Key Points
Question
Are surgical procedures that require general anesthesia in early childhood associated with adverse child development outcomes?
Findings
This population-based study of 10 897 sibling pairs aged 5 to 6 years used the Early Development Instrument (a population-based measure of child development before primary school entry) data for Ontario, Canada, and found no differences in the adjusted odds of early developmental vulnerability or scores in major developmental domains between biological siblings when children were exposed to surgical procedures that require general anesthesia.
Meaning
After mitigating for biological vulnerability and environmental factors, exposure to surgical procedures that require general anesthesia in early childhood was not associated with increased risks of adverse child development outcomes.
This population-based sibling-matched study assesses the association between surgical procedures that require anesthesia in children aged 5 to 6 years and child development in Ontario, Canada.
Abstract
Importance
Substantial preclinical evidence suggests that the developing brain is susceptible to injury from anesthetic drugs. Findings from clinical studies of the neurotoxic effects of anesthesia are mixed, but these effects can be influenced by unmeasured confounding from biological and environmental risk and protective factors on child development.
Objective
To examine the association between surgical procedures that require general anesthesia before primary school entry and child development in biological siblings.
Design, Setting, and Participants
This retrospective sibling-matched cohort study included sibling pairs aged 5 to 6 years with the same birth mother who had Early Development Instrument (EDI) data completed. The EDI is a population-based measure of child development that assesses children’s readiness to learn in 5 major domains (physical health and well-being, social knowledge and competence, emotional health and maturity, language and cognitive development, and communication skills and general knowledge). All eligible children in public and Catholic schools in Ontario, Canada, from 2004 through 2012 were included. Data were analyzed from December 13, 2017, through July 27, 2018.
Exposures
Surgical procedures that require general anesthesia from the date of birth to EDI completion.
Main Outcomes and Measures
Early developmental vulnerability, defined as any major domain of the EDI in the lowest 10th percentile of the Ontario population.
Results
Of the 187 226 eligible children for whom the EDI was completed, a total of 10 897 sibling pairs (21 794 children; 53.8% female; mean [SD] age, 5.7 [0.3] years) were subsequently identified, including 2346 with only 1 child exposed to surgery. No significant differences were found between exposed and unexposed children in early developmental vulnerability (697 of 3080 [22.6%] vs 3739 of 18 714 [20.0%]; adjusted odds ratio [aOR], 1.03; 95% CI, 0.98-1.14; P = .58) or for each of the 5 major EDI domains (aOR for language and cognitive development, 0.96 [95% CI, 0.80-1.14]; aOR for physical health and well-being, 1.09 [95% CI, 0.96-1.24]; aOR for social knowledge and competence, 0.98 [95% CI, 0.84-1.14]; aOR for emotional health and maturity, 0.98 [95% CI, 0.84-1.14]; and aOR for communication skills and general knowledge, 0.90 [95% CI, 0.77-1.05]), after adjusting for confounding factors (age at EDI completion, sex, mother’s age at birth, and eldest sibling status).
Conclusions and Relevance
In this provincial cohort study, children who had surgical procedures that require general anesthesia before primary school entry were not found to be at increased risk of adverse child development outcomes compared with their biological siblings who did not have surgery. These findings further support that anesthesia exposure in early childhood is not associated with detectable adverse child development outcomes.
Introduction
Substantial preclinical evidence suggests that the developing brain is susceptible to injury from anesthetic drugs and painful interventions.1,2 The US Food and Drug Administration has issued warnings about using general anesthetic drugs in young children,3 and this issue remains a priority for pediatric health care professionals.4 Multiple mechanisms have been implicated in the development of neurotoxic effects of pediatric anesthesia,5 and with rapid neurodevelopment in childhood there is a distinct potential for a range of neurologic deficits to occur.6 As a consequence, translation of preclinical findings to humans has been difficult, and clinical studies have provided heterogeneous and mixed findings.7 In general, large cohort studies have found no or very modest associations between exposure to surgical procedures that require general anesthesia in early childhood and neurodevelopmental or educational outcomes.8,9,10 However, despite using rich data sources, these studies are often criticized for being at risk of confounding due to other factors affecting child development.11
Adverse child development is a function of the complex interaction between de novo genetic, familial, and environmental risk and protective factors.12 Most often, these covariates cannot be directly or comprehensively addressed in the types of observational cohorts used to investigate neurotoxic effects of pediatric anesthesia. For example, an association between exposure to anesthetics and attention-deficit/hyperactivity disorder has been demonstrated repeatedly.13,14 However, although susceptible to environmental factors,15 attention-deficit/hyperactivity disorder has a strong genetic component and is considered to be highly familial. As a result of these findings and potential differences in the use of health care services among children with developmental problems,16 the cumulative risk burden can differ significantly among children and sampling of even large cohorts may not reduce these inherent biases.17
Previous investigators have used sibling-controlled cohorts to mitigate for these factors,18,19 but have not detected differences in neurodevelopmental and educational outcomes measured between exposed and unexposed siblings. However, what has become more evident from recent studies is that the magnitude of risk associated with neurotoxic effects of pediatric anesthesia represents only a small fraction of the variability seen and much less than other perinatal, home environmental, and social covariates (eg, smoking while pregnant, maternal educational level, or family income assistance).9,10,20 As a result, larger sample populations may be required to detect potentially small differences in child development outcomes. The aim of this study was to investigate in siblings whether anesthesia and surgery in early childhood are associated with adverse child development as measured by the Early Development Instrument (EDI), a population-based measure of child development.21 By examining differences between biological siblings in Ontario, Canada, we sought to mitigate risks of unmeasured confounding from biological vulnerability and environmental factors to provide a more accurate estimate of the risk of adverse child development after surgery performed before primary school entry.
