Abstract
Objective
The prevention and treatment of the common complication of cerebrospinal fluid leakage (CSFL) during anterior approach cervical spine surgery for severe ossification of the posterior longitudinal ligament (OPLL) is documented.
Method
A retrospective analysis of 47 patients with severe cervical OPLL aged 39 to 73 years (average, 56.4 years) who underwent anterior operations was conducted. All patients were classified as local or segmental types based on the thickness of the ossified mass (>5 mm) and canal stenosis rates exceeding 50%. Fifteen cases underwent discectomy and fusion and 32 cases corpectomy and fusion. Preoperative CT images were analyzed for operation breakthrough and decompression range. During surgery, the ossified mass was excised or floated and the arachnoid reserved in order to reduce dural damage. Sutures coupled with gelatin sponge or muscle pedicle were applied to repair dural defects. After surgery, patients were confined to bed to allow for drainage or puncture.
Results
Fifteen cases of dural defects were recognized intraoperatively. Postoperatively, 5 patients developed CSFL. Partially cut dura with intact arachnoid occurred in one patient who developed a CSF pseudocyst. No cases required secondary operations or shunt placement and all exhibited good wound healing, with JOA scores increasing from 7.30 ± 1.08 to 13.70 ± 0.81 points and improvement rates of 65.98% ± 7.35%.
Conclusion
During anterior surgical treatment for severe OPLL, CSFL can be prevented and treated through careful analysis of CT images, meticulous operative technique, careful handling of the ossified mass, and intra‐operative repair of dural damage.
Keywords: Cerebrospinal fluid leakage, Complication, Ossification of posterior longitudinal ligament
Introduction
Cerebrospinal fluid leakage (CSFL) is the most common and serious complication of anterior spinal surgery1, 2, 3, 4. This problematic complication can lead to postoperative issues such as wound infection, purulent meningitis, and increased mortality rates1, 4. In particular, severe ossification or adhesion of the dura mater can result in increased risk of CSFL in cases of severe ossification of the posterior longitudinal ligament (OPLL), which is characterized by an ossified mass exceeding 5 mm and over 50% canal stenosis. Because of the clinical importance of preventing and treating CSFL, we conducted this retrospective study of 47 patients with severe OPLL. The information we provide here includes a detailed account of our experiences of treating dural damage and CSFL that is of potential use to researchers and clinicians in this field.
Materials and Methods
Patients
In all, 47 patients with severe OPLL were treated with anterior spinal surgery between January 2008 and May 2011. There were 33 men and 14 women aged from 39 to 73 years (mean, 56.4 years) with an average degree of canal stenosis of 62.13%. All cases presented with spinal cord compression and progressive pyramidal tract symptoms. Plain radiography, CT imaging and reconstruction, and MRI were used to make a definite diagnosis of OPLL. According to the Hirabayashi classification5, the patients were divided into groups based on type: localized (22 patients) and segmental (25). Of the 22 patients with localized OPLL, 15 underwent discectomy and fusion according to location: C3–4 (three cases), C4–5 (five), C5–6 (four), and C6–7 (three). The remaining seven patients with localized OPLL and the 25 with segmental OPLL underwent corpectomy and fusion according to location: C3 (two cases), C4 (five), C5 (seven), C6 (four), C3 + C4 (two), C4 + C5 (seven), and C5 + C6 (five).
Operative Technique
The initial operative approach was made from under the cervical plexus with potentiated anesthesia. The patient was positioned in a supine position with the head slightly extended.
For patients undergoing discectomy and fusion, the anterior longitudinal ligament was severed in order to excise the disc for decompression. Decompression was performed from the Luschka joint, reaching back as far as the posterior surfaces of the adjacent bodies. Any osteophytes and the ossified posterior longitudinal ligament (PLL) were resected. At this point, a cage was carefully implanted and fixed using screws and a titanium plate.
For patients undergoing corpectomy and fusion, a rongeur was used to partially remove the part of the vertebral bodies within the Luschka joint. The posterior cortex of the bodies was drilled until the ossified PLL was visible. An elevator was then used to identify and enlarge the space between the PLL and spinal cord. The PLL was then lifted and severed, followed by careful piece‐by‐piece resection of the ossified mass using a Kerrison rongeur.
In some cases, adhesion of the dura to the ligament or dural ossification made the separation more difficult. In these cases, the “floating method” was approach of choice. An abrasive drill was used to carefully abrade the ossified mass, thus slowly removing the ossified tissue until the mass had been sufficiently thinned (generally “paper‐thin”) to permit its separation. Bulging or pulsation of the dura mater was observed in some cases (as shown in Fig. 1).
