Background
Osteoarthritis (OA) is the most common form of arthritis and a cause of chronic disability. OA remains an exciting challenge in terms of the development of this diagnosis and its disease‐modifying drugs, and the search for more effective treatment for OA‐related pain. This guideline provides practitioners with recommendations on diagnosis and treatment for people with OA.
On October 12, 2001, the Chinese government established a Health Ministry Fund for an Arthritis Education Program. In 2003, with this fund's support, a group of Chinese experts on orthopaedics and rheumatology were organized to draw up a draft guideline on the diagnosis and treatment of osteoarthritis. Although the published draft provided recommendations, it must be recognized that there has been considerable progress in the development of diagnosis and treatment for this disease 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 . Therefore, the draft requires extensive modification.
This Guideline is just an academic reference, and is to be be implemented according to the specific characteristics of patients, and the available medical equipment and environmental conditions. Please always read and follow relevant instructions before implementing any control and treatment measures.
Overview
OA is a joint disease characterized by fibrosis, osteophyte development, and articular cartilage damage. The exact causes of OA are unknown. However, a number of risk factors are commonly associated with the onset of the disease; these include ageing, obesity, inflammation, trauma, genetics, and other factors. The pathological features of the disorder are articular cartilage deterioration, subchondral cyst formation and bone sclerosis, osteophytes around joint margins, synovial hyperplasia, joint capsule contracture, ligament laxity or contracture, and muscle atrophy and weakness. Osteophytes or knee joint effusion may cause swelling in the joints of the hand.
OA most commonly affects patients who are middle‐aged and older, and is more common in female than male patients. The prevalence rate of OA reaches 50% among the people over 60 years of age. The rate hits 80% among the people over 75 years, with a disability rate as high as 53%. OA most often affects the hands, feet, spine, and large weight bearing joints, such as the knees and hips.
Classification
OA can be divided into primary and secondary types. Primary OA is commonly linked to middle and old age, when cartilage failure of unknown cause develops, predisposing factors including genetic factors and physical activity. Secondary OA is due to trauma, inflammation, joint instability, developmental diseases, repetitive mechanical stress, certain metabolic dysfunctions, or congenital diseases.
Clinical manifestations
Symptoms and physical signs
Joint pain and pain on pressure
In the early stages, joint pain is intermittent and dull, and rated as mild‐to‐moderate in severity. It may improve after rest but worsen after activity and is often related to the weather. In the advanced stages, the pain can be continuous and at night. Pressure on the joint can produce pain, which tends to be more marked when there is swelling of the joint.
Joint stiffness
Affected joints are stiff and tight when the patient gets up in the morning. This joint stiffness, also known as morning stiffness, can be alleviated by joint activity. The symptom can last from a few to ten min, rarely lasting more than 30 min. Low atmospheric pressure or high air humidity can worsen this symptom.
Joint swelling
Obvious swelling and deformation in the hand joints are often observed. Bony nodules may develop at the distal and proximal interphalangeal joints (called Heberden's and Bouchard's nodes, respectively).
Bony crepitation (fremitus)
There is bony crepitation (fremitus) during movement of the joint if the cartilage surface is damaged or has become rough. Typically, this symptom presents in OA of the knee.
Muscle weakness and impairment of movement
Muscle weakness around the affected joint is a common finding. Loss of strength in the muscles supporting the joint and impairment of surrounding soft tissue lead to uncoordinated movements which overstress the joint.
Laboratory tests
There is no definitive laboratory test for the diagnosis of OA. Routine blood test results are normal. Results of protein electrophoresis and values for immune complexes, complement, and so on are within the normal range. An OA patient with synovitis may have a slight increase in serum C‐creative protein (CRP) and erythrocyte sedimentation rate (ESR).
Radiology
Plain film radiographic findings include asymmetrical narrow joint spaces, subchondral sclerosis, bone cysts, osteophytosis, and hyperosteogeny at the joint margins. Loose bodies or dysarthrosis can be observed in some cases.
Essentials of diagnosis
Diagnosis of OA is based on the symptoms, physical signs, X‐ray manifestations and laboratory tests. A flow chart is proposed to aid the diagnosis (Fig. 1). The proposed diagnostic criteria for OA of the knee and hip joints are listed in 1, 2, respectively.
Figure 1.