Methods
The research ethics boards at The Hospital for Sick Children, Toronto, Ontario, and McMaster University, Hamilton, Ontario, approved the study. Data housed at the Institute for Clinical Evaluative Sciences (ICES), Toronto, are deidentified and a waiver of participant consent was obtained.
Study Design
This sibling-controlled cohort study used the EDI and population-based health and demographic administrative databases housed at ICES. The linkage and assembly of the Ontario EDI-ICES database have been previously described.8 The EDI is a 103-item teacher-completed questionnaire used to assess child development before primary school entry (ages 5-6 years) in 5 major domains (physical health and well-being, social knowledge and competence, emotional health and maturity, language and cognitive development, and communication skills and general knowledge).22 The EDI is a validated, population-based measure of child development, demonstrates high levels of reliability,22,23 can distinguish between children of different levels of ability,24 and has moderate concurrent validity with direct measures of child development.22 A complete description of the EDI development, domains, and validation is available from the Offord Centre for Child Studies.25
Data Collection Periods
Data collection for the EDI in Ontario was undertaken in all public and Catholic schools in 3 consecutive cycles from 2004 through 2012. All health care interventions recorded in the Discharge Abstract Database and Same Day Surgery database from birth to the date of EDI completion were considered for eligibility.
Study Population
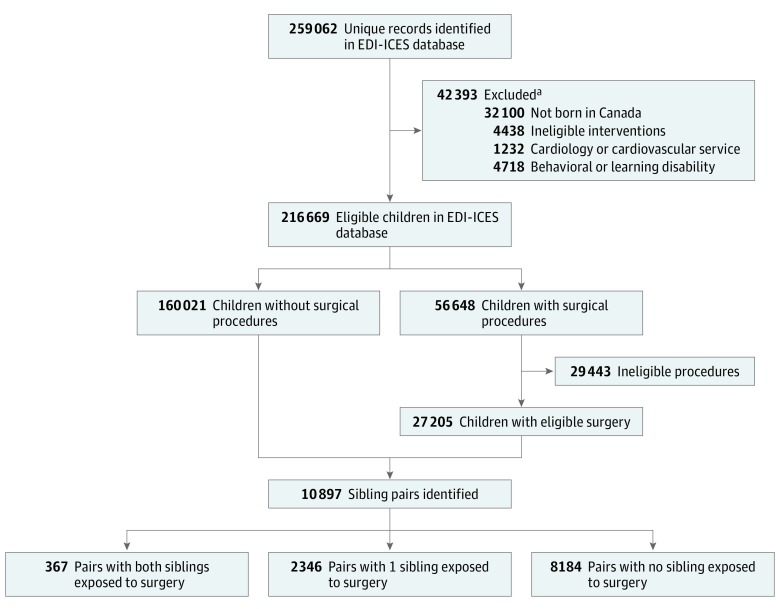
This study cohort was assembled from 259 062 unique records for children in the EDI-ICES database. Children not born in Canada, children with potential health care–related causes of impaired child development (eg, a history of fetal intervention, radiation therapy, brachytherapy, pharmacotherapy, or chemotherapy and those followed by a cardiology or cardiovascular service), and children who had behavioral, learning, or developmental problems recorded in the EDI were first excluded (Figure). Children with EDI-recorded behavioral, learning, or developmental problems were excluded in the primary analysis because the time of diagnosis relative to exposure could not be confirmed, and the tendency for increased use of health care services among this population could introduce a risk of selection bias. Procedures were identified by exclusion using either Canadian Classification of Health Interventions therapeutic intervention codes or Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures codes (eTable 1 in the Supplement). Siblings with the same birth mother were identified from the mother’s ICES number—a unique confidential identifier assigned to each individual for use across ICES databases—from the hospital admission at the time of the child’s birth. Sibling pairs were then classified by exposure to surgery (both siblings had surgery, neither sibling had surgery, or only 1 sibling had surgery).