Figure 1.

A 47 year‐old man with OPLL, underwent anterior corpectomy (C5, C6) and fusion, was treated by floating the ossified dura intraoperatively without CSFL. (a) Preoperative CT image (sagittal view) showed a C5–6 segmental OPLL with double‐layer sign. (b) Axial CT image showing degree of canal stenosis of 71%. (c) Sagittal CT image one week postoperatively showed the ossified PLL has been resected. (d) Axial CT image showing the local dura is floating.
Occasionally, the dura mater was partially cut to preserve the integrity of the arachnoid (as shown in Fig. 2). Titanium mesh with autogenous bone was then inserted and fixed using screws and a titanium plate.
Figure 2.
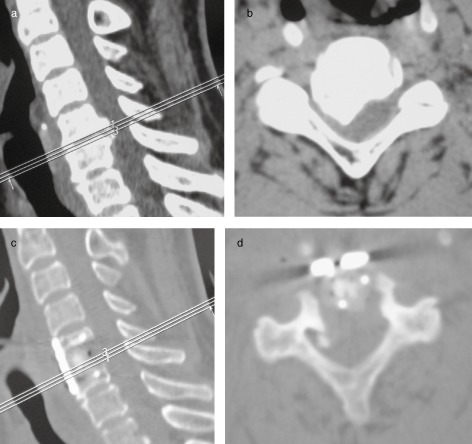
A 56 year‐old man with OPLL underwent discectomy (C5–6) and fusion. Because of severe adhesions, the dura was partially cut, keeping the arachnoid intact. (a) Preoperative CT image (sagittal view) showed a local‐type OPLL on the C5–6 level. (b) Axial CT image showing a hill‐type mass. (c) Sagittal CT image one week postoperatively showing a cage has been implanted. (d) Axial CT image showing the cord has been decompressed well.
Data in Records
All postoperative general features and symptoms were recorded for each patient. Follow‐up was conducted in all patients, including both lateral X‐ray and CT scan imaging, 1 week, 3 months, 6 months, and 1 year after surgery. The Japanese Orthopedic Association (JOA) scoring system5 was used to evaluate the patients' neurological statuses.
Statistical Analysis
For statistical analysis of the results, the Wilcoxon signed ranks test was performed using SPSS version 13.0. The results were considered significant when the P‐value was less than 0.05.
Results
Intraoperative Findings and Procedures
In 24 patients, we observed no adherence between the PLL and dura and could therefore easily resect the ossified masses without damage to the dura. However, in eight patients, the ossified masses had adhered extensively to the dura, often complicated by dural ossification. In these patients, we used the “floating method” to avoid dural damage. In 10 patients, cerebral spinal fluid (CSF) began to leak during separation of the PLL, likely due to tearing or other injury to the dura. In five patients, we observed severe localized dural adherence to the ligament; however, we observed no leakage when we partially cut the dura mater and we were able to preserve the arachnoid in a fully intact condition.
Postoperative Complications of Cerebrospinal Fluid Leakage
Cerebrospinal fluid leakage occurred in five patients following anterior spinal surgery operations. In four of these cases, the cause was dural tearing or other injury. These patients recovered fully within 4–6 days of bed rest, drainage, and administration of vinegar chlorine amide to reduce secretions, resulting in normal wound healing. In the one case in which the dura had been partially cut, a CSF pseudocyst caused by severe coughing developed. We repeatedly punctured and drained the pseudocyst and bandaged the wound with pressure; healing occurred within three weeks. No patient developed spinal canal or intracranial infection, cutaneous fistula, secondary operations, or required insertion of a shunt (Table 1).
Table 1.
Data concerning characteristics and management of postoperative CSFL
| Case no. | Sex/Age (years) | Location of surgery | Double‐layer sign | Cause | Dural resection | Intraoperative CSFL | Intraoperative treatment | Postoperative treatment | Healing time |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male/63 | C4 | + | Separation, tearing | No | Yes | Muscle + gelatin sponge + fibrin glue | Conventional treatment | 5 days |
| 2 | Male/57 | C5–6 | + | Separation, injured by a dissector | No | Yes | Gelatin sponge + fibrin glue | Conventional treatment | 6 days |
| 3 | Female/49 | C5 | − | Separation, tearing | No | Yes | Suturing + gelatin sponge + fibrin glue | Conventional treatment | 4 days |
| 4 | Male/51 | C4 + C5 | − | Adhesions, injured by a dissector | No | Yes | Muscle + gelatin sponge + fibrin glue | Conventional treatment | 6 days |
| 5 | Male/56 | C4 | − | Postoperative cough | Yes | No | Gelatin sponge + fibrin glue | Conventional treatment + reduce cough + repeated puncture + antibiotic infusion | 3 weeks |
Conventional treatment: Drainage, confined to bed, local pressure, oral acetazolamide and so on.