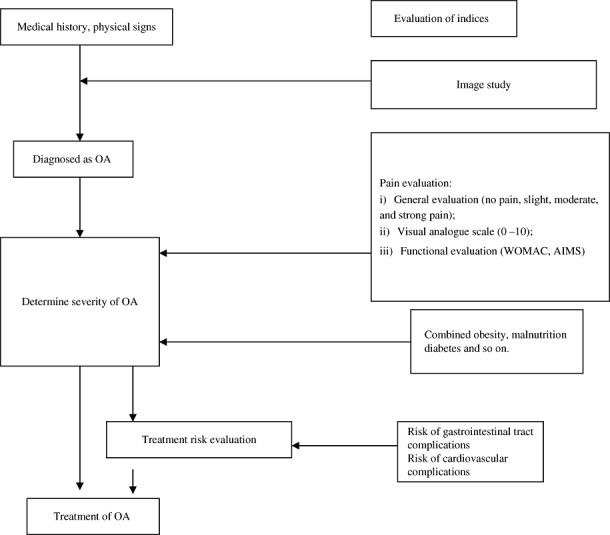
Flow chart for diagnosis and evaluation of OA.
Table 1.
Diagnostic criteria for OA in the knee joint
| Number | Characteristics |
|---|---|
| 1 | Recurrent knee joint pain in the last month. |
| 2 | Narrowed joint space, subchondral cyst formation and bone sclerosis, or osteophytosis around joint margin on the radiographs in standing or load position. |
| 3 | Evidence of clear, transparent, and viscous joint effusion at least twice; white cell count <2000/ml. |
| 4 | Middle‐aged and aged patients (40 years old or older). |
| 5 | Morning stiffness ≤30 min. |
| 6 | Palpable bone crepitation (fremitus) on movement of joint. |
Note: Diagnosis of knee OA can be made if the following conditions are satisfied: 1 + 2, 1 + 3 + 5 + 6 or 1 + 4 + 5 + 6.
Table 2.
Diagnostic criteria for OA in the hip joint
| Number | Characteristics |
|---|---|
| 1 | Recurrent hip joint pain in the last month. |
| 2 | ESR ≤20 mm/h. |
| 3 | Osteophytosis and hyperplasia in the acetabular margin on radiograph. |
| 4 | A narrowed hip joint space on plain radiograph. |
Note: Diagnosis of hip OA can be made if the following conditions are satisfied: 1 + 2 + 3 or 1 + 3 + 4.
Treatment
There is still no cure for OA. However, treatment can reduce pain, correct deformities, and improve joint function so as to improve the quality of the patient's life. A combination of non‐drug and drug treatments is superior to drug treatment alone. Surgical treatment is appropriate when conservative therapy fails, or is inadequate. Treatment regimens should be chosen according to age, gender, body weight, risk factors, lesion site and severity, and expectations of the treatment.
Non‐drug treatment
Non‐drug treatment should always be initiated first, especially for patients with mild OA. Many options exist for individuals with the disorder. Some of the most common are briefly described below.
Patient education
Education programs should be designed to provide patients with information about behavioural changes which could benefit them (such as avoiding inappropriate activities, exercise, postures, or stair climbing, as well as avoiding running, jumping or crouching for long periods of time), body weight reduction, aerobic exercise (such as swimming and bicycling), joint exercises (such as non‐weight‐bearing knee flexion and extension to maintain a maximum range of movement in the joint), and muscle strength training (such as training the abductor muscles of hip joint).
Physiotherapy
The main goal of physiotherapy is to increase local blood circulation and reduce inflammatory reactions. Physiotherapies include thermotherapy, hydrotherapy, ultrasound therapy, acupuncture, massage, traction, and transcutaneous electric nerve stimulation.
Use of supportive devices
A hand crutch, walking stick, or walking frame can be used to reduce weight bearing of the affected joint during walking.
Change of weight‐bearing line
Orthoses or orthopaedic shoes designed for correcting varus or valgus deformity can be used to change weight‐bearing patterns in the lower extremity.
Drug treatment
If non‐drug treatment is ineffective, drug treatment should be conducted according to the severity of joint pain.
Topical drug treatment
In the treatment of hand and knee OA, topical treatment is recommended prior to administration of oral medications. Non‐steroidal anti‐inflammatory drug (NSAID) emulsions, pastes, patches, and liniments can effectively alleviate mild to moderate joint pain with minimal adverse reactions. Topical and oral NSAIDs can be used in combination to relieve moderate to severe pain.
Systemic analgesics
Various routes of administration are available, including oral, parenteral and rectal routes.
