Abstract
Importance
The clinical high-risk state in psychosis is most often characterized by subthreshold psychotic symptoms (STPS) and represents a target for psychosis prevention. However, evidence suggests that between 30% and 50% of patients with a first episode of psychosis (FEP) report no prior history of STPS, indicating that not all patients with FEP experience a previous clinical high-risk phase. As with other early characteristics of illness onset, this diversity in the early course of symptoms may offer prognostic value for subsequent clinical trajectories.
Objective
To determine whether a history of pre-onset STPS is associated with differential 1-year treatment outcomes in an early intervention service for FEP.
Design, Setting, and Participants
Data on 195 patients 15 to 35 years of age who were recruited between January 17, 2003, and October 17, 2013, were collected from a catchment-based specialized early intervention service for FEP. Patients who reported experiencing at least 1 STPS prior to the onset of FEP were identified as STPS present (STPSp; n = 135); those who reported no such history were identified as STPS absent (STPSa; n = 60). Statistical analysis was conducted from December 15, 2016, to February 15, 2018.
Main Outcomes and Measures
Summary scores on the Scale for the Assessment of Positive Symptoms and the Scale for the Assessment of Negative Symptoms, Calgary Depression Scale for Schizophrenia, Hamilton Anxiety Rating Scale, Global Assessment of Functioning scores, and Social and Occupational Functioning Assessment Scale scores at baseline and after 1 year of treatment were analyzed to evaluate 1-year outcomes.
Results
Individuals in the STPSp group (39 female and 96 male participants; mean [SD] age, 23.4 [4.2] years) and the STPSa group (20 female and 40 male participants; mean [SD] age, 23.9 [5.1] years) did not differ in symptom severity or functioning at baseline. Although both groups improved by 1 year of treatment, mixed analyses of covariance (controlling for duration of untreated psychosis) revealed group-by-time interactions for scores on the Scale for the Assessment of Negative Symptoms (F1,192 = 6.17; P = .01), the Global Assessment of Functioning (F1,188 = 7.54; P = .006), and the Social and Occupational Functioning Assessment Scale (F1,192 = 3.79; P = .05). Mixed analyses of covariance also revealed a group effect for scores on the Scale for the Assessment of Positive Symptoms (F1,192 = 5.31; P = .02). After controlling for multiple comparisons, all significant results indicate poorer 1-year outcomes for patients with STPSp compared with patients with STPSa.
Conclusions and Relevance
A history of pre-onset STPS consistent with a prior clinical high-risk state is associated with poorer outcomes in psychotic symptoms and global functioning for patients after 1 year of treatment for FEP. The presence or absence of pre-onset STPS therefore has prognostic value for treatment outcomes, even during a later stage of psychotic illness.
This cohort study examines whether a history of pre-onset subthreshold psychotic symptoms is associated with differential 1-year treatment outcomes in an early intervention service for patients with a first episode of psychosis.
Key Points
Question
Do the outcomes of patients with a first episode of psychosis differ based on the experience of a prior clinical high-risk state?
Findings
In this cohort study of 195 patients with a first episode of psychosis, those recalling pre-onset subthreshold psychotic symptoms presented similarly at baseline compared with patients without such a history, but they exhibited poorer psychotic symptom outcomes and functional outcomes after 1 year of specialized early intervention.
Meaning
A history of subthreshold psychotic symptoms, consistent with a prior clinical high-risk state, is associated with poorer psychotic symptom outcomes and functional outcomes during treatment for a first episode of psychosis; the presence or absence of pre-onset subthreshold psychotic symptoms has prognostic value for treatment outcomes even during a later stage of psychosis.