Figure. Study Flow Diagram.
The EDI-ICES database consists of Early Development Instrument (EDI) data housed at the Institute for Clinical Evaluative Sciences (ICES), Toronto, Ontario, Canada.
aSome children had multiple reasons for exclusions.
Exposures and Outcomes
The primary outcome was early developmental vulnerability, defined as any major domain of the EDI in the lowest 10th percentile of the Ontario population.26 Secondary outcomes included performance in major EDI domains and a multiple challenge index, defined as vulnerability in at least 9 subdomains of the EDI.21 Normative data for the Ontario population were determined using the first (2004 through 2006) cycle of the EDI.25
Covariates
Demographic characteristics available included aboriginal status, age at completion of EDI, age at the time of first surgery, eldest sibling status (among all siblings in the same family), median neighborhood household income quintile, rurality of residence, and sex. Birth characteristics included gestational age at birth, mother’s age at birth, and multiple births; surgical admission characteristics included admission category (elective, newborn, or urgent), cumulative length of hospital stay, type of surgery, and physiological complexity of surgical procedures (based on the relative value guide for anesthesia billing). Relative value guides for billing are validated for discriminating the physiological complexity of surgical procedures. Similar to previous studies, the physiological complexity of surgical procedures in this Ontario-based cohort was classified using anesthesia basic unit values for individual Canadian Classification of Health Interventions therapeutic intervention codes in the 2015 Ontario Health Insurance Plan Schedule of Benefits,27 and surgical procedures with no more than 7 basic units were classified as nonphysiologically complex.28,29 Examples of commonly performed surgical procedures with no more than 7 basic units include tonsillectomy (6 basic units), herniotomy (6-7 basic units), and circumcision (6 basic units).
Statistical Analysis
Data were analyzed from December 13, 2017, through July 27, 2018. Baseline characteristics of sibling groups were reported using standard descriptive statistics and compared using 2-tailed t tests and χ2 tests as appropriate. Unadjusted differences in EDI outcomes were estimated for each of the sibling groups. To account for clustering, multivariable mixed-effects linear and logistic models each with pair-level random intercepts were used to determine the adjusted association between exposure to surgery (independent variable) and EDI outcomes (early developmental vulnerability [primary outcome], a multiple challenge index, and scores in specific EDI domains). Covariates (age at EDI completion, sex, mother’s age at birth, and eldest sibling status) adjusted for in the models were specified a priori based on multivariable analyses of demographic and health care–related factors (eTable 2 in the Supplement) and differences in baseline characteristics between exposure groups. Subgroup and secondary analyses were performed using discordant sibling pairs (ie, only 1 sibling had surgery). A post hoc sensitivity analysis including children who had EDI-recorded behavioral, learning, or developmental problems in the cohort was performed to examine whether the exclusion of these children from the primary analysis had introduced a risk of bias toward the null. Adjusted associations are presented as adjusted odds ratios (aORs) and 95% CIs. Statistical significance was defined as 2-tailed P < .005, correcting for multiple comparisons. All analyses were performed using SAS software (version 9.4; SAS Institute Inc).
Results
Characteristics
From 259 062 unique records in the EDI-ICES database, 187 226 children with or without eligible surgery were identified (Figure). Of these, 27 205 (14.5%) underwent eligible surgical procedures and 160 021 (85.5%) had no exposure to surgery. The characteristics of all eligible children are summarized in eTable 3 in the Supplement.
The study cohort consisted of 21 794 children classified by sibling and exposure status (10 897 sibling pairs) (53.8% female and 46.2% male; mean [SD] age, 5.7 [0.3] years). A total of 8184 sibling pairs had no exposure to surgery; 367 sibling pairs, exposure for both; and 2346 sibling pairs, exposure for 1 sibling (Figure). Descriptive characteristics of the sibling groups are summarized in Table 1. Overall, 3080 (14.1%) of 21 794 children were exposed to surgery that required general anesthesia. Most children who had surgery were 2 years or older at the time of first surgery (1835 [59.6%]), had a same-day surgery and discharge (2429 [78.9%]), had only 1 surgery performed before EDI completion (2489 [80.8%]), and had a nonphysiologically complex procedure performed (2813 [91.3%]). The most common anatomical categories of surgical procedures performed (based on Canadian Classification of Health Interventions or Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures codes) were ear and mastoid (1173 [38.1%]), oral cavity and pharynx (1035 [33.6%]), male genital organs (461 [15.0%]), and musculoskeletal (427 [13.9%]). Among children in the discordant group (ie, only 1 sibling had surgery), children exposed to surgery were more likely to be male (1547 [65.9%] vs 881 [37.6%]; P < .001), to be the eldest child in their family (1323 [56.4%] vs 1139 [48.6%]; P < .001), to have the EDI completed at an older age (P = .04), and have a younger mother at birth (median age, 30 years [interquartile range, 26-33 years] vs 30 years [interquartile range, 27-33 years]; P = .007).
