb) of nitrogen dioxide | |||
| Unadjusted full pregnancy model | 131 437 | 1300 | 1.11 (1.05-1.17) |
| Adjusted full pregnancy model | 129 436 | 1276 | 1.06 (0.99-1.12) |
| Adjusted full pregnancy model: male children | 66 611 | 1064 | 1.07 (1.00-1.13) |
| Adjusted full pregnancy model: female children | 62 825 | 212 | 1.00 (0.86-1.16) |
Models adjusted for child sex, birth month, birth year, maternal age, maternal birthplace, and neighborhood-level urbanicity and income band.
We conducted sensitivity analyses to examine whether adjusting for gestational age, status as small for gestational age, birth weight, multiple birth status, maternal parity, and maternal smoking status altered outcome estimates. We also replaced birth month with conception month and compared temporally adjusted exposure estimates using truncated and nontruncated LUR models. Adjusting for the additional covariates added to the base models and using exposure estimates derived from nontruncated LUR models resulted in nearly identical ORs and CIs for all adjustments for the 3 pollutants, with nearly all ORs and CIs for NO retaining statistical significance and most ORs and CIs for PM2.5 and NO2 showing no significant association (eTable 2 in the Supplement).
We had limited ability to differentiate between trimester exposures because of their high correlation. Likelihood ratio tests on adjusted models with mutually adjusted trimesters (eTable 3 in the Supplement) indicated no differences in odds of developing ASD between trimester-specific exposures for any of the pollutants.
Discussion
We observed increased risk of ASD associated with exposure to PM2.5, NO, and NO2 in what is, to our knowledge, one of the largest population-based cohort studies of prenatal exposures to air pollution and the subsequent development of ASD. Exposures to PM2.5 and NO2 were not significantly associated with risk for ASD. Notably, this study was conducted in a population with low levels of air pollution and relied on strict, established, clinically verified ASD diagnostic criteria.
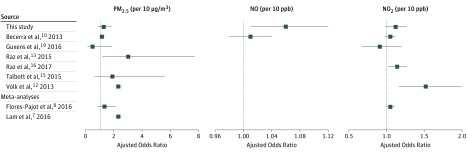
We found an association between ASD and prenatal exposures to NO (Figure). While the OR was small, a small increase in relative risk for high prevalence exposures can result in a large burden of disease. Consistent with the meta-analysis by Flores-Pajot et al,8 we did not find a significant association between ASD and PM2.5 or NO2 exposure during full pregnancy. Although NO is not a regulated air pollutant, we included NO as an indicator of traffic-associated air pollution because our exposure models were capable of distinguishing between NO and NO2 as primary and secondary traffic-associated air pollutants.
Figure. Forest Plot of Odds Ratios of the Association of Autism Spectrum Disorder and Prenatal Exposure to Air Pollutants During Pregnancy.
If multiple models were presented in a study, the chosen models were those that used temporally adjusted exposure estimates to account for temporal variation and models that used full pregnancy exposure estimates without mutually adjusting for other periods. For comparability, effect estimates were standardized to 10 μg/m3 unit change for 2.5-μm particulate matter (PM2.5) and 10 parts per billion (ppb) unit change for nitric oxide (NO) and nitrogen dioxide (NO2). For the conversion of NO2, 10 μg/m3 is equivalent to 5.32 ppb, based on an ambient pressure of 1 atm and a temperature of 25°C. The Flores-Pajot et al meta-analysis8 summary risk ratio for PM2.5 includes Guxens et al,19 Becerra et al,10 Raz et al,13 Talbott et al,15 and Volk et al,12 and the Flores-Pajot et al meta-analysis8 summary risk ratio for NO2 includes Guxens et al,19 Becerra et al,10 and Volk et al.12 The Lam et al7 meta-analysis summary odds ratio for PM2.5 includes Raz et al,13 Volk et al,12 and Becerra et al.10 Blue lines indicate the 95% CI of each depicted odds ratios (blue squares).
Like 1 prior study,13 our sex-stratified analyses did not show differences in ORs among male children compared with female children, because the test for interactions was not significant. Because of the smaller number of female children with ASD in our study, the sex-stratified models with female children had wider CIs.