Medication principles: (i) Prior to administration of drugs, patient‐related risk factors such as other medical conditions should be assessed. (ii) Dosage should be individualized according to the characteristics of the patient. (iii) As far as possible, the minimum effective dose should be prescribed, and excessive and repeated doses, as well as use of more than one drug in the same category, should be avoided. (iv) Three months after drug administration, routine blood and stool tests, stool occult blood tests, and hepatic and renal function tests should be performed selectively according to the patient's individual characteristics.
Drug selection: (i) Paracetamol is recommended as the first line drug for pain relief in OA, the maximum daily dose should not exceed 4000 mg. (ii) If paracetamol provides inadequate pain relief, NSAIDs (Table 3) can be used on patients who are not at high risk on the basis of gastrointestinal, hepatic, renal or cardiovascular diseases. There are two main types of NSAIDs; non‐selective NSAIDs and selective cyclo‐oxygenase‐2 (COX‐2) inhibitors. As different types of NSAIDs have different efficacies and adverse reactions, oral administration of these drugs should be adjusted according to individual patient risk factors, and patient and drug characteristics (Table 4). Selective COX‐2 inhibitors or non‐selective NSAIDs plus gastric mucosal protective agents, such as histamine (H2) receptor antagonists, proton pump inhibitors or misoprostol, can be of benefit in patients at high risk of adverse gastrointestinal reactions. (iii) Other analgesic drugs, such as tramadol, opioid analgesics, and paracetamol compounds, can be used if NSAIDs are ineffective or poorly tolerated.
Table 3.
Common NSAIDs for OA treatment
| Drugs | Half‐life (h) | Total dosage | Dosage / dose | Times /day |
|---|---|---|---|---|
| Propionic acid derivatives | ||||
| Ibuprofen | 2 | 1200–2400 mg | 400–600 mg | 3–4 |
| Naproxen | 14 | 500–1000 mg | 250–500 mg | 2 |
| Loxoprofen | 1.2 | 180 mg | 60 mg | 3 |
| Benzoyl acid derivatives | ||||
| Diclofenac | 2 | 75–150 mg | 25–50 mg | 2–3 |
| Indolyl acids | ||||
| Sulindac | 18 | 400 mg | 200 mg | 2 |
| Acemetacin | 3 | 90–180 mg | 30–60 mg | 3 |
| Pyronecarboxylic acids | ||||
| Etodolac | 8.3 | 400–1000 mg | 400–1000 mg | 1 |
| Non‐acids | ||||
| Nabumetone | 24 | 1000–2000 mg | 1000 mg | 1–2 |
| Xicams | ||||
| Meloxicam | 20 | 7.5–15 mg | 7.5–15 mg | 1 |
| Sulfonanilides | ||||
| Nimesulide | 2–5 | 400 mg | 100–200 mg | 2 |
| Coxibs | ||||
| Cimicoxib | 11 | 200 mg | 100–200 mg | 1–2 |
| Other analgesics | ||||
| Tramadol amino‐phenol | 6–7 | 3–6 tablets | 1–2 tablets | 2–3 |
| Tramadol hydrochloride | 6–7 | 3–6 tablets | 1–2 tablets | 2–3 |
Table 4.
High risk factors for NSAIDs
| Number | High‐risk of upper gastrointestinal complications | High‐risk of heart, brain, and kidney complications |
|---|---|---|
| 1 | Over 65 years of age | Over 65 years of age |
| 2 | Long‐term administration of NSAIDs | Past history of cerebrovascular disease (stroke or transient cerebral ischemia attack) |
| 3 | Receiving an oral glucocorticoid | History of cerebrovascular disease |
| 4 | History of upper gastrointestinal ulcer or bleeding | History of renal disease |
| 5 | Using anticoagulant drugs | Using ACEI and hydragogue simultaneously |
| 6 | History of alcoholism | Perioperative period of a CABG (the use of NSAIDs is contraindicated) |
ACEI, angiotensin converting enzyme inhibitors; CABG, coronary artery bypass grafting.
Injection into the articular space
(i) When oral drugs have resulted in inadequate pain relief, the viscoelastic extender sodium hyaluronate can be injected into the articular cavity after draining the joint fluid. (ii) Where NSAIDs are poorly tolerated or the treatment is ineffective after 4–6 weeks of treatment, a glucocorticoid can be injected into the articular cavity. Because articular cartilage damage can be worsened by constant use of glucocorticoids, injection should not be performed more than three to four times a year.
Medications which help to improve the disease condition and cartilage‐protective agents
Medications such as diacerein, dextrosamine, avocado soybean unsaponifiables, and doxycycline can slow down the course of the disease and improve the patients' symptoms to a certain degree. Diacerein plays a role in regulating or adjusting joint structure.