Table 1. Characteristics of Sibling Pairs, Classified by Exposure to Surgery.
| Characteristic | Siblingsa | |||
|---|---|---|---|---|
| Concordant Pairs | Discordant Pairs | |||
| Both Surgery (n = 734) | Neither Surgery (n = 16 368) | No Surgery (n = 2346) | Surgery (n = 2346) | |
| Age at EDI completion, mean (SD), y | 5.7 (0.3) | 5.7 (0.3) | 5.7 (0.3) | 5.7 (0.3) |
| Male, No. (%) | 434 (59.1) | 7210 (44.0) | 881 (37.6) | 1547 (65.9) |
| Gestational age at delivery, mean (SD), wk | 39.1 (3.2) | 39.2 (1.5) | 38.9 (1.5) | 38.9 (2.3) |
| Multiple births, No. (%) | ≤5 (0.1) | 74 (0.4) | 118 (5.0) | 122 (5.2) |
| Neighborhood income quintile, No. (%) | ||||
| Missing | 94 (12.8) | 2179 (13.3) | 10 (0.4) | 12 (0.5) |
| 1 | 109 (14.8) | 2781 (17.0) | 285 (12.1) | 298 (12.7) |
| 2 | 154 (21.0) | 3586 (21.9) | 365 (15.6) | 382 (16.3) |
| 3 | 192 (26.2) | 4085 (25.0) | 537 (22.9) | 503 (21.4) |
| 4 | 183 (24.9) | 3663 (22.4) | 637 (27.2) | 640 (27.3) |
| 5 | ≤5 (0.3) | 74 (0.4) | 512 (21.8) | 511 (21.8) |
| Aboriginal, No. (%) | 11 (1.5) | 140 (0.8) | 19 (0.8) | 20 (0.8) |
| Rural home location, No. (%) | 107 (14.6) | 1696 (10.4) | 251 (10.7) | 258 (11.0) |
| Mother’s age at birth, median (IQR), y | 29 (26-33) | 30 (27-33) | 30 (27-33) | 30 (26-33) |
| Eldest child | NA | NA | 1139 (48.6) | 1323 (56.4) |
| No. of surgery exposure(s), No. (%) | ||||
| 0 | 0 | 16 368 (100) | 2346 (100) | 0 |
| 1 | 525 (71.5) | 0 | 0 | 1964 (83.7) |
| ≥2 | 209 (28.5) | 0 | 0 | 382 (16.3) |
| Age at time of first surgery, y, No. (%) | ||||
| <2 | 320 (43.6) | 0 | 0 | 925 (39.4) |
| ≥2 | 414 (56.4) | 0 | 0 | 1421 (60.6) |
| Surgical procedures with ≤7 OHIP anesthesia basic units, No. (%) | 691 (94.1) | 0 | 0 | 2122 (90.4) |
| Same day surgery and discharge, No. (%) | 604 (82.3) | 0 | 0 | 1825 (77.8) |
| Cumulative hospital LOS, median (IQR), d | 1 (1-2) | 0 | 0 | 1 (1-2) |
Abbreviations: EDI, Early Development Instrument; IQR, interquartile range; LOS, length of stay; OHIP, Ontario Health Insurance Plan; NA, not applicable.
Cell counts of 5 or less cannot be reported.
Primary Analysis: Association Between Surgery and Early Developmental Vulnerability
Unadjusted EDI outcomes for all sibling pairs classified by exposure to surgery are summarized in Table 2. Overall, children exposed to surgery had a higher unadjusted risk of early developmental vulnerability compared with children not exposed to surgery (697 of 3080 [22.6%] vs 3739 of 18 714 [20.0%]; P < .001). After adjusting for potential confounding (age at EDI completion, sex, mother’s age at birth, and eldest sibling status), exposure to surgery before primary school age was not found to increase the adjusted risk of early developmental vulnerability (aOR of early developmental vulnerability, 1.03; 95% CI, 0.93-1.14; P = .58). No significant differences were found in the adjusted risk of a multiple challenge index (aOR, 1.16; 95% CI, 0.89-1.51; P = .27), vulnerability (<10th percentile) in major EDI domains or scores in major EDI domains after exposure to surgery (Table 3).
