Abstract
Importance
Many medicines prescribed to children have not been studied or formally approved for pediatric use. The Pediatric Research Equity Act of 2003 authorized the US Food and Drug Administration (FDA) to require pediatric clinical studies.
Objective
To evaluate the characteristics, completion rate, and transparency of study design and results for mandatory pediatric postmarketing studies required under the Pediatric Research Equity Act.
Design and Setting
A retrospective cohort study was conducted of pediatric postmarketing studies required for new drugs and new indications approved by the FDA between January 1, 2007, and December 31, 2014, with follow-up through December 1, 2017. Information on the status, design, and results of pediatric studies was obtained from publicly available FDA databases and ClinicalTrials.gov, direct communication with the FDA, and searches of MEDLINE, EMBASE, and Web of Science for peer-reviewed publications.
Main Outcomes and Measures
Characteristics and transparency of pediatric studies, results reporting (in ClinicalTrials.gov, peer-reviewed literature, or FDA documents), and availability of pediatric information in drug labels. Rates and times to study completion were evaluated using Cox proportional hazards regression models.
Results
Between 2007 and 2014, the FDA approved 114 new drugs and new indications for already approved drugs that were subject to Pediatric Research Equity Act requirements. These drugs were associated with 222 required pediatric postmarketing clinical studies. Overall, 75 pediatric studies (33.8%) were completed as of December 1, 2017. The rates of completion were significantly lower for efficacy studies (38 of 132 [28.8%]) compared with pharmacokinetic studies (19 of 34 [55.9%]; adjusted hazard ratio, 0.31; 95% CI, 0.12-0.82). Information on randomization, blinding, comparator, end point, and study size could not be identified for 74 studies (33.3%), and no reason for discontinuation was provided for 29 of the 42 discontinued studies (69.0%). Among the completed studies, the results were reported for 57 (76.0%). At the time of approval, 18 of 114 drug approvals (15.8%) had any pediatric efficacy, safety, or dosing information in their labels. After a median duration of follow-up of 6.8 years (interquartile range, 4.7-9.1 years), 47 of 114 of drug labels (41.2%) had any pediatric information.
Conclusions and Relevance
Only 33.8% of mandatory pediatric postmarketing studies have been completed after a median follow-up of 6.8 years, and most drug labels do not include information important for pediatric use. To improve evidence-based prescribing of medicines to children, more timely completion of pediatric drug studies is needed.
This cohort study evaluates the characteristics, completion rate, and transparency of study design and results for mandatory pediatric postmarketing studies required under the Pediatric Research Equity Act.
Key Points
Question
What are the characteristics, completion rate, and transparency of pediatric postmarketing studies required under the US Pediatric Research Equity Act for drug approvals from 2007 to 2014?
Findings
This cohort study found that only 33.8% of pediatric postmarketing studies mandated by the Pediatric Research Equity Act have been completed after a median follow-up of 6.8 years, and 41.2% of drug labels include any pediatric data. Information about the characteristics and study design could not be identified for 33% of pediatric studies.
Meaning
Many pediatric studies mandated by the Pediatric Research Equity Act are not completed in a timely fashion, and many drug labels continue to lack any pediatric information.
Introduction
Many medicines prescribed to children have not been studied in this patient population or formally approved for pediatric use.1,2 Across outpatient and inpatient pediatric care, rates of off-label prescribing are estimated to range from 62% to 85% in the United States.3,4,5 Widespread off-label use of medicines in pediatric care raises several concerns. Patients may be prescribed treatments that are not effective or physicians may withhold potentially effective therapies because they are not indicated for pediatric use. In addition, the off-label use of medicines is associated with an increased risk of adverse drug reactions in children.6,7,8
To address this gap in the evidence base, the Pediatric Research Equity Act (PREA), signed into law in 2003, authorized the US Food and Drug Administration (FDA) to require clinical studies assessing the safety and efficacy of new drugs—as well as new indications, dosage forms, and routes of administration of already approved products—in all relevant pediatric subpopulations (Box). The statute required pediatric studies to be submitted before the drug can be approved, reflecting the hope that patients and physicians will have access to pediatric data as soon as a new drug becomes available on the market.9 However, the legislation allowed pharmaceutical companies to request deferrals of pediatric studies until after approval in certain cases. In 2007, the FDA Amendments Act amended PREA to designate all deferred studies as postmarketing studies and to require better tracking by the FDA of these required pediatric studies. It also established financial and other penalties for companies that do not comply with the pediatric postmarketing requirements.
Box. Summary of the Pediatric Research Equity Act.
Requirement
Companies must conduct studies “(i) to assess the safety and effectiveness of the drug or the biological product for the claimed indications in all relevant pediatric subpopulations; and (ii) to support dosing and administration for each relevant pediatric subpopulation.”