There is insufficient evidence of clear trends in increased odds by trimester. A systematic review on PM and ASD reported no definitive conclusions between exposure periods, PM, and risk of ASD.9 Based on a small number of studies, Flores-Pajot et al8 reported an increase in the strength of association between PM2.5 and ASD from the first to the third trimester, from a risk ratio of 1.10 to 1.33 per 10 μg/m3, and little difference across trimesters for NO2.
To date, positive associations between ASD and air pollution have been observed across 7 studies in the United States,10,12,13,14,15 Israel,16 and Taiwan,11 but not in 3 European studies.17,18,19 Inconsistent findings could be because of differences in case definition, air pollution levels, or composition of airborne pollutants.16,17,19 Our study was in an area with relatively low air pollution and had similar levels of air pollution as the European cohorts; Guxens et al19 observed median air pollution levels during pregnancy ranging from 8.4 to 22.4 μg/m3 of PM2.5 and 17.9 to 42.2 μg/m3 of NO2 across cohorts in Sweden, the Netherlands, Italy, and Spain. Gong et al18 reported yearly mean levels from local traffic in Stockholm, Sweden, ranging from 3.3 to 4.2 μg/m3 of PM10 and 5.4 to 12.7 μg/m3 of NOx, and, in another study,17 Gong et al reported 4.2 to 4.4 μg/m3 of PM10 and 9.8 to 11.0 μg/m3 of NOx. However, Guxens et al19 and Gong et al18 used measures of autistic traits, not diagnoses of ASD, and therefore their findings may not be directly comparable with the other studies described above.
We did not adjust for birth weight, status as small for gestational age, and preterm birth in our primary analyses, because these could be on the causal pathway between air pollution and ASD. Studies have found adverse associations between traffic-associated air pollution and birth weight, status as small for gestational age, and preterm birth,37,38 and some evidence that these may be associated with ASD.39,40,41
With a linkage of our cohort to Ministry of Education data that contain ASD diagnoses from both BCAAN and private practitioners for children in the public-school system, 1967 children were identified with ASD, although only 1307 (66.4%) are identified by the stricter BCAAN criteria. We compared mean exposures for the full pregnancy period among children with positive diagnoses of ASD in both BCAAN and Ministry of Education data (n = 1178; PM2.5, 3.7 μg/m3; NO, 21.8 ppb; NO2, 15.8 ppb), only in BCAAN data (n = 129; PM2.5, 3.6 μg/m3; NO, 21.7 ppb; NO2, 15.8 ppb), and only in Ministry of Education data (n = 660; PM2.5, 3.5 μg/m3; NO, 20.5 ppb; NO2, 15.1 ppb). These differences were not meaningful, and we did not suspect differential misclassification by exposure.
Limitations
This study has strengths and limitations. In our study, which is, to our knowledge, one of the largest population-based cohorts on ASD and air pollution, all children who were diagnosed with ASD received a clinical diagnosis by qualified diagnosticians who have undergone extensive training to provide standardized clinical assessments. However, children in British Columbia who received an ASD diagnosis from a private practitioner were not included in this case definition.
We included covariates (social class, urban residence, maternal age, season of conception or birth, and calendar time) based on a list identified by the Environmental Epidemiology of Autism Research Network.7 Although we controlled for these potential confounders, we did not test for multiple comparisons and residual confounding may remain, likely biasing our effect estimates toward the null.
