Abstract
Gouty arthritis is an extremely painful condition that causes functional impairment. Gouty arthritis has become increasingly complex because of multiple comorbidities, iatrogenic factors and hyperuricemia that is refractory to treatment. In this review, we present a general overview of gouty arthritis including its pathophysiology, clinical presentations, diagnosis, predisposing factors and prophylactic therapy for preventing gouty arthritis flares.
Keywords: Diagnosis, Gouty arthritis, Tophi, Treatment, Uric acid lowering therapy
Introduction
A metabolic disorder, gouty arthritis is characterized by hyperuricemia and precipitation and deposition of inflammatory monosodium urate (MSU) crystals in synovial and other tissues, accompanied by extreme pain. It is bone damaging if not treated adequately. Gouty arthritis is the common inflammatory arthritis in men over the age of 40 years, with a 1.4% prevalence rate in the UK1.
Gouty arthritis, one of the oldest diseases, was first identified by Egyptians in 2640 B.C. and is consistently poorly managed, especially in refractory patients2. Historically, it was termed the “disease of kings” because it was associated with eating rich foods and consuming excessive alcoholic beverages. Acute gouty arthritis is triggered when MSU crystals are deposited in a joint space; this can occur as a result of any condition that leads to rapid increases/decreases in serum urate (SU, uric acid) concentrations3. Patients with advanced tophaceous gouty arthritis have constant pain and swelling of affected joints. Gouty arthritis is associated with a 38% increase in risk of cardiovascular disease‐related death, 55% increase in risk of coronary heart disease‐related death and 28% increase in risk of death from any cause4. Gouty arthritis‐associated pain and functional impairments significantly lower the quality of life, causing a substantial clinical and economical burden5.
The following issues are both crucial and challenging in management of gouty arthritis: (i) modification of lifestyle; (ii) use of urate‐lowering therapy (ULT) to minimize gouty arthritis flares; (iii) use of alternative drugs for patients who are unresponsive or have adverse reactions to traditional drugs such as colchicine and nonsteroidal anti‐inflammatory drugs (NSAIDs); and (iv) surgical management for gout‐related complications. Recently, an improved understanding of the pathophysiology of gouty arthritis, including the identification of many genetic factors, has contributed to facilitating new therapeutic modalities6. New innovative drugs such as anti‐interleukin (IL) inhibitors and non‐xanthine oxidase inhibitors have been developed for treating gouty arthritis7, 8, 9, 10. Guidelines that have been developed by the European League Against Rheumatology (EULAR)11 and the British Society for Rheumatology12 are available for both specialists and non‐specialists. With the development of new treatment modalities, these pragmatic guidelines require updating to include current best management.
This review aims to present advances in translational, genetic and clinical research on gouty arthritis.
Clinical Pathophysiology
The manifestations of gouty arthritis range from asymptomatic hyperuricemia to advanced tophaceous gouty arthritis. In the early stage, individuals with SU concentrations > 6.8 mg/dL may remain asymptomatic or develop MSU crystal formation and deposits in joints and other tissues13. Despite the fact that hyperuricemia substantially increases the risk of gouty arthritis flares, it is still relatively difficult to predict who will develop them14. It must be emphasized that not all individuals with hyperuricemia develop gouty arthritis. MSU‐induced inflammation is driven by components of the innate immune system. Recent research has shown that factors such as IL‐1β play important roles in this pathway10, 13. Once sufficient urate deposits have developed around joints, patients will have intermittent attacks of gouty arthritis13. MSU crystals continue to deposit in synovial fluid and tissue, resulting in synovial inflammation and bone destruction during the intervals between such attacks13 (Fig. 1a,b).
Figure 1.
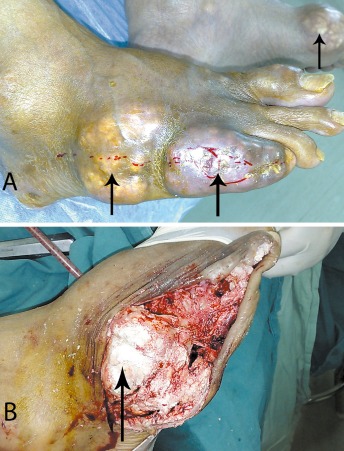
(A) Photographs of both feet of a male patient with gouty arthritis showing joint abnormalities and subcutaneous tophi (arrows) in multiple joints; and (B) Intraoperative photograph showing the presence of intracellular MSU crystals in a joint of the right foot.