Scope
-
Applies to FDA approvals of new drugs or biologics, as well as any product application or supplements to applications for:
New active ingredient
New indication
New dosage form or dosing regimen
New route of administration
Excluded Products
Does not apply to products designated as treating a rare disease (affecting fewer than 200 000 people in the United States) under the Orphan Drug Acta
The FDA may also waive requirements for some or all pediatric age groups if necessary studies would be “impossible or highly impracticable” (eg, disease occurs primarily in adult populations), evidence strongly suggests the drug is ineffective or unsafe, or if reasonable attempts to produce a pediatric formulation have failed.
Indication
Limited to indication(s) approved in adults
Deferral
The FDA can grant deferral of submission of required pediatric data until after approval. In addition, sponsors may request extensions of deferrals.
Despite PREA’s goal of ensuring earlier access to information on drug approvals in the pediatric population, most studies required under PREA have been deferred,10,11 mirroring the broader shift of evidence collection from before to after regulatory approval in recent years.12,13,14,15 In 2012, the National Academy of Medicine recommended that the FDA improve the timeliness of completion of pediatric studies required under PREA and expand public access to information from these studies.1 However, in December 2017, it was reported that there was a backlog of more than 500 pediatric studies for approved drugs.16
As PREA is the primary mechanism for generating evidence on the safety, efficacy, and appropriate use of new drugs in children, we sought to examine the performance of this policy. In this study, we evaluated the characteristics, completion rate, and transparency of the design and results of pediatric postmarketing studies required by the FDA under PREA for new drugs and new indications for already approved drugs in the 7-year period from 2007 to 2014. In addition, we assessed the availability of pediatric information in product labels.
Methods
Study Cohort
We identified all new drugs and supplemental indications (ie, new indications for previously approved drugs) approved by the FDA between January 1, 2007, and December 31, 2014, using the FDA’s Drugs@FDA database.17,18 The study time frame was chosen to allow at least 3 years of follow-up from the date of FDA approval.
For all identified new drugs and supplemental indications, we reviewed the FDA’s approval letters and review dossiers to determine whether any pediatric studies had been required under PREA. Drugs intended to treat rare diseases affecting fewer than 200 000 people in the United States (Orphan Drug Act–designated drugs) are statutorily exempted from PREA requirements.19 In addition, the FDA may waive the need for pediatric studies in certain cases; for example, if a disease occurs primarily in adults (eg, pediatric study requirements were waived for aflibercept when it was approved for a new indication of macular edema after retinal vein occlusion, because pediatric studies for this indication were considered impossible or highly impracticable) (Box). Our study cohort comprised all pediatric studies required by the FDA at the time of approval for new drugs and supplemental indications with PREA requirements.
Data Extraction and Outcome Measures
Using the FDA’s approval letters and public databases, we extracted information on the scope of the pediatric study requirements: planned completion date, indication, study population, and, if provided by the FDA, study design and number of participants. We included both clinical trials and observational clinical studies. To obtain additional information on study design and conduct, we linked all included pediatric studies to ClinicalTrials.gov entries by searching the registry for the study name or a combination of the drug name, indication, study population and age group, study type, and planned completion date. We categorized studies into 3 mutually exclusive groups, as relating primarily to efficacy (ie, inclusion of at least 1 efficacy outcome as a primary end point), safety, or pharmacokinetics and pharmacodynamics (PK/PD). We identified whether any pediatric subpopulation was planned to be enrolled, using the following FDA definitions for pediatric age groups: neonates (<28 days), infants (1-23 months), children (2-11 years), and adolescents (12-17 years).20,21 Using methods described previously,18,22 we categorized study end points as clinical outcomes (clinical outcomes or scales) or surrogate measures (laboratory, radiographic, or other measures believed to predict clinical benefit).
We used a comprehensive search strategy to identify the status of all included studies through the date of database lock on December 1, 2017. First, we matched our database of pediatric studies to the FDA’s Postmarket Requirements and Commitments data file, which is updated on a quarterly basis and provides information on postmarketing studies for drugs regulated by the FDA.23,24 We also identified the current study status on ClinicalTrials.gov and, for studies that appeared to be not yet completed, supplemented this with a manual review of all FDA labeling and approval documents on the Drugs@FDA database to identify any notice from the FDA that the study had been completed or discontinued. Finally, for studies with a status that could not be definitively determined from FDA databases or on ClinicalTrials.gov (approximately 5% of cases; eTable 1 in the Supplement), we communicated directly with the FDA to establish the current status.