Introduction
It is well accepted that specialized early intervention in the first 2 to 5 years of psychotic disorders, the putative “critical period,”1 can improve trajectories of illness and optimize long-term outcomes.2,3,4,5,6 Conversely, a failure to intervene during the early stages of illness is thought to lead to poorer long-term outcomes.7,8,9 This realization and the promise of initial studies regarding the early course of schizophrenia10,11 have led to the definition and widespread operationalization of stages of illness in psychosis research and clinical programming,12 including the threshold-level first episode of psychosis (FEP); a retrospectively defined prodromal period of affective symptoms, anxiety symptoms, attentional symptoms, subthreshold psychotic symptoms (STPS), and/or other symptoms that runs continuously into FEP13,14; and, more recently, the prospectively defined clinical high-risk (CHR) state.15,16 The CHR state is most often characterized by STPS17 and indicates increased risk for psychosis compared with the general population,18,19 even though only a minority of cases experiencing a CHR state ultimately transition to FEP.20
Although a CHR state thus represents an important target for preventing or delaying the onset of psychosis21,22,23 and other features of illness onset are associated with longitudinal trajectories,13,24,25,26 it remains unknown whether a history of pre-onset STPS (consistent with the CHR state) is associated with differential outcomes during treatment of FEP. Compared with patients with FEP presenting to services de novo, patients with FEP who had previously been treated for a CHR state had higher employment rates and lower rates of hospital admission.27 Yet individuals able to access early intervention services at an earlier (CHR) stage may a priori be more likely to demonstrate improved outcomes relative to those who receive services only at a later (FEP) stage. Furthermore, services for those in a CHR state have a more limited reach than services for FEP: the vast majority of patients with FEP have not received specialized treatment for a CHR state.28,29
In addition to previous critiques of the CHR field,28,29 these comparisons make the implicit assumption that services for a CHR state would have been indicated before the onset of any FEP. However, recent work has found that between 30% and 50% of cases of FEP appear to develop without any history of prior STPS,30,31 indicating 2 potentially distinct subpopulations of patients with FEP.32 Because not all patients with FEP experience pre-onset STPS, the focus on targeting these symptoms during a CHR phase (beyond the benefits of preventing or delaying psychosis) presumes that STPS have prognostic value over time. And since subpopulations of patients with or without STPS are similar in sociodemographics, symptoms, and functioning at intake for FEP services,30 it is critical to know whether individuals reporting pre-onset STPS experience differential clinical outcomes during specialized early intervention for FEP.
The objective of the present study is, therefore, to determine whether a history of pre-onset STPS is associated with differential FEP treatment outcomes. We examined symptom severity and functioning33,34 during 1 year of specialized early intervention for patients with FEP for whom the reported presence or absence of pre-onset STPS was routinely documented as part of an ongoing observational study situated in a catchment-based clinical service.30 Based on known associations between prolonged durations of untreated (threshold-level) psychosis and less favorable outcomes,7,35,36,37,38,39,40 we hypothesized that even after correcting for differences in treatment delay, patients with pre-onset STPS would exhibit greater psychotic symptoms and poorer functioning after 1 year of specialized early intervention for FEP than patients who did not experience STPS.
Methods
Setting
Patients who participated in this study were recruited between January 17, 2003, and October 17, 2013, from the Prevention and Early Intervention Program for Psychosis (PEPP-Montreal), an early intervention clinic of the Douglas Mental Health University Institute that serves a catchment area of approximately 300 000 individuals in the southwest of Montreal, Québec, Canada. In keeping with recent guidelines,41,42 patients were treated with low-dose second-generation antipsychotic medications on a naturalistic basis. Dosage was recorded in chlorpromazine hydrochloride equivalents (dosage × adherence). In addition, patients received modified assertive case management and had access to referrals for family-focused treatment, individual cognitive behavioral therapy, and groups focused on anxiety and other psychosocial functioning. Patients were eligible to participate in PEPP-Montreal’s ongoing naturalistic, longitudinal research study if they (1) met the diagnostic criteria for a nonaffective (schizophrenia spectrum) or affective psychotic disorder (not attributable to substance use alone) based on the Structured Clinical Interview from the DSM-IV,43 (2) had received less than 1 month of antipsychotic medication, (3) had an IQ of 70 or greater, (4) had no organic mental disorder (eg, epilepsy), and (5) were between 14 and 35 years of age. The research study was approved by the Douglas Mental Health University Institute’s ethics review board. Participants provided written informed consent, or assent with written parental consent if they were younger than 18 years.
