Introduction
Since Guinto et al. reported a case of spontaneous regression of a herniated lumbar disc in 1984 1 , this phenomenon in lumbar discs has been well documented and discussed 2 , 3 , 4 . However, there have been fewer reports of spontaneous regression of cervical disc herniation (CDH) 5 , 6 , 7 , especially ones confirmed by magnetic resonance imaging (MRI) 8 , 9 , 10 . Recently, a patient with CDH attending our hospital experienced this exceptional condition after 6 months of conservative treatment. In the following report, we will present this case and discuss the condition.
Case report
A 32‐year‐old man was admitted to hospital because of weakness, impairment of walking, sensory disturbance in all extremities, and being woken at night by neck pain. The patient had no difficulty with micturition nor trauma history. In addition, there had been no obvious improvement in his condition after taking nonsteroidal drugs.
On physical examination, C7 sensory level hypesthesia was demonstrated, his motor power was assessed as grade 4, Hoffmann's sign was present bilaterally, and he had hyperactive patellar tendon reflexes. Ankle and patellar clonus were not observed. Although there were no remarkable findings on plain X‐ray films, a computed tomography (CT) scan and MRI showed a large disc herniation at the C6‐7 level (1, 2). In addition, the MRI showed obvious compression of the anterior aspect of the spinal cord with changes in its signal intensity. The diagnosis established, we recommended that the patient undergo anterior cervical discectomy and fusion. However, he refused and discharged himself. At a follow‐up examination 4 months later, he stated that his condition had improved without any specific care, apart from a hard cervical collar he had obtained from another hospital. His symptoms had begun to show rapid improvement and he had regained his motor strength within 3 weeks, while the numbness in all his extremities had resolved within 3 months. A follow‐up MRI 6 months later showed complete regression of the herniated cervical disc (Fig. 3).
Figure 1.
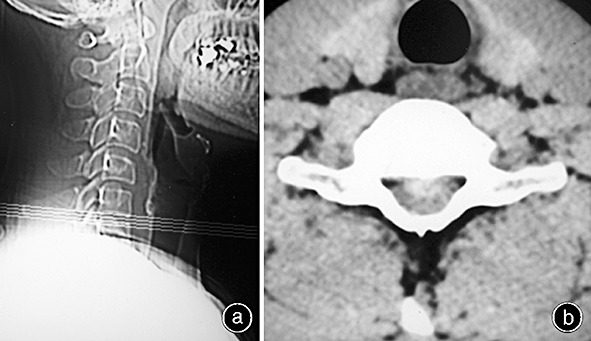
(a) A lateral X‐ray film showing no remarkable findings. (b) CT scan showing large disc herniation at the C6‐7 level.
Figure 2.
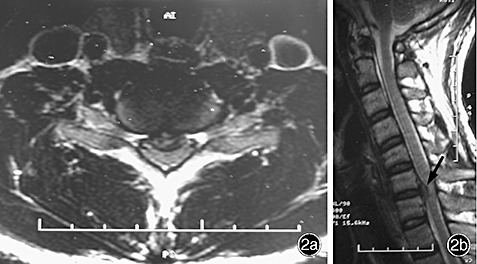
(a, b) T2‐weighted imaging showing compression of the spinal cord at C6‐7 with obvious changes in signal intensity.
Figure 3.
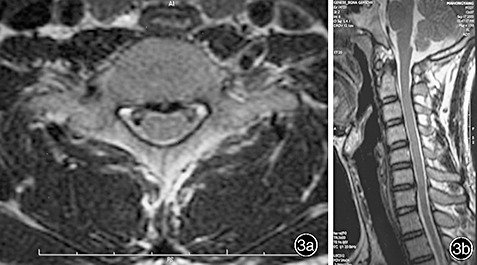
(a, b) After 6 months of conservative treatment, MRI shows complete regression of the herniated disc.
Discussion
Since the first report of spontaneous regression of a herniated cervical disc by Krieger and Maniker in 1992 5 , several other authors have also reported this rare phenomenon 6 , 7 , 8 , 9 , 10 . Usually, patients with myelopathy, a condition which is more severe than radiculopathy, are impatient for their neurological deficits to resolve. Therefore, in the absence of knowledge about the natural history of such patients, doctors are apt to choose surgical treatment. To our knowledge, only 13 cases of spontaneous regression of herniated cervical disc with detailed supportive MRI findings have been reported in the literature. The time between initial presentation and spontaneous regression varied from 2–28 months 5 , 6 , 7 , 8 , 9 , 10 . These cases of spontaneous regression of CDH in patients without surgical treatment can be regarded as a benign natural course which occurs in some patients with herniated cervical disc.
Many factors relating to the resorption process have been recognized, including the age of the patients, dehydration of the expanded nucleus pulposus, resorption of hematoma, revascularization, penetration of herniated cervical disc fragments through the posterior longitudinal ligament (PLL), size of disc herniation, and existence of cartilage and annulus fibrosus tissue in the herniated material. Resorption of a herniated nucleus pulposus is thought to occur via an inflammatory reaction in the outermost layer of the herniation, with macrophages as the major cellular population.
In previously reported cases, spontaneous regression of a herniated cervical disc has been found more frequently in extruded than in protruded discs 9 . Larger type herniations are likely to regress more readily than smaller ones because of their tendency to penetrate the annulus fibrosus and PLL, thereby being exposed to the systemic circulation in the epidural space. Evidence of regression has been seen more frequently in patients who underwent imaging soon after the onset of symptoms than in those in whom imaging was delayed 6 . Herniated disc regression detected on MRI might represent in part dehydration of the expanded nucleus pulposus and resorption of hematoma, which can occur subsequent to annulus rupture.