In gouty arthritis, bone destruction seems to result from an imbalance in bone remodeling. The relationship between MSU crystals and increased numbers of osteoclasts is not yet fully understood. Zhao et al. reported that in patients with gouty arthritis, serum Dickkopf‐1 concentrations are remarkably increased and there is a positive correlation between concentrations of Dickkopf‐1 and tartrate‐resistant acid phosphatase 5b, which is involved in gouty arthritis‐associated bone destruction15. Lee and colleagues proposed that bone destruction in chronic gouty arthritis is at least in part dependent on expression by T cells of receptor activator of NF‐κB ligand (RANKL)16. Overexpression of RANKL promotes osteoclastogenesis, which is characterized by T cell infiltrates in a proinflammatory environment. Concomitantly, the RANKL antagonist osteoprotegerin is downregulated. Tophus‐associated monocytes contribute tumor necrosis factor‐α, IL‐1β and IL‐6, which play roles as additional promoters of osteoclast differentiation.
Clinical Features and Diagnosis
Although clinical symptoms include swelling, redness and warmth, acute gouty arthritis is typically characterized by rapid onset of pain in the affected joint. These attacks are often self‐limiting, lasting 3–14 days13, 17. Attacks of gouty arthritis usually affect only one joint, a metatarsophalangeal joint being involved in about 50% of initial gouty attacks13. Chronic gouty arthritis is characterized by intense inflammation, persistent joint abnormalities, tophi and nephrolithiasis18.
The presence of intracellular MSU crystals in synovial fluid or in aspirates from tophi is diagnostic of gouty arthritis. If infection is suspected, aspiration is recommended19.
Gouty arthritis commonly involves extremity joints such as the first metatarsophalangeal joint, ankle, knee, wrist and elbow20. However, Konatalapalli et al. reported that axial gout may be a common feature of chronic gouty arthritis21. Clinicians should be aware of spinal involvement with associated back pain in patients with gouty arthritis. In their study, all patients with tophi in the spine had abnormal hand or feet radiographs (P = 0.005). Characteristic findings of gouty arthritis are found in extremity radiographs of 45% of patients, this correlates strongly with CT evidence of axial gout (P < 0.001).
Although the SU concentration during an acute flare of gouty arthritis may not accurately reflect chronic hyperuricemia, the presence of persistent hyperuricemia (SU > 6.8 mg/dl) implies gouty arthritis in patients who present with a history of previous monoarticular arthritic attacks19. Of note, studies have noted that SU concentrations in patients with gout may be significantly lower during acute flares than during the intercritical phase, owing to an increase in the urinary excretion of uric acid22.
Conventional radiography, ultrasound, CT, and MRI contribute to the diagnosis and assessment of gouty arthritis23. Vasil'ev and Obramenko reported that MRI enhances the information obtained by radiographic examination of patients with gouty arthritis and that this improves the ratio of correctly diagnosed cases24. They pointed that both X‐ray films and MRI images show tophus formation in affected joints. Ito et al. reported that 18 F‐fluorodeoxyglucose positron emission tomography demonstrated focal uptake in multiple joints, including the bases of both big toes, and in dense masses in the juxta‐articular soft tissue of the elbows in a patient with gouty arthritis with tophi25. They recommended that gouty arthritis be considered when focal 18 F‐fluorodeoxyglucose uptake is found in dense masses in juxta‐articular soft tissue with or without additional erosions. The radiologic features in soft tissue, bone and joints of patients with chronic tophaceous gout are not usually seen until 6–12 years after the initial attack. The soft tissue findings consist of calcific deposits and eccentric juxta‐articular lobulated masses (hand, elbow, knee, ankle and foot). Bone findings include “mouse bites” from erosion of long‐standing soft‐tissue tophi, bone infarction, “punch‐out” lytic bone lesions and sclerosis of margins. Joint findings comprise erosion of joint margins with sclerosis, cartilage destruction and periarticular swelling20, 26, 27.
Management of Gouty Arthritis
The protocol for management of gouty arthritis focuses on the following objectives: (i) alleviating the pain and inflammation that accompanies acute gouty arthritis flares; (ii) preventing recurrence of gouty arthritis flares; and (iii) preventing urate crystal deposition by prescribing long‐term ULT.
Lifestyle Modifications
Gouty arthritis has been referred to as the “arthritis of the rich” because of its association with rich food and excessive alcohol consumption2. Lifestyle modification alone has a small urate‐lowering effect. Obesity is the commonest comorbid risk with gout1. Therefore, modifications of lifestyle and diet are key components of gout management. However, Su et al. have pointed out that, regardless of the many other relevant environmental and nutritional factors, gout involves homeostatic imbalance of essential trace elements caused by genetic factors and has a unique expression profile of these elements6. Much epidemiological research has demonstrated links between gouty arthritis and a variety of foods and drinks, including beer, meat, seafood and fructose5, 28, whereas high intake of low‐fat food, coffee and ascorbate is thought to be protective29. Supplementation with vitamin C reportedly reduces SU concentrations30. It is to be emphasized that consumption of beer and fructose‐rich beverages also confers a higher risk of gout on women31, 32. A randomized study by Dalbeth et al. reported that intact cow's milk (not soy) decreases SU concentrations by 10% three hours after consumption33. Based on another controlled trial, Dalbeth and colleagues suggested that milk fat extract or skimmed milk enriched with glycomacropeptides significantly reduces the risk of gout flares34.