Table 2. Unadjusted EDI Outcomes for Sibling Pairs, Classified by Exposure to Surgery.
| Outcome by Domain | Siblings | |||
|---|---|---|---|---|
| Concordant Pairs | Discordant Pairs | |||
| Both Surgery (n = 734) | Neither Surgery (n = 16 368) | No Surgery (n = 2346) | Surgery (n = 2346) | |
| Early developmental vulnerability, No. (%) | 154 (21.0) | 3307 (20.2) | 432 (18.4) | 543 (23.1) |
| Multiple challenge index, No. (%) | 9 (1.2) | 294 (1.8) | 35 (1.5) | 66 (2.8) |
| Language and cognitive development | ||||
| Domain score, mean (SD) | 8.93 (1.32) | 8.94 (1.43) | 8.97 (1.42) | 8.85 (1.52) |
| ≤10th Percentile, No. (%) | 33 (4.5) | 884 (5.4) | 131 (5.6) | 146 (6.2) |
| Physical health and well-being | ||||
| Domain score, mean (SD) | 8.97 (1.16) | 9.07 (1.15) | 9.06 (1.16) | 8.97 (1.20) |
| ≤10th Percentile, No. (%) | 75 (10.2) | 1571 (9.6) | 225 (9.6) | 272 (11.6) |
| Social knowledge and competence | ||||
| Domain score, mean (SD) | 8.63 (1.52) | 8.70 (1.56) | 8.75 (1.50) | 8.51 (1.66) |
| ≤10th Percentile, No. (%) | 41 (5.6) | 934 (5.7) | 123 (5.2) | 161 (6.9) |
| Emotional health and maturity | ||||
| Domain score, mean (SD) | 8.32 (1.25) | 8.35 (1.33) | 8.43 (1.29) | 8.17 (1.42) |
| ≤10th Percentile, No. (%) | 44 (6.0) | 1074 (6.6) | 147 (6.3) | 201 (8.6) |
| Communication skills and general knowledge | ||||
| Domain score, mean (SD) | 8.22 (2.13) | 8.16 (2.28) | 8.27 (2.20) | 8.10 (2.31) |
| ≤10th Percentile, No. (%) | 47 (6.4) | 1290 (7.9) | 165 (7.0) | 198 (8.4) |
Abbreviation: EDI, Early Development Instrument.
Table 3. Adjusted Odds of Early Developmental Vulnerability, a Multiple Challenge Index, or Major Domains in the Lowest Tenth Percentile and Adjusted Estimates for EDI Major Domain Scores After Exposure to Surgery Before Primary School Entry.
| EDI Outcome | ≤10th Percentile | Score | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Overall early developmental vulnerability | 1.03 (0.98 to 1.14) | .58 | NA | NA |
| Multiple challenge index | 1.16 (0.89 to 1.51) | .27 | NA | NA |
| Major EDI domains | ||||
| Language and cognitive development | 0.96 (0.80 to 1.14) | .61 | 0.00 (−0.06 to 0.05) | .86 |
| Physical health and well-beinga | 1.09 (0.96 to 1.24) | .19 | −0.04 (−0.09 to −1.95) | .05 |
| Social knowledge and competence | 0.98 (0.84 to 1.14) | .83 | −0.04 (−0.10 to 0.01) | .13 |
| Emotional heath and maturity | 0.98 (0.84 to 1.14) | .81 | −0.02 (−0.07 to 0.03) | .40 |
| Communication skills and general knowledge | 0.90 (0.77 to 1.05) | .17 | 0.05 (−0.03 to 0.14) | .20 |
Abbreviations: EDI, Early Development Instrument; NA, not applicable; OR, odds ratio.
Not adjusted for eldest sibling status due to lack of model convergence.
A total of 323 sibling pairs were otherwise eligible but not included in the primary analysis because 1 of the children (52 exposed and 271 unexposed) had an EDI-recorded behavioral, learning, or developmental problem. In a sensitivity analysis including these children (11 220 sibling pairs), we found no significant differences between exposure groups in the adjusted risk of early developmental vulnerability, overall or for any major domains of the EDI (eTable 4 in the Supplement).
Subgroup Analysis: Exposure to Surgery Among Discordant Sibling Pairs and Child Development Outcomes
In the 2346 discordant sibling pairs after adjusting for potential confounding (age at EDI completion, sex, mother’s age at birth, and eldest sibling status), we found no significant difference between biological siblings for early developmental vulnerability. No significant differences in the aOR of early developmental vulnerability (1.14; 95% CI, 0.98-1.33; P = .09) or a multiple challenge index (1.51; 95% CI, 0.98-2.32; P = .06) were found after exposure to surgery before primary school entry. In addition, no significant differences in vulnerability (<10th percentile) or adjusted estimates for any major EDI domains were found (aOr for language and cognitive development, 0.92 [95% CI, 0.71-1.19; P = .53]; aOR for physical health and well-being, 1.12 [95% CI, 0.92-1.37; P = .27]; aOR for social knowledge and competence, 1.09 [95% CI, 0.85-1.41; P = .49]; aOR for emotional health and maturity, 1.07 [95% CI, 0.84-1.34; P = .60]; and aOR for communication skills and general knowledge, 1.03 [95% CI, 0.81-1.30; P = .82]).