Follow‐up
We followed up all patients for an average of 14.8 months (range, 12–18 months). Within 6 months, all patients had evidence of bony union. We observed no instrument subsidence, shifting, breakage, or extrusion in any of the patients in this study. Preoperative JOA scores averaged 7.30 ± 1.08 points. We observed a statistically significant improvement in postoperative JOA scores, with an average of 13.70 ± 0.81 points (Z = −6.081, P = 0.00). The average improvement rate ([postoperative JOA score—preoperative JOA score]/[17—preoperative JOA score] × 100%) was 65.98% ± 7.35%.
Discussion
Cerebrospinal Fluid Leakage Risk during Anterior Surgical Approaches for Treating Ossification of the Posterior Longitudinal Ligament
Ideal OPLL therapy involves reduction of the pressure on the ventral spinal cord. Unfortunately, in this relatively narrow surgical field, minor errors in operative manipulations can easily cause serious complications1, such as CSFL. In severe OPLL, patients are at increased risk of dural damage and CSFL because of increased dural adhesion and dural ossification. Joseph et al. reported that the rate of CSFL after anterior corpectomy ranged from 12.5% to 32%2, making CSFL far more common than after other general spinal operations (0% to 8.3%). Hannallah et al. reported that OPLL is the most dangerous risk factor for surgical cervical CSFL, stating that its occurrence during OPLL is 13.7‐fold higher than during other surgeries3. Following occurrence of CSFL, secondary complications, such as delayed healing, meningitis, airway obstruction, cutaneous fistula, and pseudomeningocele, often pose significant barriers to patient recovery4. Thus, it is not sufficient to simply reduce the morbidity of CSFL by postoperative measures. Careful preoperative preparation and attention to meticulous preventive measures during the intraoperative procedure can also improve outcomes.
Preoperative Computed Tomography Guidance for Treatment Planning
Computed tomography scanning is vital to the diagnosis and treatment of OPLL6. CT scans can clearly reveal the unique characteristics of OPLL cases, including the extent, shape, and degree of maturity, spinal cord compression, and dural ossification6, 7. In axial CT images, flat‐, mushroom‐, hill‐, and bouquet‐like types can be clearly distinguished8. In 1997, Hida et al. first described the single‐ and double‐layer signs that are associated with dural ossification7. Epstein considered that the double‐layer sign is the most significant and accurate indicator of dural ossification9. Mizuno et al. proposed that separating OPLL tissue from ossified dural tissue through a thin layer of non‐ossified and degenerated PLL may reduce dural damage, thus reducing the risk of CSFL occurring10.
In consideration of these previous reports, the current study initially analyzed CT findings in order to guide our surgical procedures, thus reducing dural damage and risk of CSFL. In CT scans that did not reveal dural ossification, mushroom‐ or bouquet‐like types with narrow bases were visible. In these cases, we were able to perform decompression from the lateral weak area. In cases where the typical double‐layer sign was present, we performed separation through the non‐ossified degenerated PLL, thus avoiding the dural ossification and reducing the chance of CSFL. We treated 14 patients with double‐layer signs on CT images in this manner, achieving smooth separation of ossified PLL issues. In cases where CT scans had revealed the single‐layer sign with a large homogeneous mass, separation of ossified PLL was difficult. In these cases, we preferred the floating method and partial cutting of the dura.
Computed tomography findings can also be used to ascertain the decompression range necessary. In local types, the ossified mass appears on the disc level. When this is the case, it indicates that the disc, osteophytes, and ossified mass can be directly resected. If a large ossified PLL is present in the posterior marginal zone, part of the vertebral bodies can be removed. In segmental types, CT scans can be used to determine whether one or two‐level corpectomy is indicated. Such preoperative assessment not only facilitates successful surgery, but also aids in avoidance of CSFL. Thus, in the current study, patients with localized OPLL underwent discectomy and fusion with either one‐level or two‐level corpectomy, while patients with segmental OPLL underwent corpectomy and fusion.
Reducing Dural Damage
Spinal surgeons face the challenge of operating on severe cases of OPLL through an anterior approach. Careful observance of three factors can help them to improve their results: sufficient decompression range, use of the floating method, and cutting the dura while keeping the arachnoid intact.