Treatment of Acute Gout
Acute attacks of gout should be treated resting the affected joint(s) and treating them with ice, which has a significant additional anti‐inflammatory effect35. Pharmacological treatments, including NSAIDs and colchicine, are the most frequently used. However, steroids and IL‐1 inhibitors can also be used in patients who are resistant to NSAIDs and colchicine or for whom these agents are contraindicated7, 8, 36. The xanthine oxidase inhibitors febuxostat or allopurinol are indicated in patients with acute attacks, tophi, and joint destruction; they reduce production of uric acid through inhibiting the enzyme11. However, these agents are not approved for treatment of patients with asymptomatic hyperuricemia.
In addition, Zhou et al. reported that acupuncture combined with infrared irradiation is more effective than oral indomethacin for acute gouty arthritis. An additional advantage is that the former combination provides significant analgesia without impairing liver function37.
Treatment of Chronic Gout
Urate‐lowering Therapy
It is now generally accepted that persistent chronic inflammation is often present in patients with gouty arthritis, even when the patient is asymptomatic20. It is therefore important to use ULT to maintain SU concentrations well below the point of saturation (6.8 mg/dL) in asymptomatic patients; this may prevent continued gouty arthritis flares and urate crystal deposition38. Rapid changes in SU concentrations during the initial stages of ULT therapy are associated with an increased risk of acute gouty arthritis flares; this provides a challenge to the successful management of gouty arthritis39. Initiation of ULT requires appropriate dosage and monitoring of SU concentration. In the early weeks and months of ULT, there is a high incidence of acute flares in patients whose SU concentrations decrease dramatically40. Much research has confirmed the correlation between likelihood of flares and the speed with which SU concentrations drop. In a 14 week randomized controlled trial, 88% of patients had ≥ one flare when their SU concentrations were rapidly reduced (to <6 mg/mL within 6 h). Therefore, many authors advise that reducing SU concentrations slowly to modestly sub‐saturation levels (e.g. 4.6–6.6 mg/dL) reduces the likelihood of flares40, 41. To dissolve tophi, it is necessary to increase the dose of ULT once moderately improved SU concentrations have been achieved40.
An increase in flares is frequently linked with suboptimal patient compliance with ULT42, 43. This phenomenon has been observed regardless of which particular form of ULT is used (e.g. febuxostat, allopurinol, pegloticase, probenecid)44, 45. Randomized controlled studies of febuxostat46, 47, 48 and pegloticase49, 50 have identified the association between initiation of ULT and acute flares. The exact mechanism by which ULT triggers acute flares remains unclear. Becker and colleagues proposed that the increased flare rate that occurs when SU concentrations change rapidly because of ULT might be attributable to alterations in the physical or chemical state of preexisting MSU crystals40. According to this theory, when SU concentrations decrease the relative balance of remodeling and mechanical disruption alters the stability of tophi in the affected joint. Superficial MSU crystals dissolve, which exposes uncoated MSU crystals to synoviocytes and monocytes, stimulating expression of proinflammatory cytokines including IL‐1 and IL‐851.
The following issues remain controversial: (i) which agents to use when initiating ULT; and (ii) the duration of ULT. Basically, ULT is strongly recommended in patients with severe gouty arthritis (presence of tophi, structural bone damage, polyarticular joint involvement and recurrent flares). While taking ULT, NSAIDs, colchicine or corticosteroids should be provided to patients as required. If colchicine and NSAIDs are contraindicated, IL‐1 inhibitors such as canakinumab and rilonacept are useful alternatives for preventing flares. Rilonacept inhibits IL‐1, including IL‐1α and IL‐1RA, and canakinumab selectively binds the IL‐1β pathway.
There is controversy about the optimal protocol for ULT. The aim of treatment is to reduce SU concentrations below 6.8 mg/dL (the ideal point of saturation of uric acid, <6.0 mg/dl)38. It is important to note that ULT alone is not sufficient treatment for gouty arthritis; long term anti‐inflammatory treatment also should be considered.
Ferraz and O'Brien presented analysis of a hypothetical decision model according to which ULT was identified as cost‐saving in patients who had been having ≥ two flares a year52. Neogi recommends that ULT be initiated once flares are well controlled and the patient has been symptom‐free for at least 2 weeks17. There is as yet no consensus on when to start ULT therapy to maintain SU concentrations. Nevertheless, it is crucial to maintain them below 6.8 mg/dL; this helps to prevent future gouty arthritis flares and ensures resorption of tophi18, 38.
Alternative Prophylactic Agents
Colchicine
Colchicine, which blocks microtubule assembly and reduces transport of MSU crystals, is the standard medication for preventing flares during initiation of ULT11. The US Food and Drug Administration approved colchicine for prophylaxis of gouty arthritis flares in 200953.