Our temporally adjusted LUR models had high spatial and temporal resolutions to help reduce measurement error. Spatially, exposure estimates were resolved to small geographic areas at a postal code level. Exposure errors derived from these models are typically Berkson-like, in which case the error has little to no effect on ORs but rather increases the width of CIs.42 However, trimester-specific analyses were limited by the high correlation between trimester exposures. Incorporating residential history during pregnancy into exposure estimates could potentially increase exposure variation and reduce correlation of trimester exposures if pregnant women move between areas with noticeably different air pollution concentrations. Direct exposure assessment is not possible in a study of this size, particularly for exposures for the full 9-month duration of a pregnancy and a study using a retrospective design. Sampling pregnant women from the time of conception and prospectively measuring their exposures would not be possible, and any direct measurement during pregnancy would be especially challenging, because ASD is a rare outcome. In a study comparing short-term personal exposures to air pollution in pregnant women vs ambient air pollution estimates from the LUR models, the ability of these models to characterize high-resolution spatial variability in exposure was also reflected in personal exposure measurements, with increased correlation when both home and work locations were considered.33 Accounting for time-activity patterns during pregnancy could help improve personal exposure estimates,33 although pregnant women tend to increasingly spend more time at home during the more advanced part of pregnancy.43
Our study design only tested associations for single pollutants. Assessment of pollutant mixtures are an important future research direction.
Conclusions
Using strict person-specific diagnostic criteria and high-resolution exposure estimates applied to a large population-based birth cohort, we observed an association between exposure to NO and increased risk of ASD in a metropolitan area with relatively low ambient air pollution levels. These findings suggest that reducing exposure to NO for pregnant women may be associated with a reduction in ASD incidence.
eTable 1. Median monthly average prenatal exposure estimates among children born in Metro Vancouver, Canada, between 2004–2009.
eTable 2. Sensitivity analyses of odds of ASD for prenatal exposure to air pollutants.
eTable 3. Comparison of adjusted odds ratios between individual trimester models versus a mutually adjusted trimester model.
eFigure 1. Truncated land use regression model for PM2.5 (2003) in Metro Vancouver, Canada.
eFigure 2. Truncated land use regression model for NO (2003) in Metro Vancouver, Canada.
eFigure 3. Truncated land use regression model for NO2 (2003) in Metro Vancouver, Canada.
eFigure 4. Monthly average concentrations of PM2.5, NO, and NO2 from Air Quality Monitoring Stations in Metro Vancouver, 2003–2009.
eFigure 5. Correlation matrix of air pollutant exposures across pregnancy and trimesters.
eFigure 6. Overall and sex-stratified odds of ASD for prenatal exposure to air pollutants during pregnancy and trimesters among children born in Metro Vancouver, Canada, between 2004–2009.
eReferences.
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Christensen DL, Baio J, Van Naarden Braun K, et al. ; Centers for Disease Control and Prevention (CDC) . Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23. doi: 10.15585/mmwr.ss6503a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Autism spectrum disorder (ASD): data & statistics. https://www.cdc.gov/ncbddd/autism/data.html. Published 2016. Accessed March 1, 2018.
- 4.Ofner M, Coles A, Decou M Lou, et al. Autism spectrum disorder among children and youth in Canada 2018: a report of the National Autism Spectrum Disorder Surveillance System. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/autism-spectrum-disorder-children-youth-canada-2018.html. Published 2018. Accessed August 20, 2018.
- 5.Dietert RR, Dietert JM, Dewitt JC. Environmental risk factors for autism. Emerg Health Threats J. 2011;4(1):7111. doi: 10.3402/ehtj.v4i0.7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120(7):a258-a260. doi: 10.1289/ehp.1104285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam J, Sutton P, Kalkbrenner A, et al. . A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11(9):e0161851. doi: 10.1371/journal.pone.0161851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: a review and meta-analysis. Environ Res. 2016;151:763-776. doi: 10.1016/j.envres.2016.07.030 [DOI] [PubMed] [Google Scholar]