By ensuring a sufficient decompression range, the PLL can be directly resected or its resection facilitated by floating manipulation, thus reducing damage to the dura. Even when CSFL does occur, this method makes repair much easier. Generally speaking, the extent of decompression should be greater than 80% of the distance between vertebral pedicles11. In the current study, we performed decompression within the medial border of the Luschka joint, which allows for visualization of the margins of ossified foci. For severe local types, we sometimes removed sections of the adjacent vertebral bodies to enable direct decompression. Approaching through the middle or posterior sections of the bodies made drilling or resection of the PLL more convenient and was effective in reducing dural damage.
The floating method was originally suggested by Yamaura et al.12 When the dura is widely adhesive or ossified, the floating method minimizes the extent of surgical invasion and damage to the venous plexus, thus avoiding disturbance of the nervous tissues and decreasing acute dural inflation and CSFL13. Chen et al. reported noting no apparent differences in clinical outcomes between surgery with removal versus non‐removal of dural ossification using the floating method14. In a retrospective study of 144 patients, Joseph et al. found that the incidence of CSFL using the floating method via an anterior approach was 6.3%2. In this study, 10 patients underwent surgery using the floating method; all had satisfactory neurological recoveries.
The dura may also be partially cut, keeping the arachnoid intact, if necessary. Cases requiring this procedure have umbrella‐ or hill‐like masses, often with punctiform dural ossification and local adhesion of the dura, in their CT scans. The dura can be carefully cut in a manner that preserves the arachnoid. This region is then covered with a gelatin sponge and sprayed with fibrin glue. In the current study, we used this method in five cases and only one of them developed CSFL because of a racking cough. When using this method, it is important to note that the range of the dural cut should be as small as possible; otherwise the floating method may be preferable.
Treatment of Intraoperative Dural Damage
In surgical treatment of severe OPLL by an anterior approach, dural injury or tearing often necessitates difficult repairs. Joseph et al. reported repair of dural defects using only grafts of crushed muscle/fascia and a layer of gelatin sponge2. They combined this with a subsequent lumbar subarachnoid drain to achieve satisfactory outcomes. Hyun et al. suggested that the muscle pedicle flap technique be employed as an alternative to direct “water‐tight” closure of ventral cervical dural defect repairs15. Other alternative measures, such as autologous fascia, dural substitute, lumber subarachnoid drain, and micro‐dural stapling, have also been reported2, 16.
Of the 15 patients in the current study who experienced either tearing or excision intraoperative dural damage, all achieve positive outcomes. Improved outcomes are, in part, attributable to the use of bright and clear surgical fields to avoid further damage. During discectomy procedures, the use of sutures is generally impractical. Thus, we used gelatin sponges and sprayed fibrin glue to close the gaps between the cage and the longus colli muscle in three patients. During corpectomy procedures, breakage can generally be repaired using sutures applied longitudinally provided there is no dural ossification or adhesion. In cases of unfavorable sutures or large defects, we used crushed muscle (similar to using the platysma muscle) to form a region larger than the defect. We subsequently covered this region with a gelatin sponge and sprayed it with fibrin glue. We then tamped the gelatin sponge into the gaps adjacent to the mesh. We treated three patients by this technique. In the five patients in whom we partially cut the dura and kept the arachnoid intact, we used gelatin sponge and fibrin glue similar to that described above. We placed drainage beside the wound to prevent sinus formation.
Postoperative Management of Cerebrospinal Fluid Leakage
Postoperative management of CSFL must be comprehensive and careful to achieve best results. In general, clinicians should: (i) limit neck activity, employ pressure bandaging, and apply dry and clean dressings regularly; (ii) enforce prolonged bed rest, prevent dehydration, observe temperature variations, and assess for development of intracranial hypotension or central nervous system infection; (iii) observe color, volume, and character of drainage fluid (light red or pale yellow is indicative of CSFL); (iv) remove drainage within 48 hours in most cases; and (v) administer oral medications, such as vinegar chlorine amide, to reduce the amount of CSF. In the current study, we treated four patients by these methods, resulting in stoppage of CSFL within 4–6 days and superior overall wound healing. In the one patient with cut dura and intact arachnoid in which a CSF pseudocyst developed around the wound after coughing, the above‐listed general therapies were coupled with oral medication to reduce cough and sputum production, repeated puncture, and antibiotic infusion. The pseudocyst disappeared within 3 weeks, and the patient recovered fully. Other studies have proposed that lumbar subarachnoid drains or lumboperitoneal and wound peritoneal shunts, can be used to treat stubborn CSFL2, 4. Unfortunately, these methods pose potential hazards, including infection, blockage, fracture, displacement, and over‐ or under‐drainage4. Thus, these alternatives should be employed only as a last resort in extreme cases.