Instruments
To evaluate whether patients with FEP experienced STPS, data collected in the Topography of Psychotic Episode were reviewed for the onset and course of 27 early psychiatric signs and symptoms within the first 3 months of entry into PEPP-Montreal. The Topography of Psychotic Episode is part of the Circumstances of Onset and Relapse Schedule,44 which includes detailed interviews with the patient (and whenever possible, a close family member) as well as a review of all available health and educational records for the purpose of determining pathways to care. Although the availability of collateral information and written records differed from patient to patient, trained research assistants (with at least an undergraduate degree) synthesized this information into a Circumstances of Onset and Relapse Schedule narrative prior to a regular meeting chaired by a research psychiatrist (A.M. or R.J.), at which a consensus on critical dates (eg, date of onset) and early symptoms was reached.30 Training for the Circumstances of Onset and Relapse Schedule and the Topography of Psychotic Episode included orientation, rating videotapes, role-playing, conducting interviews under supervision, and identifying key dates related to the course of illness. Interrater reliability was based on 12 randomly selected cases evaluated separately by 3 raters (intraclass correlation coefficient, 0.81-0.98).30
Of 27 signs and symptoms, the following 9 were selected by international experts as constituting STPS “if they appeared at a time when an individual would not have met criteria for a syndromal level psychotic episode”30(p1048): suspiciousness or odd ideas of reference; odd or bizarre ideas that are not delusional; odd, unusual, or eccentric behavior; unusual perceptual experiences that are not clearly psychotic; disorganized or odd speech; inappropriate affect; hallucinations or delusions (subthreshold); and passivity experiences. As previously described,30 these early signs and symptoms map onto the various subscales of the Comprehensive Assessment of At-Risk Mental States45 and the Structured Interview for Psychosis-Risk Syndrome.46 Patients were classified as STPS present (STPSp) if they reported 1 or more STPS occurring prior to the onset of FEP and STPS absent (STPSa) if they reported none.30
The Scale for the Assessment of Positive Symptoms (SAPS)47 and the Scale for the Assessment of Negative Symptoms (SANS)48 were administered by trained research staff to measure the severity of psychotic symptoms. With the exception of the attention item in the SANS (which has been shown to reflect cognition49 and whose exclusion provides greater internal consistency50), summary scores51 (sum of global scores; range, 0-20) for each metric were calculated. Depression was evaluated with the Calgary Depression Scale for Schizophrenia (CDSS),52 and anxiety was evaluated with the Hamilton Anxiety Rating Scale (HAM-A)53; total scores (CDSS: range, 0-27; HAM-A: range, 0-54) were considered for analyses.
To assess functioning, the Social and Occupational Functioning Assessment Scale (SOFAS)54 and the Global Assessment of Functioning (GAF; a comprehensive measure of both functioning and symptom severity)55 were administered (for both, total scores range from 1 to 100). Data collected at baseline (mean [SD] 7.7 [7.8] days after entry to PEPP-Montreal) and after 1 year of treatment were used for analyses.
Exclusion
Of the 469 eligible patients who provided signed informed consent between 2003 and 2013, 468 completed at least 1 baseline clinical assessment, and 350 completed the Circumstances of Onset and Relapse Schedule and the Topography of Psychotic Episode. Of these, 204 participants completed both symptom and functioning evaluations at baseline and after 1 year of treatment. To minimize confounders introduced by prior exposure to specialized early intervention, 4 participants were excluded who had received previous services for a CHR state, and 5 were excluded because information on the duration of untreated psychosis (DUP), a key covariate in the statistical model used, was not collected. Owing to these limitations, the following analyses of outcome are restricted to data collected from 195 participants with FEP (135 STPSp and 60 STPSa).