Among children exposed to surgery in this subgroup (n = 2346), health care–related factors—age category at first exposure (<2 vs ≥2 years), multiple exposures (1 vs >1), and physiological complexity (≥7 vs >8 Ontario Health Insurance Plan basic units)—did not alter the risk of adverse EDI outcomes after adjusting for other potential confounding factors (eTable 2 in the Supplement). Children younger than 2 years at the time of surgery had lower adjusted odds of vulnerability in the domain of social competence (aOR, 0.63; 95% CI, 0.44-0.91), but this factor did not reach statistical significance (P = .01).
Discussion
In this sibling-matched cohort of children in Ontario, Canada, we found no differences in the adjusted odds of early developmental vulnerability or performance in any major developmental domain between biological siblings after exposure to surgical procedures that require general anesthesia. Health care–related factors—age at first exposure, number of exposures, and physiological complexity of surgical procedures—were not associated with increased risks of adverse child development outcomes among children who underwent surgical procedures. This study further supports that surgery in early childhood should not be delayed for consideration of neurologic injury due to anesthetic drugs.
These findings support the conclusions of previous research using the same outcome measure8,9 and highlight the important contribution of genetic and home environmental factors to child development. Similar to previous studies, most children in this cohort underwent a single nonphysiologically complex surgical procedure. As a consequence, although the findings are generalizable to most children who undergo surgical procedures before primary school age, we do not know whether they can be applied to children with repeated or lengthy exposures to surgery and anesthesia or other health care–related risk factors for altered neurodevelopment. In a similar cohort of 84 276 children in Ontario, O’Leary et al8 previously found small differences in the rate and adjusted odds of early developmental vulnerability between exposure groups after accounting for multiple demographic and socioeconomic covariates, but not biological and home environment factors. The magnitude of adverse changes associated with exposure to surgery and anesthesia in that larger cohort was modest and, notably, the risk of early developmental vulnerability was found to be higher among older children (age, ≥2 years) and not younger children who are hypothesized to be at higher risk of neurologic injury due to the extensive neurodevelopment that occurs in early childhood.30 These findings were also replicated in a separate cohort study of 4470 children who underwent surgery in Manitoba, Canada, by Graham et al,9 who also found consistently lower scores in EDI major domains using a similar methodologic approach; again, these differences were considered small, and no adverse changes were found in younger children. Together, these studies suggested that anesthesia is not a strong causative pathway for adverse child development outcomes.31 Nonetheless, considering the substantial number of children who require general anesthesia every year (almost 3 million in the United States annually32,33), the small differences in child development outcomes in these studies still had potential public health implications. On the other hand, this present study finds no significant differences between biological siblings for any EDI outcomes, when considered as continuous or categorical variables, after exposure to surgery and anesthesia.
Although previous large cohort studies8,9,10 have attempted to mitigate for differences in socioeconomic and environmental factors, it is difficult to eliminate the burden of heritable and lifestyle differences on child development. The PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study18 used a similar approach (ie, examining relevant outcomes in siblings) to this study and had similar findings. Across multiple neuropsychological and behavioral outcomes in later childhood (ages 8 to 15 years), Sun et al18 found no differences between young children who had a single anesthetic exposure before 3 years of age and their unexposed sibling. To account for biological plausibility, Bartels et al19 used the Netherlands Twin Registry to measure educational outcomes for 1143 monozygotic twin pairs and found that twins who were exposed to anesthesia before 3 years of age had significantly lower educational achievement scores and significantly more cognitive problems than twins not exposed to anesthesia. However, discordant (for anesthesia exposure) twin pairs did not differ from each other.19 The negative findings of the current study are also consistent with the interim findings of the GAS (General Anesthesia Compared With Spinal Anesthesia) study,34 which randomized infants undergoing inguinal hernia repair to a sevoflurane-based general anesthetic or a neuraxial block without sedation. These investigators found no evidence of a difference in the secondary outcome of neurodevelopment at 2 years of age measured with the Bayley III Scales of Infant and Toddler Development.34 Together, these data support the premise that, although anesthesia exposure can be associated with worsened outcomes in cohort studies, it is a marker of vulnerability and does not reflect a causative pathway for adverse child development. Whether these findings can be generalized to children with longer cumulative exposures to anesthetic and sedative drugs is unknown, because most children included in these cohort studies had only a single exposure to surgery and anesthesia.