Surgical treatment
When conservative measures fail to control pain and improve joint function, surgery can be considered. Surgical intervention may assist in making the diagnosis or be an integral part of the whole treatment process.
Surgery is aimed at alleviating or even eliminating pain, preventing or correcting malformation, preventing worsening joint damage, and improving joint function.
There are various options for surgical treatment of OA, including extirpation of loose bodies, arthrotomy and joint debridement, osteotomy, joint fusion, and artificial joint replacement.
The advantages of arthroscopy over traditional open surgery are the small incisions required and the ability to safely inspect the joint.
Disclosure
A Chinese edition of this guideline was published in Chinese J. Orthopaedics, 2007, 27: 793–796.
References
- 1. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum, 2000, 43: 1905–1915. [DOI] [PubMed] [Google Scholar]
- 2. Schnitzer TJ, American College of Rheumatology . Update of ACR guidelines for osteoarthritis: role of the coxibs. J Pain Symptom Manage, 2002, 23 (Suppl. 4): S24–S34. [DOI] [PubMed] [Google Scholar]
- 3. Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum, 1995, 38: 1541–1546. [DOI] [PubMed] [Google Scholar]
- 4. Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part I. Osteoarthritis of the hip. American College of Rheumatology. Arthritis Rheum, 1995, 38: 1535–1540. [DOI] [PubMed] [Google Scholar]
- 5. Simon LS, Lipman AG, Jacox AK, eds. Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis, 2nd ed. Glenview, IL: American Pain Society (APS), 2002. [Google Scholar]
- 6. Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis, 2007, 66: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang W, Doherty M. EULAR recommendations for knee and hip osteoarthritis: a critique of the methodology. Br J Sports Med, 2006, 40: 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis, 2000, 59: 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis, 2005, 64: 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chevalier X, Marre JP, De Butler J, et al. Questionnaire survey of management and prescription of general practitioners in knee osteoarthritis: a comparison with 2000 EULAR recommendations. Clin Exp Rheumatol, 2004, 22: 205–212. [PubMed] [Google Scholar]
- 11. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis, 2003, 62: 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mazieres B, Bannwarth B, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials. Joint Bone Spine, 2001, 68: 231–240. [DOI] [PubMed] [Google Scholar]
- 13. Altman RD. The classification of osteoarthritis. J Rheumatol Suppl, 1995, 43: 42–43. [PubMed] [Google Scholar]
- 14. Brand C, Cox S. Systems for implementing best practice for a chronic disease: management of osteoarthritis of the hip and knee. Intern Med J, 2006, 36: 170–179. [DOI] [PubMed] [Google Scholar]
- 15. Jawad AS. Analgesics and osteoarthritis: are treatment guidelines reflected in clinical practice? Am J Ther, 2005, 12: 98–103. [DOI] [PubMed] [Google Scholar]
- 16. Brosseau L, Wells GA, Tugwell P, et al. Ottawa panel evidence‐based clinical practice guidelines for therapeutic exercises and manual therapy in the management of osteoarthritis. Phys Ther, 2005, 85: 907–971. [PubMed] [Google Scholar]
- 17. Roddy E, Zhang W, Doherty M, et al. Evidence‐based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee – the MOVE consensus. Rheumatology (Oxford), 2005, 44: 67–73. [DOI] [PubMed] [Google Scholar]
- 18. Wegman A, Van Der Windt D, Van Tulder M, et al. Nonsteroidal anti‐inflammatory drugs or acetaminophen for osteoarthritis of the hip or knee? A systematic review of evidence and guidelines. J Rheumatol, 2004, 31: 344–354. [PubMed] [Google Scholar]
- 19. Roddy E, Doherty M. Guidelines for management of osteoarthritis published by the American College of Rheumatology and the European League against Rheumatism: why are they so different? Rheum Dis Clin North Am, 2003, 29: 717–731. [DOI] [PubMed] [Google Scholar]
- 20. Schnitzer TJ. Update on guidelines for the treatment of chronic musculoskeletal pain. Clin Rheumatol, 2006, 25 (Suppl. 1): S22–S29. [DOI] [PubMed] [Google Scholar]
- 21. Pencharz JN, Grigoriadis E, Jansz GF, et al. A critical appraisal of clinical practice guidelines for the treatment of lower‐limb osteoarthritis. Arthritis Res, 2002, 4: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum, 1991, 34: 505–514. [DOI] [PubMed] [Google Scholar]
- 23. Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis, 2007, 66: 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