Statistical Analysis
Statistical analysis was conducted from December 15, 2016, to February 15, 2018. All statistical tests were 2-tailed and were performed on SPSS Statistics 22,56 with P < .05 considered statistically significant except in the case of multiple comparison correction. χ2 Tests, unpaired t tests, and Mann-Whitney tests were first applied to sociodemographic characteristics (sex, age, educational level, and patient and parental socioeconomic status measured by the Hollingshead Index of Socioeconomic Status57) and baseline clinical scores (SAPS, SANS, GAF, SOFAS, CDSS and HAM-A) to compare the 195 participants who were included in the longitudinal analyses of outcomes with the remaining participants. Tests were similarly applied among the included sample of 195 participants to assess for sociodemographic differences between groups (STPSp vs STPSa). To examine medication dosage between groups, a mixed analysis of variance was applied to chlorpromazine equivalents across time points.
Because DUP, defined as the time elapsed between the start of a psychotic episode and the administration of antipsychotic medication, is an established predictor of outcome,7,8,9 a Mann-Whitney test was applied to test group effects. It was decided a priori that if significant at P < .05, DUP would be included as a covariate in the analyses of covariance (ANCOVA) for the outcomes described.
To analyze changing symptoms and functioning, mixed ANCOVA, controlling for significant covariates, were applied with group (STPSp and STPSa) as the between-participants variable and time point (baseline and after 1 year of treatment) as the within-participants variable. The following 3 domains of outcome (and corresponding dependent variables) were considered: psychotic symptoms (SAPS and SANS), nonpsychotic symptoms (CDSS and HAM-A), and functioning (GAF and SOFAS). Bonferroni corrections were applied for multiple comparisons within each domain of outcome, yielding a P < .025 for significance.
Significant group-by-time interactions and main group effects were analyzed post hoc, with Bonferroni-corrected simple effects tests (P < .025) comparing groups at each time point. For all mixed analyses of variance and ANCOVA, Greenhouse-Geisser corrections were applied to correct for sphericity violations.
Results
Sample Characteristics
No significant group differences in mean age at baseline evaluation, educational level, patient and parental socioeconomic status, ratio of affective to nonaffective cases, and medication dosage were observed between patients with STPSp and patients with STPSa included in longitudinal analyses (Table 1). Furthermore, the total (combined STPSp and STPSa) sample included in the longitudinal analyses of outcome did not differ in age, sex, parental socioeconomic status, or most clinical characteristics at baseline from the sample excluded owing to incomplete STPS or other evaluations (Table 2). Compared with the excluded sample, those included had completed more years of education (mean [SD], 11.7 [2.5] vs 11.2 [2.5] years; P = .045).
Table 1. Sample Characteristics by First Episode of Psychosis.
| Characteristic | Value | Statistic | P Value |
|---|---|---|---|
| Age at baseline, mean (SD), y | |||
| 135 Patients with STPSp | 23.4 (4.2) | t = −0.80 | .43 |
| 60 Patients with STPSa | 23.9 (5.1) | ||
| Female, No. (%) | |||
| 135 Patients with STPSp | 39 (28.9) | χ2 = 0.39 | .53 |
| 60 Patients with STPSa | 20 (33.3) | ||
| Educational level, mean (SD), y | |||
| 132 Patients with STPSp | 11.6 (2.4) | t = −0.74 | .46 |
| 54 Patients with STPSa | 11.8 (2.8) | ||
| Socioeconomic status, median (IQR)a | |||
| 113 Patients with STPSp | 4.00 (3.00-5.00) | U = 2417 | .47 |
| 46 Patients with STPSas | 4.00 (3.00-5.00) | ||
| Parental socioeconomic status, median (IQR)a | |||
| 77 Patients with STPSp | 2.50 (1.50-4.00) | U = 1242 | .50 |
| 35 Patients with STPSa | 3.00 (1.50-4.00) | ||
| Affective psychosis, No. (%) | |||
| 135 Patients with STPSp | 31 (23.0) | χ2 = 2.31 | .13 |
| 60 Patients with STPSa | 20 (33.3) | ||
| Nonaffective psychosis, No. (%) | |||
| 135 Patients with STPSp | 104 (77.0) | ||
| 60 Patients with STPSa | 40 (66.7) | ||
| Duration of untreated psychosis, median (IQR), wk | |||
| 135 Patients with STPSp | 17.6 (7.14-62.00) | U = 3315 | .043b |
| 60 Patients with STPSa | 11.4 (4.33-26.30) | ||
| Baseline CPZ | |||
| 135 Patients with STPSp | 165 (142) | F = 0.595c | .44 |
| 60 Patients with STPSa | 150 (109) | ||
| 1-y CPZ | |||
| 135 Patients with STPSp | 165 (196) | ||
| 60 Patients with STPSa | 124 (155) |
Abbreviations: CPZ, chlorpromazine hydrochloride equivalents; IQR, interquartile range; STPSa, subthreshold psychotic symptoms absent; STPSp, subthreshold psychotic symptoms present.