Limitations
This study has some limitations. As an observational study, the ability to infer causality is limited, and the retrospective study design introduces risk of biases and unmeasured sources of confounding from other factors that were not mitigated by using siblings. Using provincial demographic and administrative databases, bias from missing data are unlikely, but these administrative databases prevent us from investigating relevant clinical factors (eg, duration and type of anesthetic). Full biological sibling status could not be confirmed in this study (ie, whether sibling pairs had the same biological father), and as a result the genetic burden on child development cannot be assumed to be identical across all pairs, but this was unlikely to be a common issue. Children who had EDI-recorded behavioral, learning, or developmental problems were excluded from the cohort because the time of diagnosis relative to exposure could not be confirmed, and use of health care services tends to increase among this population. This situation had the potential of biasing the results toward the null, but a sensitivity analysis including these children in the cohort had similar findings to our primary analysis. Finally, the EDI is a validated instrument to assess children’s developmental health before primary school entry, demonstrates moderate concurrent validity with direct measures of neurodevelopment, and is used to guide population-level interventions for vulnerable children who may not meet individual diagnostic criteria for developmental delay22; however, the EDI is completed by kindergarten teachers and has not been designed to identify specific neurodevelopmental deficits in individual children.
Conclusions
Children who had surgical procedures that require general anesthesia before primary school entry were not found to be at increased risk of adverse child development outcomes compared with their biological siblings who did not have surgery. This sibling cohort mitigated for unmeasured biological vulnerability and home environmental influences on child development, and these findings further support that anesthesia exposure in early childhood is not associated with detectable adverse child development.
eTable 1. Definition of Surgical Procedures
eTable 2. Adjusted Odds of Early Developmental Vulnerability, a Multiple Challenge Index, or Major Domains in the Lowest Tenth Percentile for Demographic and Health Care–Related Factors in Children Exposed to Surgery (n = 2346) From Discordant Sibling Pairs
eTable 3. Characteristics of All Eligible Children Available From the EDI-ICES Database (n = 187 226)
eTable 4. Adjusted Odds of Early Developmental Vulnerability, a Multiple Challenge Index, or Major Domains in the Lowest Tenth Percentile After Exposure to Surgery Before Primary School Entry for a Sensitivity Analysis
References
- 1.Lin EP, Lee JR, Lee CS, Deng M, Loepke AW. Do anesthetics harm the developing human brain? an integrative analysis of animal and human studies. Neurotoxicol Teratol. 2017;60:117-128. doi: 10.1016/j.ntt.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 2.Walker SM. Biological and neurodevelopmental implications of neonatal pain. Clin Perinatol. 2013;40(3):471-491. doi: 10.1016/j.clp.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration Drug safety communication: FDA approves label changes for use of general anesthetic and sedation drugs in young children. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM554644.pdf. Published April 27, 2017. Accessed April 20, 2018.
- 4.Orser BA, Suresh S, Evers AS. SmartTots update regarding anesthetic neurotoxicity in the developing brain. Anesth Analg. 2018;126(4):1393-1396. doi: 10.1213/ANE.0000000000002833 [DOI] [PubMed] [Google Scholar]
- 5.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17(11):705-717. doi: 10.1038/nrn.2016.128 [DOI] [PubMed] [Google Scholar]
- 6.Hofacer RD, Deng M, Ward CG, et al. Cell age–specific vulnerability of neurons to anesthetic toxicity. Ann Neurol. 2013;73(6):695-704. doi: 10.1002/ana.23892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson AJ, Sun LS. Clinical evidence for any effect of anesthesia on the developing brain. Anesthesiology. 2018;128(4):840-853. doi: 10.1097/ALN.0000000000001972 [DOI] [PubMed] [Google Scholar]
- 8.O’Leary JD, Janus M, Duku E, et al. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology. 2016;125(2):272-279. doi: 10.1097/ALN.0000000000001200 [DOI] [PubMed] [Google Scholar]
- 9.Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 years: a retrospective matched cohort study. Anesthesiology. 2016;125(4):667-677. doi: 10.1097/ALN.0000000000001245 [DOI] [PubMed] [Google Scholar]
- 10.Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2017;171(1):e163470. doi: 10.1001/jamapediatrics.2016.3470 [DOI] [PubMed] [Google Scholar]
- 11.O’Leary JD, Warner DO. What do recent human studies tell us about the association between anaesthesia in young children and neurodevelopmental outcomes? Br J Anaesth. 2017;119(3):458-464. doi: 10.1093/bja/aex141 [DOI] [PubMed] [Google Scholar]
- 12.Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol. 2008;20(4):1161-1175. doi: 10.1017/S0954579408000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu D, Flick RP, Zaccariello MJ, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127(2):227-240. doi: 10.1097/ALN.0000000000001735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87(2):120-129. doi: 10.1016/j.mayocp.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54(1):3-16. doi: 10.1111/j.1469-7610.2012.02611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBar LL, Lynch FL, Boles M. Healthcare use by children with attention deficit/hyperactivity disorder with and without psychiatric comorbidities. J Behav Health Serv Res. 2004;31(3):312-323. doi: 10.1007/BF02287293 [DOI] [PubMed] [Google Scholar]
- 17.Efron D, Vutskits L, Davidson AJ. Can we really suggest that anesthesia might cause attention-deficit/hyperactivity disorder? Anesthesiology. 2017;127(2):209-211. doi: 10.1097/ALN.0000000000001736 [DOI] [PubMed] [Google Scholar]
- 18.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315(21):2312-2320. doi: 10.1001/jama.2016.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12(3):246-253. doi: 10.1375/twin.12.3.246 [DOI] [PubMed] [Google Scholar]
- 20.de Heer IJ, Tiemeier H, Hoeks SE, Weber F. Intelligence quotient scores at the age of 6 years in children anaesthetised before the age of 5 years. Anaesthesia. 2017;72(1):57-62. doi: 10.1111/anae.13687 [DOI] [PubMed] [Google Scholar]
- 21.Janus M, Walsh C, Duku E. Early Development Instrument: factor structure, sub-domains and multiple challenge index. https://edi.offordcentre.com/wp/wp-content/uploads/2015/11/RESULTS.Normative_Data_II.pdf. Published March 2005. Accessed April 20, 2018.