The efficacy of colchicine for reduction or prevention of gouty arthritis flares on ULT initiation has been shown by a randomized study39. Borstad and colleagues performed a randomized, double‐blind, placebo‐controlled study on colchicine. Patients who receiving colchicine (0.6 mg twice a day) experienced fewer flares than did those receiving placebo (P = 0.008) over 6 months. In addition, the flares were less severe in the colchicine than in the placebo group (P = 0.018). However, the incidence of diarrhea was higher in the colchicine group39.
Adverse events such as nausea, abdominal cramps, diarrhea, blood disorders, neuropathy and myopathy have been reported. Generally, low‐dose colchicine prophylaxis is well tolerated54. The recommended dose for colchicine prophylaxis (0.6 mg once or twice daily) is better tolerated than the higher doses that are used for managing acute gouty arthritis flares (1.2 mg immediately followed by 0.6 mg 1 h later)55. Tolerance for colchicine is dose‐dependent. Of note, dosage reduction is recommended in patients with advanced chronic kidney disease55. When administering colchicine with inhibitors of CYP3A4 or P‐glycoprotein, dosage adjustment is also necessary because of the increased risk of colchicine‐induced toxic effects. Generally, colchicine's efficacy is well established with fewer and less severe flares than with placebo. Prophylactic doses of colchicine are well tolerated18, 45.
Nonsteroidal Anti‐inflammatory Drugs
Schlesingerpointed out that treatment of chronic gouty arthritis is not just about ULT56. It is well accepted that, even in asymptomatic patients, chronic inflammation is usually present in patients with chronic gouty arthritis. Accordingly, long‐term anti‐inflammatory therapy should be combined with ULT. Clinical trials indicate that prophylaxis, including colchicine and NSAIDs, can substantially reduce the severity and incidence of bouts of gouty arthritis; however, there is controversy about whether NSAIDs is an ideal option for prophylaxis of gouty arthritis flares11, 12, 57. Because long‐term NSAIDs are not safe, Schmidt et al.58 and Trelle et al.57 suggest that they are not appropriate choices for prophylaxis. However, the British Society for rheumatology12 and EULAR11 recommend NSAIDs as an option for prophylaxis of gouty arthritis flares.
Corticosteroids
Corticosteroids are a good alternative when patients fail to respond to colchicine and NSAIDs or these agents are contraindicated in refractory cases. Systemic corticosteroids are approved for short‐term administration in patients with acute gouty arthritis flares as adjunctive therapy11. Of note, there is no conclusive evidence that corticosteroids are more effective than other anti‐inflammatory treatments in patients with chronic gouty arthritis28. Doses of corticosteroids should be individualized for each patient. Once symptoms are controlled, maintenance therapy is based on minimum doses and the shortest possible duration to avoid side‐effects28.
Investigational Prophylactic Agents: Interleukin‐1β inhibitors
Recently, IL‐1β blockers such as anakinra9, canakinumab7, 8, 36 and rilonacept10 have been studied for the management of acute gouty arthritis flares. This novel concept stems from the discovery that MSU crystals stimulate attacks of gout by causing IL‐1β secretion3. In a study involving two randomized, multicenter, double‐blind trails, Schlesinger et al. reported that canakinumab provides significant relief of pain and inflammation and reduces the risk of new flares in patient for whom non‐steroidal anti‐inflammatory drugs and colchicine are inappropriate7. In this study, they assessed the efficacy and safety of canakinumab (150 mg, 230 cases) or triamcinolone acetonide (40 mg, 226 cases) at baseline in patients who had been subject to frequent flares. Canakinumab significantly delayed the time to the first new flare and reduced the risk of flare by 62% compared to acetonide. Thus, when NSAIDs and colchicine are contraindicated or poorly tolerated, trials suggest that the IL‐1 inhibitors rilonacept and canakinumab may be useful alternatives for flare prevention. It is important to note that, although both inhibit the IL‐1β pathway, rilonacept also binds IL‐1α whereas canakinumab binds selectively to IL‐1β.
Recommendations for Drug Treatment of Gout
Guidelines for management of gouty arthritis are available from many societies11, 12. EULAR recommend administrated colchicine (0.5–1.0 mg daily) and/or an NSAID (with gastric protection if indicated) as prophylaxis for acute flares during the first month of ULT11. The British Society for Rheumatology and British Health Professionals in Rheumatology recommend administering colchicine 0.5 mg twice daily following initiation of treatment with allopurinol, and continuing it for at least 6 months. Cyclo‐oxygenase inhibitors or NSAIDs can be substituted in patients who cannot tolerate colchicine, but the duration of treatment with NSAIDs or cyclooxygenase 2 inhibitors should be limited to 6 weeks12.