Our co-primary outcome measures were study completion rate and results reporting. We considered a study to be completed if it was designated by the FDA as fulfilled (meaning the FDA was satisfied that the company had met the terms of the postmarketing study requirement) or, if that information was unavailable from the FDA, the study was marked as completed in ClinicalTrials.gov. Discontinued studies included those that had been terminated by the company before or after initiation and those classified as terminated or withdrawn in ClinicalTrials.gov. Pending studies were those that had not been completed or discontinued.
Results reporting was defined as results posted on ClinicalTrials.gov, publication in a peer-reviewed journal, and/or results published in FDA documents. To identify peer-reviewed publications, we searched PubMed/Medline, EMBASE, and Web of Science using the study name or a combination of the drug name, indication, study population and age group, study type, planned completion date, and, if available, ClinicalTrials.gov identifier. We extracted the date that results were posted on ClinicalTrials.gov and the date of first publication (earlier of the first publication online or publication date in the citation). We also assessed the drug labeling at the time of approval and at the time of database lock to determine whether any pediatric efficacy, safety, and/or dosing information was available.17
Finally, we evaluated the transparency of the pediatric study design by assessing whether any information was available from the FDA or on ClinicalTrials.gov for each of 5 key study characteristics (randomization [yes or no], blinding [double, single, or none or open label], comparator [placebo, active, or other or none], end point [clinical or surrogate], and study size). For discontinued studies, we determined whether any reason for discontinuation was provided.
All data were independently collected by 2 of us (T.J.H. and L.O.), with validation of data extraction by another of us (F.T.B.). Disagreements were resolved by consensus among all authors.
Statistical Analysis
Descriptive statistics were used to characterize the size and design of pediatric studies as well as the proportion of drug labels that had any pediatric information at the time of approval and as of December 1, 2017. We calculated the unadjusted rates and times to study completion and results reporting, both from the date of initial FDA approval. We used Cox proportional hazards regression models to assess differences in time to study completion for efficacy studies vs safety and PK/PD studies, with censoring of studies not completed before the end of follow-up as of December 1, 2017. To compare completed studies with and without results reporting, the Fisher exact test was used to assess differences in the proportion of studies that were efficacy studies and the Mann-Whitney test was used to assess differences in median study duration. Statistical analyses were performed using Stata, version 12 (StataCorp). All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
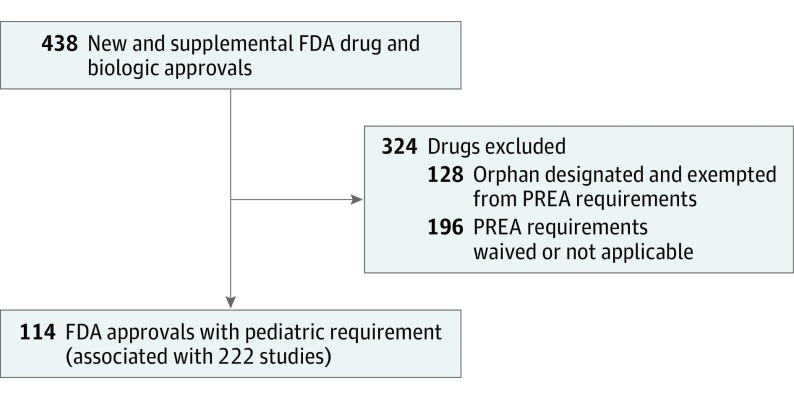
The FDA approved 438 new drugs and new indications for already approved drugs between 2007 and 2014, of which 114 were subject to PREA study requirements, including 84 new drugs and 30 supplemental indications (Figure 1; eTable 2 in the Supplement). The FDA required 222 pediatric postmarketing clinical studies for these 114 drug approvals (median, 2 studies per approval; interquartile range [IQR], 1-2) (Table 1). The 3 most common clinical indications were seizures (21 studies), type 2 diabetes (20 studies), and HIV (17 studies). By study type, 132 studies (59.5%) were efficacy studies, 56 (25.2%) were safety studies, and 34 (15.3%) were PK/PD studies. There were 62 studies (27.9%) that planned enrollment of neonates, 80 studies (36.0%) that planned enrollment of infants, 162 studies (73.0%) that planned enrollment of children, and 176 studies (79.3%) that planned enrollment of adolescents. Only 27 studies (12.2%) planned to enroll only neonates or infants, and 6 studies (2.7%) planned to enroll neonates exclusively.
Figure 1. Study Flowchart.
FDA indicates US Food and Drug Administration; PREA, Pediatric Research Equity Act.