- 22.Janus M, Brinkman S, Duku E, et al. The Early Development Instrument: a population-based measure for communities. a handbook on development, properties, and use Toronto, Ontario: Offord Centre for Child Studies; 2007. http://www.academia.edu/6314289/The_Early_Development_Instrument_A_Population-based_Measure_for_Communities_A_Handbook_on_Development_Properties_and_Use. Accessed September 16, 2018.
- 23.Janus M, Offord D. Development and psychometric properties of the Early Development Instrument (EDI): a measure of children’s school readiness. Can J Behav Sci. 2007;39(1):1-22. doi: 10.1037/cjbs2007001 [DOI] [Google Scholar]
- 24.Curtin M, Browne J, Staines A, Perry IJ. The Early Development Instrument: an evaluation of its five domains using Rasch analysis. BMC Pediatr. 2016;16:10. doi: 10.1186/s12887-016-0543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Offord Centre for Child Studies , McMaster University. Early Development Instrument. https://www.edi.offordcentre.com. Accessed April 20, 2018.
- 26.Janus M, Duku E. The school entry gap: socioeconomic, family, and health factors associated with children’s school readiness to learn. Early Educ Dev. 2007;18(3):375-403. doi: 10.1080/10409280701610796a [DOI] [Google Scholar]
- 27.Ontario Ministry of Health and Long-Term Care. Ontario Health Insurance Plan schedule of benefits. 2016. http://www.health.gov.on.ca/en/pro/programs/ohip/sob/. Accessed April 20, 2018.
- 28.Dexter F, Macario A, Penning DH, Chung P. Development of an appropriate list of surgical procedures of a specified maximum anesthetic complexity to be performed at a new ambulatory surgery facility. Anesth Analg. 2002;95(1):78-82. doi: 10.1097/00000539-200207000-00014 [DOI] [PubMed] [Google Scholar]
- 29.O’Leary JD, Dexter F, Faraoni D, Crawford MW. Incidence of non-physiologically complex surgical procedures performed in children: an Ontario population-based study of health administrative data. Can J Anaesth. 2018;65(1):23-33. doi: 10.1007/s12630-017-0993-y [DOI] [PubMed] [Google Scholar]
- 30.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1-3):241-257. doi: 10.1016/S0301-0511(00)00058-2 [DOI] [PubMed] [Google Scholar]
- 31.Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 2015;12:14. doi: 10.1186/s12982-015-0037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabbitts JA, Groenewald CB, Moriarty JP, Flick R. Epidemiology of ambulatory anesthesia for children in the United States: 2006 and 1996. Anesth Analg. 2010;111(4):1011-1015. doi: 10.1213/ANE.e3181ee8479 [DOI] [PubMed] [Google Scholar]
- 33.Tzong KY, Han S, Roh A, Ing C. Epidemiology of pediatric surgical admissions in US children: data from the HCUP Kids Inpatient Database. J Neurosurg Anesthesiol. 2012;24(4):391-395. doi: 10.1097/ANA.0b013e31826a0345 [DOI] [PubMed] [Google Scholar]
- 34.Davidson AJ, Disma N, de Graaff JC, et al. ; GAS Consortium . Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239-250. doi: 10.1016/S0140-6736(15)00608-X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definition of Surgical Procedures
eTable 2. Adjusted Odds of Early Developmental Vulnerability, a Multiple Challenge Index, or Major Domains in the Lowest Tenth Percentile for Demographic and Health Care–Related Factors in Children Exposed to Surgery (n = 2346) From Discordant Sibling Pairs
eTable 3. Characteristics of All Eligible Children Available From the EDI-ICES Database (n = 187 226)
eTable 4. Adjusted Odds of Early Developmental Vulnerability, a Multiple Challenge Index, or Major Domains in the Lowest Tenth Percentile After Exposure to Surgery Before Primary School Entry for a Sensitivity Analysis