Prophylaxis with colchicine and NSAIDs during ULT initiation substantially reduces the incidence and severity of gouty arthritis flares. However, most clinicians do not prescribe anti‐inflammatory prophylaxis regularly59. A study by Terkeltaub59 reported an analysis of gouty arthritis treatment in the USA. About 2.8 million prescriptions for allopurinol were recommended, whereas 700,000 prescriptions for NSAIDs and approximately 381,000 prescriptions for colchicine were actually written, which illustrates that many physicians do not prescribe prophylaxis when treating gouty arthritis. One survey found that rheumatologists are more likely to prescribe prophylaxis than are non‐rheumatologists60.
Current gouty arthritis treatment recommendations and the existing evidence suggest that prophylaxis should be started 1 to 2 weeks prior to ULT and continued for at least 6 months61. There are two apparently contradictory aspects to use of ULT: (i) gouty arthritis flares occur as a result of manipulation of the uric acid pool; and (ii) there is evidence that gouty arthritis flares are significantly reduced or eliminated if low SU concentrations are maintained62, 63. It is important to note that, when prophylaxis is discontinued after 8 weeks of ULT, the risk of flares increases, the risk depending on the ULT dosage. The higher the dosage, the more flares the patient experiences47, 48. Polyarticular disease and tophi may occur in non‐compliant patients and those with uncontrolled disease.
The management of gouty arthritis in patients with chronic kidney disease (CKD) remains a challenge for clinicians. Curiel and Guzman pointed out that there is an unmeet need for additional treatment options for gouty arthritis patients with CKD64. The main challenges are as follows: (i) the commonly used drugs such as NSAIDs and colchicine are not appropriate in patients with CKD; (ii) corticosteroids may be an effective alternative in these patients; however, their efficacy has not been confirmed by randomized controlled trials and they can cause serious side effects; (iii), febuxostat and pegloticase are possible treatment options for chronic urate‐lowering prophylaxis; however, their safety in patients with CKD is so far unconfirmed reported; and (iv) allopurinol is an alternative for the prophylactic management of chronic hyperuricemia in patients with CKD, but the recommended dosage may not be effective and serious hypersensitivity reactions may preclude its use.
Surgical Treatment of Gout
Although a variety of medications are available for prevention and treatment of gouty arthritis, 5% of patients are unresponsive to medical management and progress to the tophaceous stage65, 66. MSU crystals accumulate slowly and gradually enlarge cavities in bones and joints. Eventually, the cortexes of affected bones become thinner and expand into adjacent soft tissues as the walls are destroyed. These destructive changes usually occur in small bones and joints of the extremities (Fig. 2). Surgical interventions should to be considered in these patients.
Figure 2.
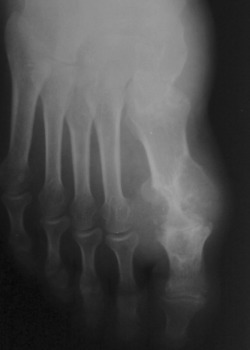
An anteroposterior X‐ray film showing tophus formation and bone destruction of the first metatarsophalangeal joint in a female patient with gouty arthritis.
The Aims of and Indications for Surgical Treatment
The aims of surgical intervention in patients with tophaceous gout are to improve function, alleviate symptomatic discomfort, improve the appearance, eradicate draining sinuses and remove large urate deposits66, 67. Surgical interventions become inevitable when the overlying soft tissue of tophus ulcerates and (or) becomes infected67.
The Methods, Timing and Results of Surgical Treatment
The timing of surgery for tophi affects the functional outcome. It is definitely unnecessary to wait until the skin over the tophaceous mass becomes infected or ulcerated. Lee et al. reported that directly shaving away tophaceous masses is a more straightforward and rapid approach to reducing the total body urate burden67. Furthermore, surgery on a septic tophaceous draining sinus is associated with a relatively high rate of complications such as poor wound healing. It is well accepted that surgery before these ulcerations development is prudent66, 67. Surgical procedures are usually for the restoration of function, cosmetic purposes and control of infection66. Limb‐salvage surgery is the primary goal in patients who warrant surgical treatment. Tophi involving metaphyses can be curetted. Bone defects should be managed with bone grafts and reconstructed with other bone substitutes. Joint fusion in a functional position is necessary for flail joints. Amputation may be considered as a last option66, 68. The risk of overlying skin necrosis and delayed wound healing are important issues in conventional surgical debridement of tophi66, 67. A minimally invasive approach to managing tophi reportedly minimizes wound complications69. Lee et al. recommend an arthroscopic shaver technique to avoid poor wound healing67. In their study, they stated that tophaceous lesions can be treated surgically during the inter‐critical phase. Although the minimally invasive approach to resecting tophi using a soft tissue shaving system has the advantage of decreased risk of wound complications, Lui points out that this is essentially a percutaneous procedure and has the risk of damaging nerves and blood vessels69. In addition, removal of tophaceous deposits might be incomplete when ligaments and joint capsule are involved. Lui strongly recommends an endoscopic approach69. An endoscopic approach allows surgeons to remove tophaceous deposits under arthroscopic visualization and preserve important structures such as ligaments, nerves, and blood vessels, which should minimize the risk of skin necrosis. In addition, the affected joint can be assessed through the portal and the concomitant intra‐articular pathology managed accordingly69. However, it should be emphasized that the surgical method does not completely remove all tophaceous tissue; therefore adjuvant long‐term anti‐urate drug therapy is still recommended67.