Table 1. Characteristics of Pediatric Studies for Drugs Approved by the FDA in 2007-2014.
| Characteristic | No. (%) |
|---|---|
| FDA Drug Approvals (n = 114) | |
| FDA approval year | |
| 2007 | 12 (10.5) |
| 2008 | 22 (19.3) |
| 2009 | 13 (11.4) |
| 2010 | 7 (6.1) |
| 2011 | 15 (13.2) |
| 2012 | 13 (11.4) |
| 2013 | 13 (11.4) |
| 2014 | 19 (16.7) |
| Approval type | |
| New drug or biologic | 84 (73.7) |
| Supplemental | 30 (26.3) |
| Drug type | |
| Pharmacologic | 98 (86.0) |
| Biologic | 16 (14.0) |
| Associated Pediatric Studies (n = 222) | |
| Study type | |
| Efficacy | 132 (59.5) |
| Safety | 56 (25.2) |
| PK/PD | 34 (15.3) |
| Current study status | |
| Completed | 75 (33.8) |
| Pending | 105 (47.3) |
| Discontinued | 42 (18.9) |
| Enrollment of pediatric subpopulationa | |
| Any neonates (age, <28 d) | 62 (27.9) |
| Any infants (age, 28 d to <24 mo) | 80 (36.0) |
| Any children (age, 2-11 y) | 162 (73.0) |
| Any adolescents (age, ≥12 y) | 176 (79.3) |
Abbreviations: FDA, US Food and Drug Administration; PD, pharmacodynamics; PK, pharmacokinetics.
Studies may enroll multiple pediatric subpopulations.
Characteristics of Pediatric Clinical Studies
For the 157 studies with publicly available information on study size, 17 333 pediatric participants were enrolled or planned to be enrolled, with a median of 83 participants per study (IQR, 38-160) (Table 2). Overall, 88 studies (56.1%) included fewer than 100 participants and 129 studies (82.2%) included fewer than 200 participants. The median number of participants was 132 (IQR, 78-230) for efficacy studies, 49 (IQR, 29-84) for safety only studies, and 33 (IQR, 17-59) for PK/PD studies.
Table 2. Characteristics of Pediatric Studies Required Under PREA.
| Characteristic | Study, No./Total No. (%) | |
|---|---|---|
| All Studiesa | Efficacy Studies Only | |
| Randomization | ||
| Yes | 96/188 (51.1) | 88/114 (77.2) |
| No | 92/188 (48.9) | 26/114 (22.8) |
| Blinding | ||
| Double-blinded | 80/187 (42.8) | 75/113 (66.4) |
| Single-blinded | 7/187 (3.7) | 6/113 (5.3) |
| None (open-label) | 100/187 (53.5) | 32/113 (28.3) |
| Comparator | ||
| Active | 38/182 (20.9) | 35/109 (32.1) |
| Placebo | 44/182 (24.2) | 43/109 (39.4) |
| None | 100/182 (54.9) | 31/109 (28.4) |
| End point | ||
| Clinical outcome | 21/162 (13.0) | 21/90 (23.3) |
| Clinical scale | 19/162 (11.7) | 19/90 (21.1) |
| Surrogate measure and otherb | 122/162 (75.3) | 50/90 (55.6) |
| Study participants, median (IQR), No. | ||
| Total | 83 (38-160) | 132 (78-230) |
| Intervention group | 60 (34-104) | 86 (50-135) |
Abbreviations: IQR, interquartile range; PREA, Pediatric Research Equity Act.
Values are for studies with available information only.
Includes studies with safety and pharmacokinetics and pharmacodynamics outcomes.
Among studies with information available on any of the other key study design characteristics, 51.1% (96 of 188) of pediatric studies were randomized, 46.5% (87 of 187) had some blinding, 45.1% (82 of 182) had an active or placebo comparator, and 24.7% (40 of 162) used clinical end points. Most efficacy studies had randomized clinical designs (78 of 109 [71.6%]), although 55.6% of efficacy studies (50 of 90) used surrogate outcomes and only 32.1% (35 of 109) used an active comparator.
Completion of Mandatory Pediatric Studies
Pediatric studies were planned to be completed at a median of 5.1 years (IQR, 3.8-7.0 years) after approval. The median planned study durations were 5.4 years for efficacy studies, 5.5 years for safety only studies, and 3.3 years for PK/PD studies.