- 9.Morales-Suárez-Varela M, Peraita-Costa I, Llopis-González A. Systematic review of the association between particulate matter exposure and autism spectrum disorders. Environ Res. 2017;153:150-160. doi: 10.1016/j.envres.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 10.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles County, California. Environ Health Perspect. 2013;121(3):380-386. doi: 10.1289/ehp.1205827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung C-R, Lin Y-T, Hwang B-F. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One. 2013;8(9):e75510. doi: 10.1371/journal.pone.0075510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70(1):71-77. doi: 10.1001/jamapsychiatry.2013.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raz R, Roberts AL, Lyall K, et al. . Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect. 2015;123(3):264-270. doi: 10.1289/ehp.1408133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalkbrenner AE, Windham GC, Serre ML, et al. . Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26(1):30-42. doi: 10.1097/EDE.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 15.Talbott EO, Arena VC, Rager JR, et al. . Fine particulate matter and the risk of autism spectrum disorder. Environ Res. 2015;140:414-420. doi: 10.1016/j.envres.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 16.Raz R, Levine H, Pinto O, Broday DM, Weisskopf Y. Traffic-related air pollution and autism spectrum disorder: a population-based nested case-control study in Israel. Am J Epidemiol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong T, Dalman C, Wicks S, et al. . Perinatal exposure to traffic-related air pollution and autism spectrum disorders. Environ Health Perspect. 2017;125(1):119-126. doi: 10.1289/EHP118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong T, Almqvist C, Bölte S, et al. . Exposure to air pollution from traffic and neurodevelopmental disorders in Swedish twins. Twin Res Hum Genet. 2014;17(6):553-562. doi: 10.1017/thg.2014.58 [DOI] [PubMed] [Google Scholar]
- 19.Guxens M, Ghassabian A, Gong T, et al. . Air pollution exposure during pregnancy and childhood autistic traits in four European population-based cohort studies: the ESCAPE project. Environ Health Perspect. 2016;124(1):133-140. doi: 10.1289/ehp.1408483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlayne R, Green B, Barer ML, Hertzman C, Lawrence WJ, Sheps SB. Creating a population-based linked health database: a new resource for health services research. Can J Public Health. 1998;89(4):270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canadian Institute for Health Information Population Data BC: discharge abstract database (hospital separations); data extract from the Ministry of Health, 2015. https://www.popdata.bc.ca/data/health/dad. Published 2016. Accessed August 22, 2018.
- 22.Canadian Institute for Health Information Population Data BC: consolidation file (MSP registration & premium billing); data extract from the Ministry of Health, 2015. https://www.popdata.bc.ca/data/population/consolidationfile. Published 2015. Accessed August 22, 2018.
- 23.Canadian Institute for Health Information Population Data BC: medical services plan (MSP) payment information file; data extract from the Ministry of Health, 2015. https://www.popdata.bc.ca/data/health/msp. Published 2015. Accessed August 22, 2018.
- 24.Perinatal Services BC Population Data BC: British Columbia perinatal data registry; data extract from Perinatal Services BC, 2015. https://www.popdata.bc.ca/data/health/PSBC. Published 2015. Accessed August 22, 2018.
- 25.BC Vital Statistics Agency Population Data BC: vital statistics births: data extract from BC Vital Statistics Agency, 2015. https://www.popdata.bc.ca/data/population/vsbirths. Published 2015. Accessed August 22, 2018.
- 26.BC Vital Statistics Agency Population Data BC: vital statistics births: data extract from BC Vital Statistics Agency, 2015. https://www.popdata.bc.ca/data/population/vsdeaths. Published 2015. Accessed August 22, 2018.
- 27.Statistics Canada Statistics Canada income band data (catalogue number 13C0016); data extract from Population Data BC, 2016. https://www.popdata.bc.ca/data/demographic/incomeband. Published 2009. Accessed August 22, 2018.
- 28.Perinatal Services BC Resources for health authority analysts. http://www.perinatalservicesbc.ca/health-professionals/data-surveillance/perinatal-data-registry/resources-for-analysts. Published 2015. Accessed January 1, 2018.
- 29.Clark C, Sbihi H, Tamburic L, Brauer M, Frank LD, Davies HW. Association of long-term exposure to transportation noise and traffic-related air pollution with the incidence of diabetes: A prospective cohort study. Environ Health Perspect. 2017;125(8):087025. doi: 10.1289/EHP1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson SB, Beckerman B, Jerrett M, Brauer M. Application of land use regression to estimate long-term concentrations of traffic-related nitrogen oxides and fine particulate matter. Environ Sci Technol. 2007;41(7):2422-2428. doi: 10.1021/es0606780 [DOI] [PubMed] [Google Scholar]
- 31.Dua V. Standards and guidelines for the assessment and diagnosis of young children with autism spectrum disorder in British Columbia: an evidence-based report prepared for the British Columbia Ministry of Health Planning. http://www.phsa.ca/Documents/asd_standards_0318.pdf. Published 2003. Accessed August 20, 2018.