Rim enhancement around the herniated disc seen in contrast‐enhanced MRI is thought to represent a neovascularized zone with macrophage infiltration, macrophages playing an essential role in phagocytosis and herniation regression. An enhanced rim around the outermost areas of a herniated nucleus pulposus (HNP) in a gadolinium diethylenetriamine penta‐acetic acid (Gd‐DTPA) MRI is thought to be a major determinant of spontaneous resorption of HNP. Thickness of rim with Gd‐DTPA enhancement is a more reliable prognostic indicator of spontaneous resorption than is the circumferential extent of rim enhancement 11 . However, if thickness of rim enhancement cannot be assessed, the Komori classification 12 is a useful predictive sign in MRI because herniations with a higher degree of displacement according to the Komori classification have a more rapid resorption rate; especially when the CDH extends more than 67% above or below the height of adjacent vertebrae 11 .
In older patients, the immunologic response and angiogenesis needed for CDH resorption may be weaker. Herniations tend to have less nucleus pulposus and more hardened annulus fibrosus and cartilaginous endplate material 6 , the latter is able to inhibit neovascularization of the herniated disc 13 . In younger patients, the inflammatory response may be less pronounced. Indeed, in an experimental dog model, the younger animals had no neovascularization nor inflammatory cell accumulation in sequestered disc fragments 14 .
In shorts, in five of fourteen patients, including the previous thirteen and the present one, surgery was recommended. Of these five cases, three had myelopathy and two radiculopathy. Nonoperative management was pursued for several reasons. One patient refused while the other four cases experienced relief of symptoms with conservative treatment. At the latest follow‐up, 11 patients with radiculopathy had achieved almost full recovery according to physical examination 5 , 6 , 9 , 10 .
However, one of the other three cases with myelopathy reported obvious alleviation of symptoms 6 , another experienced continuous limbs paresthesia 7 while the third one complained of no improvement in symptoms in spite of evidence of regression on MRI 6 . Eventually, this patient underwent anterior cervical discectomy and fusion. In most of the cases alleviation of clinical symptoms occurred concordantly with rapid resorption.
Although the generalization that cervical disc herniations can be successfully managed with nonoperative management would be inappropriate from the results of only a few cases, conservative observation for at least two or three months could be considered as an option for patients with cervical disc herniation, especially those with complicated medical conditions.
Spontaneous regression of herniated cervical disc is rare. As all previously reported cases have been of the extruded type, nonsurgical management may be considered as an option for the treatment of patients with a cervical extruded disc. MRI is a useful prognostic tool.
References
- 1. Guinto FC Jr, Hashim H, Stumer M. CT demonstration of disk regression after conservative therapy. AJNR Am J Neuroradiol, 1984, 5: 632–633. [PMC free article] [PubMed] [Google Scholar]
- 2. Keskil S, Ayberk G, Evliyaoglu C, et al. Spontaneous resolution of ‘protruded’ lumbar discs. Minim Invasive Neurosurg, 2004, 47: 226–229. [DOI] [PubMed] [Google Scholar]
- 3. Burke JG, Watson RW, McCormack D, et al. Spontaneous production of monocyte chemoattractant protein‐1 and interleukin‐8 by the human lumbar intervertebral disc. Spine, 2002, 27: 1402–1407. [DOI] [PubMed] [Google Scholar]
- 4. Komori H, Okawa A, Haro H, et al. Contrast‐enhanced magnetic resonance imaging in conservative management of lumbar disc herniation. Spine, 1998, 23: 67–73. [DOI] [PubMed] [Google Scholar]
- 5. Krieger AJ, Maniker AH. MRI‐documented regression of a herniated cervical nucleus pulposus: a case report. Surg Neurol, 1992, 37: 457–459. [DOI] [PubMed] [Google Scholar]
- 6. Mochida K, Komori H, Okawa A, et al. Regression of cervical disc herniation observed on magnetic resonance images. Spine, 1998, 23: 990–995. [DOI] [PubMed] [Google Scholar]
- 7. Song JH, Park HK, Shin KM. Spontaneous regression of a herniated cervical disc in a patient with myelopathy. Case report. J Neurosurg, 1999, 90: S138–S140. [DOI] [PubMed] [Google Scholar]
- 8. Westmark RM, Westmark KD, Sonntag VK. Disappearing cervical disc. Case report. J Neurosurg, 1997, 86: 289–290. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi N, Asamoto S, Doi H, et al. Spontaneous regression of herniated cervical disc. Spine J, 2003, 3: 171–173. [DOI] [PubMed] [Google Scholar]
- 10. Gurkanlar D, Yucel E, Er U, et al. Spontaneous regression of cervical disc herniations. Minim Invasive Neurosurg, 2006, 49: 179–183. [DOI] [PubMed] [Google Scholar]
- 11. Autio RA, Karppinen J, Niinimaki J, et al. Determinants of spontaneous resorption of intervertebral disc herniations. Spine, 2006, 31: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 12. Komori H, Okawa A, Haro H, et al. Contrast‐enhanced magnetic resonance imaging in conservative management of lumbar disc herniation. Spine, 1998, 23: 67–73. [DOI] [PubMed] [Google Scholar]
- 13. Kokubun S, Sakurai M, Tanaka Y. Cartilaginous endplate in cervical disc herniation. Spine, 1996, 21: 190–195. [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa T, An HS, Inufusa A, et al. The effect of age on inflammatory responses and nerve root injuries after lumbar disc herniation: an experimental study in a canine model. Spine, 2000, 25: 937–940. [DOI] [PubMed] [Google Scholar]