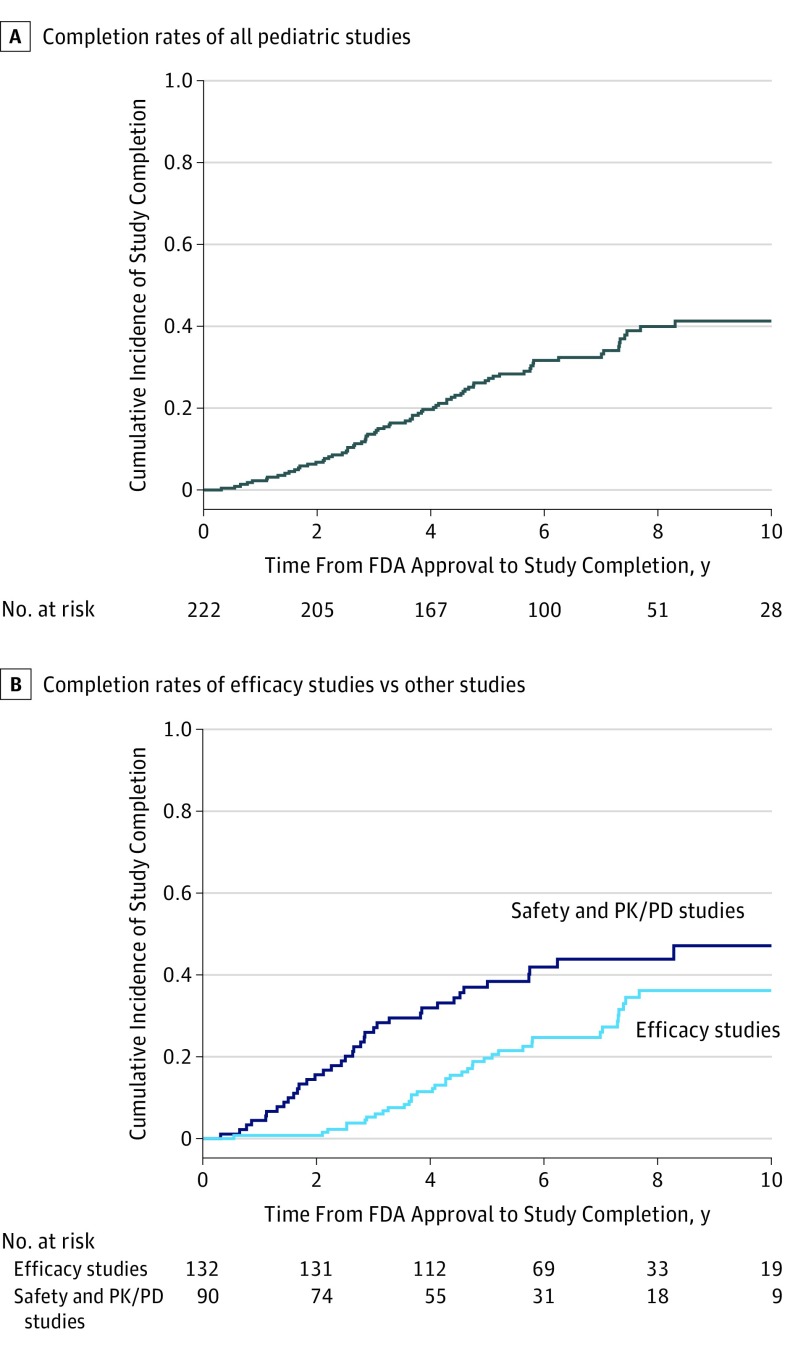
As of December 1, 2017, allowing for a median follow-up of 6.8 years (IQR, 4.7-9.1 years), 75 of 222 pediatric clinical studies (33.8%) were completed, 105 (47.3%) were pending, and 42 (18.9%) were discontinued. The rate of study completion 1 year after FDA approval was 2.3%, 2 years after FDA approval was 6.8%, and 5 years after FDA approval was 26.7% (Figure 2). The rate of completion for efficacy studies was 28.8% (38 of 132) and for safety-only studies was 32.1% (18 of 56), compared with 55.9% (19 of 34) for PK/PD studies. There were no statistically significant differences in completion rates by pediatric age group. In multivariable Cox proportional hazards regression models, efficacy studies were significantly less likely to be completed than were PK/PD studies (hazard ratio, 0.31; 95% CI, 0.12-0.82; P = .02).
Figure 2. Time to Completion of Pediatric Studies Required by the US Food and Drug Administration (FDA).
A, Cumulative incidence rates of study completion for all pediatric studies required by the FDA. B, Cumulative incidence rates of study completion stratified by efficacy studies vs safety and pharmacokinetics and pharmacodynamics (PK/PD) studies. Log-rank P = .005.
Transparency of Pediatric Study Design
With follow-up through December 1, 2017, information was available for 188 studies (84.7%) on randomization, 187 (84.2%) on blinding, 182 (82.0%) on comparator, 162 (73.0%) on end point, and 157 (70.7%) on study size (Table 3). Overall, information on all 5 of these design characteristics was available for 148 studies (66.7%). Any reason for discontinuation was provided for 13 of 42 discontinued pediatric studies (31.0%): 5 studies were discontinued because the drug was withdrawn from the market, 3 for business reasons, 3 for safety issues, and 2 for other reasons. Of the 42 discontinued studies, 13 (31.0%) were discontinued after enrollment of participants had begun.