Conclusion
The current study documents management and treatment of CSFL during anterior approach surgery for severe OPLL at a single facility. Cumulatively, these findings suggest that reactive repair is not enough. Instead, surgeons should take care in their preparations to proactively reducing patient risk of developing CSFL. The decompression methods to use can be selected according to findings on preoperative CT images, as described in the current study. This approach allows surgeons to more appropriately select either resection or floating operative methods. Additionally, we have documented the appropriate response to CSFL occurrence, which requires immediate repair. Together, these comprehensive measures can reduce the occurrence and severity of CSFL during anterior spinal surgery for treating severe OPLL.
Disclosure: No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1. Li H, Dai LY. A systematic review of complications in cervical spine surgery for ossification of the posterior longitudinal ligament. Spine J, 2011, 11: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 2. Joseph V, Kumar GS, Rajshekhar V. Cerebrospinal fluid leak during cervical corpectomy for ossified posterior longitudinal ligament: incidence, management, and outcome. Spine, 2009, 34: 491–494. [DOI] [PubMed] [Google Scholar]
- 3. Hannallah D, Lee J, Khan M, et al Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am, 2008, 90: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 4. Mazur M, Jost GF, Schmidt MH, et al Management of cerebrospinal fluid leaks after anterior decompression for ossification of the posterior longitudinal ligament: a review of the literature. Neurosurg Focus, 2011, 30: E13. [DOI] [PubMed] [Google Scholar]
- 5. Hirabayashi K, Miyakawa J, Satomi K, et al Operative results and postoperative progression of ossification among patients with ossification of posterior longitudinal ligament. Spine, 1981, 6: 354–364. [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Chen DY, Yuan W, et al CT characteristic and clinical meanings of associated dural ossification in ossification of posterior longitudinal ligament of cervical spine. Jizhu Wa Ke Za Zhi, 2006, 4: 270–273. (In Chinese) [Google Scholar]
- 7. Hida K, Iwasaki Y, Koyanagi I, et al Bone window computed tomography for detection of dural defect associated with cervical ossified posterior longitudinal ligament. Neurol Med Chir (Tokyo), 1997, 37: 173–175. [DOI] [PubMed] [Google Scholar]
- 8. Jia LS. Modern Spine Surgery. Beijing: People's Military Medical Press, 2007; 763–764. (In Chinese) [Google Scholar]
- 9. Epstein NE. Identification of ossification of the posterior longitudinal ligament extending through the dura on preoperative computed tomographic examinations of the cervical spine. Spine, 2001, 26: 182–186. [DOI] [PubMed] [Google Scholar]
- 10. Mizuno J, Nakagawa H, Song J, et al Surgery for dural ossification in association with cervical ossification of the posterior longitudinal ligament via an anterior approach. Neurol India, 2005, 53: 354–357. [DOI] [PubMed] [Google Scholar]
- 11. Cui ZM, Jia LS. Progression on surgical treatment in ossification of posterior longitudinal ligament of cervical spine. Zhongguo Jiao Xing Wa Ke Za Zhi, 2002, 14: 1414–1415. (In Chinese) [Google Scholar]
- 12. Yamaura I, Kurosa Y, Matuoka T, et al Anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Clin Orthop Relat Res, 1999, 359: 27–34. [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, Guo Y, Lu X, et al Surgical strategy for multilevel severe ossification of posterior longitudinal ligament in the cervical spine. J Spinal Disord Tech, 2011, 24: 24–30. [DOI] [PubMed] [Google Scholar]
- 14. Chen Y, Guo Y, Chen DY, et al Diagnosis and surgery of ossification of posterior longitudinal ligament associated with dural ossification in the cervical spine. Eur Spine J, 2009, 18: 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hyun SJ, Rhim SC, Ra YS. Repair of a cerebrospinal fluid fistula using a muscle pedicle flap: technical case report. Neurosurgery, 2009, 65: E1214–E1215. [DOI] [PubMed] [Google Scholar]
- 16. Hou TS, Fu Q, He SS, et al Management of cerebrospinal fluid leakage complicated in anterior cervical surgery. Zhonghua Gu Ke Za Zhi, 2003, 11: 650–652. (In Chinese) [Google Scholar]