Conclusions
Management of gouty arthritis is complex because of the need to consider adverse events, drug inter‐actions, comorbidities and contraindications. The main barrier to more successful management is the need to weigh the risks and benefits associated with different gouty arthritis therapies. Additional new drugs such as IL‐1 inhibitors have been developed to improve the management of resistant gout. A thorough understanding of pathology, diagnosis and various treatment modalities is essential for optimal individualization of management regimens.
Disclosure: All the authors have no interests related to the subject of this article.
References
- 1. Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis, 2005, 64: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nuki G. Treatment of crystal arthropathy—history and advances. Rheum Dis Clin North Am, 2006, 32: 333–357. [DOI] [PubMed] [Google Scholar]
- 3. McGonagle D, Tan AL, Shankaranarayana S, Madden J, Emery P, McDermott MF. Management of treatment resistant inflammation of acute on chronic tophaceous gout with anakinra. Ann Rheum Dis, 2007, 66: 1683–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation, 2007, 116: 894–900. [DOI] [PubMed] [Google Scholar]
- 5. Rider TG, Jordan KM. The modern management of gout. Rheumatology (Oxford), 2010, 49: 5–14. [DOI] [PubMed] [Google Scholar]
- 6. Su M, Zhang T, Zhao T, et al Human gouty arthritis is associated with a distinct serum trace elemental profile. Metallomics, 2012, 4: 244–252. [DOI] [PubMed] [Google Scholar]
- 7. Schlesinger N, Alten RE, Bardin T, et al Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active‐controlled, double‐blind trials and their initial extensions. Ann Rheum Dis, 2012, 71: 1839–1848. [DOI] [PubMed] [Google Scholar]
- 8. So A, De Meulemeester M, Pikhlak A, et al Canakinumab for the treatment of acute flares in difficult‐to‐treat gouty arthritis: results of a multicenter, phase II, dose‐ranging study. Arthritis Rheum, 2010, 62: 3064–3076. [DOI] [PubMed] [Google Scholar]
- 9. So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL‐1 inhibition by anakinra in acute gout. Arthritis Res Ther, 2007, 9: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Terkeltaub R, Sundy JS, Schumacher HR, et al The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo‐controlled, monosequence crossover, non‐randomised, single‐blind pilot study. Ann Rheum Dis, 2009, 68: 1613–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang W, Doherty M, Bardin T, et al EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis, 2006, 65: 1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jordan KM, Cameron JS, Snaith M, et al British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford), 2007, 46: 1372–1374. [DOI] [PubMed] [Google Scholar]
- 13. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med, 2008, 75 (Suppl 5): S5–S8. [DOI] [PubMed] [Google Scholar]
- 14. Martinon F. Update on biology: uric acid and the activation of immune and inflammatory cells. Curr Rheumatol Rep, 2010, 12: 135–141. [DOI] [PubMed] [Google Scholar]
- 15. Zhao W, Gao H, Zhu JX, et al Relation of serum Dickkopf‐1 with bone destruction in patients with gouty arthritis. Beijing Da Xue Xue Bao, 2012, 44: 254–258. (In Chinese) [PubMed] [Google Scholar]
- 16. Lee SJ, Nam KI, Jin HM, et al Bone destruction by receptor activator of nuclear factor κB ligand‐expressing T cells in chronic gouty arthritis. Arthritis Res Ther, 2011, 13: R164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neogi T. Clinical practice. Gout. N Engl J Med, 2011, 364: 443–452. [DOI] [PubMed] [Google Scholar]
- 18. Li‐Yu J, Clayburne G, Sieck M, et al Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout? J Rheumatol, 2001, 28: 577–580. [PubMed] [Google Scholar]
- 19. Becker MA, Ruoff GE. What do I need to know about gout? J Fam Pract, 2010, 59 (6 Suppl): S1–S8. [PubMed] [Google Scholar]
- 20. Schlesinger N. Diagnosing and treating gout: a review to aid primary care physicians. Postgrad Med, 2010, 122: 157–161. [DOI] [PubMed] [Google Scholar]
- 21. Konatalapalli RM, Lumezanu E, Jelinek JS, Murphey MD, Wang H, Weinstein A. Correlates of axial gout: a cross‐sectional study. J Rheumatol, 2012, 39: 1445–1449. [DOI] [PubMed] [Google Scholar]
- 22. Urano W, Yamanaka H, Tsutani H, et al The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol, 2002, 29: 1950–1953. [PubMed] [Google Scholar]