Table 3. Transparency of Study Design and Reason for Discontinuation of Pediatric Studies.
| Design Characteristics | Study, No. (%) |
|---|---|
| Any information on randomization | 188 (84.7) |
| Any information on blinding | 187 (84.2) |
| Any information on comparator | 182 (82.0) |
| Any information on end point | 162 (73.0) |
| Any information on study size | 157 (70.7) |
| Any information on all 5 design characteristicsa | 148 (66.7) |
| Any reason provided for discontinuation | 13/42 (31.0) |
The 5 design characteristics were randomization, blinding, comparator, end point, and study size (number of participants).
Availability of Pediatric Study Results and Labeling Information
Among the 75 completed pediatric studies, the results were reported for 57 (76.0%) in ClinicalTrials.gov, a peer-reviewed journal, or the FDA review summary. The results for 34 (45.3%) were published in a journal, the results for an additional 21 (28.0%) were posted in a trial registry, and the results for 2 (2.7%) were available only in FDA review summaries. There was no difference in study duration or proportion of efficacy studies between completed studies with vs without results reporting.
At the time of approval, 96 of 114 drug labels (84.2%) had no information on efficacy, safety, or dosing for children. The labels for 29 of these 96 drugs (30.2%) were subsequently updated with pediatric-specific information. Overall, as of December 1, 2017, a total of 47 drug labels (41.2%) included any pediatric information. Labels for 44 drug approvals (38.6%) had information on pediatric efficacy, 44 (38.6%) had information on pediatric safety, and 40 (35.1%) had information on pediatric dosing or PK/PD properties.
Discussion
In this analysis of pediatric postmarketing studies mandated by the FDA for new and supplemental drug approvals between 2007 and 2014, we found that most studies had not been completed as of December 1, 2017, and most drug labels continued to lack pediatric information. The rate of study completion 5 years after FDA approval was only 26.7%, and, after a median follow-up of 6.8 years, 41.2% of drug labels contained any pediatric efficacy, safety, or dosing information.
Despite legislation aimed at ensuring that drugs are studied in children, we found that patients and clinicians may face substantial delays after a new drug is approved before relevant pediatric information becomes available. Completion rates of pediatric studies, and particularly pediatric efficacy studies, appear to be lower than those for other FDA-mandated postmarketing studies, such as required studies of serious safety risks and confirmatory studies for drugs approved based on surrogate measures (accelerated approval) or animal studies (animal rule approvals).16,25
Once pediatric studies were completed, results from 76.0% were reported publicly, which is consistent with estimates for pediatric studies registered from 2008 to 201026 and for pediatric studies completed from 2009 to 2013.27 This rate of completion represents progress compared with the more limited reporting found in older analyses of pediatric studies completed before 2007.28,29,30 Gaps remain, however, in public availability of details related to the design of mandatory pediatric studies. We could not identify basic information about the characteristics and study design for one-third of pediatric postmarketing studies, and no reason for discontinuation was provided by either the company or the FDA in 69.0% of cases. Greater transparency is needed on the PREA requirements for all approved drugs, including the anticipated design and research questions of FDA-mandated pediatric studies. Companies already must submit pediatric study plans prior to approval (within 60 days of the end of the Phase 2 studies),31 and the FDA could disclose details of these pediatric study plans at the time of approval.
Although PREA has contributed to important new research for children, stronger policies are needed to ensure the timely availability of pediatric information for most new medicines. During the past decade, the annual number of pediatric studies required under PREA has nearly tripled.32 Two different government agencies in 201433 and 2016,34 as well as the National Academy of Medicine in 2012,1 have called for greater FDA oversight to ensure that these studies are being conducted. Although the FDA cannot withdraw approval for a drug if a manufacturer fails to comply with PREA, the FDA can determine that the drug is “misbranded” and initiate injunction or seizure proceedings. The FDA can also publicly post noncompliance letters for overdue PREA studies. However, to our knowledge, to date, no financial penalties or enforcement proceedings have been brought against manufacturers for noncompliance with PREA, and only 31 noncompliance letters have been issued.35 The FDA should be more willing to use its existing enforcement authority in cases of unreasonable delays.