- 32.Wang R, Henderson SB, Sbihi H, Allen RW, Brauer M. Temporal stability of land use regression models for traffic-related air pollution. Atmos Environ. 2013;64:312-319. doi: 10.1016/j.atmosenv.2012.09.056 [DOI] [Google Scholar]
- 33.Nethery E, Leckie SE, Teschke K, Brauer M. From measures to models: an evaluation of air pollution exposure assessment for epidemiological studies of pregnant women. Occup Environ Med. 2008;65(9):579-586. doi: 10.1136/oem.2007.035337 [DOI] [PubMed] [Google Scholar]
- 34.Marshall JD, Nethery E, Brauer M. Within-urban variability in ambient air pollution: comparison of estimation methods. Atmos Environ. 2008;42(6):1359-1369. doi: 10.1016/j.atmosenv.2007.08.012 [DOI] [Google Scholar]
- 35.Sbihi H, Allen RW, Becker A, et al. . Perinatal exposure to traffic-related air pollution and atopy at 1 year of age in a multi-center Canadian birth cohort study. Environ Health Perspect. 2015;123(9):902-908. doi: 10.1289/ehp.1408700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metro Vancouver 2014. Lower Fraser Valley air quality monitoring report. http://www.metrovancouver.org/services/air-quality/AirQualityPublications/2014_LFV_AQ_Monitoring_Report.pdf. Published 2015. Accessed August 23, 2018.
- 37.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116(5):680-686. doi: 10.1289/ehp.10952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith RB, Fecht D, Gulliver J, et al. . Impact of London’s road traffic air and noise pollution on birth weight: retrospective population based cohort study. BMJ. 2017;359:j5299. doi: 10.1136/bmj.j5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampi KM, Lehtonen L, Tran PL, et al. . Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr. 2012;161(5):830-836. doi: 10.1016/j.jpeds.2012.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol. 2012;206(4):314.e1-314.e9. doi: 10.1016/j.ajog.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leavey A, Zwaigenbaum L, Heavner K, Burstyn I. Gestational age at birth and risk of autism spectrum disorders in Alberta, Canada. J Pediatr. 2013;162(2):361-368. doi: 10.1016/j.jpeds.2012.07.040 [DOI] [PubMed] [Google Scholar]
- 42.Sheppard L, Burnett RT, Szpiro AA, et al. . Confounding and exposure measurement error in air pollution epidemiology. Air Qual Atmos Health. 2012;5(2):203-216. doi: 10.1007/s11869-011-0140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nethery E, Brauer M, Janssen P. Time-activity patterns of pregnant women and changes during the course of pregnancy. J Expo Sci Environ Epidemiol. 2009;19(3):317-324. doi: 10.1038/jes.2008.24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Median monthly average prenatal exposure estimates among children born in Metro Vancouver, Canada, between 2004–2009.
eTable 2. Sensitivity analyses of odds of ASD for prenatal exposure to air pollutants.
eTable 3. Comparison of adjusted odds ratios between individual trimester models versus a mutually adjusted trimester model.
eFigure 1. Truncated land use regression model for PM2.5 (2003) in Metro Vancouver, Canada.
eFigure 2. Truncated land use regression model for NO (2003) in Metro Vancouver, Canada.
eFigure 3. Truncated land use regression model for NO2 (2003) in Metro Vancouver, Canada.
eFigure 4. Monthly average concentrations of PM2.5, NO, and NO2 from Air Quality Monitoring Stations in Metro Vancouver, 2003–2009.
eFigure 5. Correlation matrix of air pollutant exposures across pregnancy and trimesters.
eFigure 6. Overall and sex-stratified odds of ASD for prenatal exposure to air pollutants during pregnancy and trimesters among children born in Metro Vancouver, Canada, between 2004–2009.
eReferences.