- 23. Thiele RG. Role of ultrasound and other advanced imaging in the diagnosis and management of gout. Curr Rheumatol Rep, 2011, 13: 146–153. [DOI] [PubMed] [Google Scholar]
- 24. Vasil'ev A, Obramenko IE. Syndromic approach to MR diagnosis of gouty arthritis. Vestn Rentgenol Radiol, 2011, 3: 32–35.(In Russian) [PubMed] [Google Scholar]
- 25. Ito K, Minamimoto R, Morooka M, Kubota K. A case of gouty arthritis to tophi on 18F‐FDG PET/CT imaging. Clin Nucl Med, 2012, 37: 614–617. [DOI] [PubMed] [Google Scholar]
- 26. Manger B, Lell M, Wacker J, Schett G, Rech J. Detection of periarticular urate deposits with dual energy CT in patients with acute gouty arthritis. Ann Rheum Dis, 2012, 71: 470–472. [DOI] [PubMed] [Google Scholar]
- 27. Choe JY, Lee GH, Kim SK. Radiographic bone damage in chronic gout is negatively associated with the inflammatory cytokines soluble interleukin 6 receptor and osteoprotegerin. J Rheumatol, 2011, 38: 485–491. [DOI] [PubMed] [Google Scholar]
- 28. Singh JA, Reddy SG, Kundukulam J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol, 2011, 23: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li S, Micheletti R. Role of diet in rheumatic disease. Rheum Dis Clin North Am, 2011, 37: 119–133. [DOI] [PubMed] [Google Scholar]
- 30. Juraschek SP, Miller ER 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta‐analysis of randomized controlled trials. Arthritis Care Res (Hoboken), 2011, 63: 1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaffo AL, Roseman JM, Jacobs DR Jr, et al Serum urate and its relationship with alcoholic beverage intake in men and women: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Ann Rheum Dis, 2010, 69: 1965–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi HK, Willett W, Curhan G. Fructose‐rich beverages and risk of gout in women. JAMA, 2010, 304: 2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalbeth N, Wong S, Gamble GD, et al Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis, 2010, 69: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 34. Dalbeth N, Ames R, Gamble GD, et al Effects of skim milk powder enriched with glycomacropeptide and G600 milk fat extract on frequency of gout flares: a proof‐of‐concept randomised controlled trial. Ann Rheum Dis, 2012, 71: 929–934. [DOI] [PubMed] [Google Scholar]
- 35. Schlesinger N. Response to application of ice may help differentiate between gouty arthritis and other inflammatory arthritides. J Clin Rheumatol, 2006, 12: 275–276. [DOI] [PubMed] [Google Scholar]
- 36. Schlesinger N, De Meulemeester M, Pikhlak A, et al Canakinumab relieves symptoms of acute flares and improves health‐related quality of life in patients with difficult‐to‐treat Gouty Arthritis by suppressing inflammation: results of a randomized, dose‐ranging study. Arthritis Res Ther, 2011, 13: R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou L, Xu QF, Zhang WS. Comparative observation of the efficacy on acute gouty arthritis between acupuncture combined with infrared irradiation and western medicine. Zhongguo Zhen Jiu, 2011, 31: 787–789. [PubMed] [Google Scholar]
- 38. Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum, 2004, 51: 321–325. [DOI] [PubMed] [Google Scholar]
- 39. Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol, 2004, 31: 2429–2432. [PubMed] [Google Scholar]
- 40. Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph‐Ridge N. Determinants of the clinical outcomes of gout during the first year of urate‐lowering therapy. Nucleosides Nucleotides Nucleic Acids, 2008, 27: 585–591. [DOI] [PubMed] [Google Scholar]
- 41. Yamanaka H, Togashi R, Hakoda M, et al Optimal range of serum urate concentrations to minimize risk of gouty attacks during anti‐hyperuricemic treatment. Adv Exp Med Biol, 1998, 431: 13–18. [DOI] [PubMed] [Google Scholar]
- 42. Riedel AA, Nelson M, Joseph‐Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol, 2004, 31: 1575–1581. [PubMed] [Google Scholar]
- 43. Schwabbauer ML. Use of the latent image technique to develop and evaluate problem‐solving skills. Am J Med Technol, 1975, 41: 457–462. [PubMed] [Google Scholar]
- 44. Sarawate CA, Patel PA, Schumacher HR, Yang W, Brewer KK, Bakst AW. Serum urate levels and gout flares: analysis from managed care data. J Clin Rheumatol, 2006, 12: 61–65. [DOI] [PubMed] [Google Scholar]
- 45. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate‐lowering therapy: analysis of data from three phase III trials. Clin Ther, 2010, 32: 2386–2397. [DOI] [PubMed] [Google Scholar]