Our findings may have implications for postmarketing research more broadly. The experience with PREA was cited in the passage of the FDA Amendments Act in 2007, which expanded the types of postmarketing studies that the FDA could require. New strategies to ensure completion of postmarketing studies could be tested first in the pediatric context before being applied to other types of postmarketing requirements. For example, the FDA could consider requiring certain pediatric studies (eg, those with the greatest potential importance for clinical practice) to begin enrollment of pediatric patients before approval. Such a policy would have precedent: in 2008, the FDA implemented a similar policy to ensure that companies developing antidiabetic therapies conduct cardiovascular safety trials.36,37
Limitations
Although our study allowed a median follow-up of 6.8 years, it is possible that more studies will be completed in the future, and more completed studies may report results. The reasons for study delays are multifactorial: relatively few established pediatric biomarkers, difficulties in participant recruitment, and varying obligations for pediatric studies between regulatory agencies internationally have been cited as possible contributors to study delays and noncompletion.38 Our study assesses the performance of US pediatric regulations, and future research would help elucidate the root causes of delays. In addition, we focused on studies required under PREA, and our results may not be generalizable to other types of pediatric research. In addition to PREA, Congress also passed the Best Pharmaceuticals for Children Act, which is voluntary and provides a financial incentive to companies if they perform certain requested pediatric studies. Thus, the Best Pharmaceuticals for Children Act also contributes to pediatric postmarketing studies. However, PREA is responsible for nearly 80% of pediatric drug studies completed for the FDA39 and approximately 70% of all pediatric labeling changes.40 As such, PREA remains by far the more important mechanism for pediatric postmarketing research.
Conclusions
More than a decade after enactment of pediatric legislation in the United States, important gaps remain in the availability of pediatric drug information. After a median follow-up of 6.8 years, only 33.8% of pediatric studies mandated by the FDA have been completed, and 41.2% of drug approvals include any pediatric labeling information. To reduce off-label and unsafe prescribing of medicines to children, mandatory pediatric research must be completed in a timely fashion and the results made widely available to clinicians and patients.
eTable 1. PREA Studies With Status Provided by the FDA
eTable 2. List of Included New Drugs and Supplemental Indications Approved by the FDA
Footnotes
Abbreviation: FDA, US Food and Drug Administration.
In final guidance published in July 2018, the FDA announced that it no longer intends to grant orphan drug designation to drugs for pediatric subpopulations of common diseases (eg, pediatric ulcerative colitis) unless the pediatric subpopulation can be considered a different disease from the disease in the adult population, which in the past has allowed certain companies to be exempted from required pediatric studies through orphan designation.
References
- 1.National Academy of Medicine (formerly Institute of Medicine) Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 2.Bourgeois FT, Hwang TJ. The Pediatric Research Equity Act moves into adolescence. JAMA. 2017;317(3):259-260. doi: 10.1001/jama.2016.18131 [DOI] [PubMed] [Google Scholar]
- 3.Bazzano AT, Mangione-Smith R, Schonlau M, Suttorp MJ, Brook RH. Off-label prescribing to children in the United States outpatient setting. Acad Pediatr. 2009;9(2):81-88. doi: 10.1016/j.acap.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 4.Czaja AS, Reiter PD, Schultz ML, Valuck RJ. Patterns of off-label prescribing in the pediatric intensive care unit and prioritizing future research. J Pediatr Pharmacol Ther. 2015;20(3):186-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanovska V, Rademaker CM, van Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134(2):361-372. doi: 10.1542/peds.2013-3225 [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency Evidence of Harm From Off-Label or Unlicensed Medicines in Children. EMEA/126327/2004. London, UK: European Medicines Agency; 2004. [Google Scholar]
- 7.Kirk CR, Gibbs JL, Thomas R, Radley-Smith R, Qureshi SA. Cardiovascular collapse after verapamil in supraventricular tachycardia. Arch Dis Child. 1987;62(12):1265-1266. doi: 10.1136/adc.62.12.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Noury J, Nardo JM, Healy D, et al. Restoring study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence. BMJ. 2015;351:h4320. doi: 10.1136/bmj.h4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration Guidance for industry: how to comply with the Pediatric Research Equity Act. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM077855.pdf. Accessed July 31, 2018.
- 10.US Food and Drug Administration Retrospective review of information submitted and actions taken in response to PREA 2003. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM197636.pdf. Accessed July 31, 2018.
- 11.US Food and Drug Administration CDER pediatric study deferrals and deferral extensions. https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/UCM210954.pdf. Accessed July 31, 2018.
- 12.Psaty BM, Meslin EM, Breckenridge A. A lifecycle approach to the evaluation of FDA approval methods and regulatory actions: opportunities provided by a new IOM report. JAMA. 2012;307(23):2491-2492. doi: 10.1001/jama.2012.5545 [DOI] [PubMed] [Google Scholar]
- 13.Pease AM, Krumholz HM, Downing NS, Aminawung JA, Shah ND, Ross JS. Postapproval studies of drugs initially approved by the FDA on the basis of limited evidence: systematic review. BMJ. 2017;357:j1680. doi: 10.1136/bmj.j1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woloshin S, Schwartz LM, White B, Moore TJ. The fate of FDA postapproval studies. N Engl J Med. 2017;377(12):1114-1117. doi: 10.1056/NEJMp1705800 [DOI] [PubMed] [Google Scholar]
- 15.Hudgins JD, Bacho MA, Olsen KL, Bourgeois FT. Pediatric drug information available at the time of new drug approvals: a cross-sectional analysis. Pharmacoepidemiol Drug Saf. 2018;27(2):161-167. doi: 10.1002/pds.4351 [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration Report on the performance of drug and biologics firms in conducting postmarketing requirements and commitments. https://www.gpo.gov/fdsys/pkg/FR-2017-12-08/html/2017-26470.htm. Published December 8, 2017. Accessed July 31, 2018.
- 17.US Food and Drug Administration Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed July 31, 2018.
- 18.Wang B, Kesselheim AS. Characteristics of efficacy evidence supporting approval of supplemental indications for prescription drugs in United States, 2005-14: systematic review. BMJ. 2015;351:h4679. doi: 10.1136/bmj.h4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourgeois FT, Hwang TJ. Improving the study of new medicines for children with rare diseases. JAMA Pediatr. 2018;172(1):7-9. doi: 10.1001/jamapediatrics.2017.4012 [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration Pediatric expertise for advisory panels: guidance for industry and FDA staff. https://www.fda.gov/RegulatoryInformation/Guidances/ucm082185.htm. Accessed July 31, 2018.
- 21.US Food and Drug Administration Pediatric exclusivity study age group. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/DataStandardsManualmonographs/ucm071754.htm. Accessed July 31, 2018.
- 22.Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-377. doi: 10.1001/jama.2013.282034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration Postmarket requirements and commitments. https://www.accessdata.fda.gov/scripts/cder/pmc/index.cfm. Accessed July 31, 2018.
- 24.US Food and Drug Administration Postmarketing requirements and commitments: frequently asked questions (FAQ). https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Post-marketingPhaseIVCommitments/ucm070766.htm. Accessed July 31, 2018.
- 25.Naci H, Smalley KR, Kesselheim AS. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA. 2017;318(7):626-636. doi: 10.1001/jama.2017.9415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pica N, Bourgeois F. Discontinuation and nonpublication of randomized clinical trials conducted in children. Pediatrics. 2016;138(3):e20160223. doi: 10.1542/peds.2016-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz ML, Xu J, Kashoki M, Lurie P. Publication and reporting of the results of postmarket studies for drugs required by the US Food and Drug Administration, 2009 to 2013. JAMA Intern Med. 2017;177(8):1207-1210. doi: 10.1001/jamainternmed.2017.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290(7):905-911. doi: 10.1001/jama.290.7.905 [DOI] [PubMed] [Google Scholar]
- 29.Benjamin DK Jr, Smith PB, Murphy MD, et al. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296(10):1266-1273. doi: 10.1001/jama.296.10.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin DK Jr, Smith PB, Sun MJ, et al. Safety and transparency of pediatric drug trials. Arch Pediatr Adolesc Med. 2009;163(12):1080-1086. doi: 10.1001/archpediatrics.2009.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration Pediatric study plans: content of and process for submitting initial pediatric study plans and amended initial pediatric study plans: guidance for industry. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM360507.pdf. Accessed July 31, 2018.
- 32.US Food and Drug Administration Pediatric studies—annual status summary. https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/UCM195000.pdf. Accessed July 31, 2018.
- 33.Office of Inspector General, Department of Health and Human Services FDA is issuing more postmarketing requirements, but challenges with oversight persist. https://oig.hhs.gov/oei/reports/oei-01-14-00390.pdf. Accessed July 31, 2018.
- 34.US Government Accountability Office FDA expedites many applications, but data for postapproval oversight need improvement. https://www.gao.gov/products/GAO-16-192. Published December 15, 2015. Accessed July 31, 2018.
- 35.US Food and Drug Administration Non-compliance letters under 505B(d)(1) of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/ucm343203.htm. Accessed July 31, 2018.
- 36.US Food and Drug Administration Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf. Accessed July 31, 2018.
- 37.Hwang TJ, Franklin JM, Kesselheim AS. Effect of US Food and Drug Administration’s cardiovascular safety guidance on diabetes drug development. Clin Pharmacol Ther. 2017;102(2):290-296. doi: 10.1002/cpt.705 [DOI] [PubMed] [Google Scholar]
- 38.Adamson PC. Unintended consequences of regulatory initiatives in childhood cancer drug development. JAMA Pediatr. 2013;167(10):886-887. doi: 10.1001/jamapediatrics.2013.2488 [DOI] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration Breakdown of FDAAA completed pediatric studies. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm190622.htm. Accessed July 31, 2018.
- 40.US Food and Drug Administration Pediatric and maternal health product development. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049867.htm. Accessed July 31, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. PREA Studies With Status Provided by the FDA
eTable 2. List of Included New Drugs and Supplemental Indications Approved by the FDA