- 46. Schumacher HR Jr, Becker MA, Wortmann RL, et al Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis Rheum, 2008, 59: 1540–1548. [DOI] [PubMed] [Google Scholar]
- 47. Becker MA, Schumacher HR Jr, Wortmann RL, et al Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med, 2005, 353: 2450–2461. [DOI] [PubMed] [Google Scholar]
- 48. Becker MA, Schumacher HR, Espinoza LR, et al The urate‐lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther, 2010, 12: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sundy JS, Becker MA, Baraf HS, et al Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol‐conjugated uricase) in patients with treatment‐failure gout: results of a phase II randomized study. Arthritis Rheum, 2008, 58: 2882–2891. [DOI] [PubMed] [Google Scholar]
- 50. Sundy JS, Ganson NJ, Kelly SJ, et al Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum, 2007, 56: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 51. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout‐associated uric acid crystals activate the NALP3 inflammasome. Nature, 2006, 440: 237–241. [DOI] [PubMed] [Google Scholar]
- 52. Ferraz MB, O'Brien B. A cost effectiveness analysis of urate lowering drugs in nontophaceous recurrent gouty arthritis. J Rheumatol, 1995, 22: 908–914. [PubMed] [Google Scholar]
- 53. Wertheimer AI, Davis MW, Lauterio TJ. A new perspective on the pharmacoeconomics of colchicine. Curr Med Res Opin, 2011, 27: 931–937. [DOI] [PubMed] [Google Scholar]
- 54. Kuritzky L, Panchal R. Gout: nonsteroidal anti‐inflammatory drugs and colchicine to prevent painful flares during early urate‐lowering therapy. J Pain Palliat Care Pharmacother, 2010, 24: 397–401. [DOI] [PubMed] [Google Scholar]
- 55. Yang LP. Oral colchicine (colcrys®) in the treatment and prophylaxis of gout†: profile report. Drugs Aging, 2010, 27: 855–857. [DOI] [PubMed] [Google Scholar]
- 56. Schlesinger N. Treatment of chronic gouty arthritis: it is not just about urate‐lowering therapy. Semin Arthritis Rheum, 2012, 42: 155–165. [DOI] [PubMed] [Google Scholar]
- 57. Trelle S, Reichenbach S, Wandel S, et al Cardiovascular safety of non‐steroidal anti‐inflammatory drugs: network meta‐analysis. BMJ, 2011, 342: c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schmidt M, Christiansen CF, Horvath‐Puho E, Glynn RJ, Rothman KJ, Sorensen HT. Non‐steroidal anti‐inflammatory drug use and risk of venous thromboembolism. J Thromb Haemost, 2011, 9: 1326–1333. [DOI] [PubMed] [Google Scholar]
- 59. Terkeltaub R. Gout. Novel therapies for treatment of gout and hyperuricemia. Arthritis Res Ther, 2009, 11: 236 (on‐line). http://arthritis‐research.com/content/11/4/236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barber C, Thompson K, Hanly JG. Impact of a rheumatology consultation service on the diagnostic accuracy and management of gout in hospitalized patients. J Rheumatol, 2009, 36: 1699–1704. [DOI] [PubMed] [Google Scholar]
- 61. Justiniano M, Dold S, Espinoza LR. Rapid onset of muscle weakness (rhabdomyolysis) associated with the combined use of simvastatin and colchicine. J Clin Rheumatol, 2007, 13: 266–268. [DOI] [PubMed] [Google Scholar]
- 62. Silva L, Miguel ED, Peiteado D, et al Compliance in gout patients. Acta Reumatol Port, 2010, 35: 466–474. [PubMed] [Google Scholar]
- 63. Sarawate CA, Brewer KK, Yang W, et al Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc, 2006, 81: 925–934. [DOI] [PubMed] [Google Scholar]
- 64. Curiel RV, Guzman NJ. Challenges associated with the management of gouty arthritis in patients with chronic kidney disease: a systematic review. Semin Arthritis Rheum, 2012, 42: 166–178. [DOI] [PubMed] [Google Scholar]
- 65. Kang EH, Lee EY, Lee YJ, Song YW, Lee EB. Clinical features and risk factors of postsurgical gout. Ann Rheum Dis, 2008, 67: 1271–1275. [DOI] [PubMed] [Google Scholar]
- 66. Larmon WA. Surgical management of tophaceous gout. Clin Orthop Relat Res, 1970, 71: 56–69. [PubMed] [Google Scholar]
- 67. Lee SS, Sun IF, Lu YM, Chang KP, Lai CS, Lin SD. Surgical treatment of the chronic tophaceous deformity in upper extremities—the shaving technique. J Plast Reconstr Aesthet Surg, 2009, 62: 669–674. [DOI] [PubMed] [Google Scholar]
- 68. Ertuğrul Sener E, Güzel VB, Takka S. Surgical management of tophaceous gout in the hand. Arch Orthop Trauma Surg, 2000, 120: 482–483. [DOI] [PubMed] [Google Scholar]
- 69. Lui TH. Endoscopic resection of the gouty tophi of the first metatarsophalangeal joint. Arch Orthop Trauma Surg, 2008, 128: 521–523. [DOI] [PubMed] [Google Scholar]