Measured by the Hollingshead Index of Socioeconomic Status.57
Significant group difference (P < .05).
For group-by-time interaction.
Table 2. Comparison of Sociodemographic Characteristics and Baseline Clinical Scores of Patients Included or Excluded From Primary Analysis.
| Characteristic | Value | Statistic | P Value |
|---|---|---|---|
| Age, mean (SD), y | |||
| 195 Included patients | 23.6 (4.5) | t = –0.82 | .41 |
| 274 Excluded patients | 23.2 (4.8) | ||
| Female, No. (%) | |||
| 195 Included patients | 59 (30.3) | χ2 = 0.37 | .55 |
| 278 Excluded patients | 77 (27.7) | ||
| Educational level, mean (SD), y | |||
| 186 Included patients | 11.7 (2.5) | t = –1.95 | .05 |
| 257 Excluded patients | 11.2 (2.5) | ||
| Socioeconomic status, median (IQR)a | |||
| 159 Included patients | 4.00 (3.00-5.00) | U = 16 567 | .21 |
| 194 Excluded patients | 4.00 (3.00-5.00) | ||
| Parental socioeconomic status, median (IQR)a | |||
| 112 Included patients | 3.00 (1.50-4.00) | U = 6138 | .58 |
| 105 Excluded patients | 3.00 (2.00-4.00) | ||
| SAPS, median (IQR) | |||
| 195 Included patients | 12.0 (10.0-14.0) | U = 25 576 | .55 |
| 271 Excluded patients | 12.0 (9.0-14.0) | ||
| SANS, median (IQR) | |||
| 195 Included patients | 10.0 (8.0-13.0) | U = 26 468 | .97 |
| 272 Excluded patients | 10.0 (8.0-13.0) | ||
| CDSS, median (IQR) | |||
| 195 Included patients | 4.0 (1.0-8.0) | U = 25 097 | .35 |
| 271 Excluded patients | 3.0 (1.0-8.0) | ||
| HAM-A, median (IQR) | |||
| 185 Included patients | 10.0 (6.0-16.0) | U = 21 454 | .20 |
| 250 Excluded patients | 9.00 (4.0-16.0) | ||
| GAF, median (IQR) | |||
| 195 Included patients | 30.0 (25.0-31.0) | U = 23 618 | .06 |
| 269 Excluded patients | 30.0 (24.5-30.0) | ||
| SOFAS, median (IQR) | |||
| 195 Included patients | 40.0 (33.0-50.0) | U = 19 402 | .45 |
| 208 Excluded patients | 40.0 (31.0-50.0) |
Abbreviations: CDSS, Calgary Depression Scale for Schizophrenia, total score; GAF, Global Assessment of Functioning, total score; HAM-A, Hamilton Anxiety Scale, total score; IQR, interquartile range; SANS, Scale for the Assessment of Negative Symptoms, summary score; SAPS, Scale for the Assessment of Positive Symptoms, summary score; SOFAS, Social and Occupational Functioning Assessment Scale, total score.
Measured by the Hollingshead Index of Socioeconomic Status.57
Duration of Untreated Psychosis
Patients who experienced subthreshold psychotic symptoms exhibited a significantly longer DUP than those who did not experience such symptoms (median, 17.6 weeks [interquartile range, 7.1-62.0 weeks] vs 11.4 weeks [interquartile range, 4.3-26.3 weeks]; P = .04; Table 1). As such, DUP was introduced as a covariate in subsequent analyses examining outcomes in symptoms and functioning.
Outcomes
Psychotic Symptoms
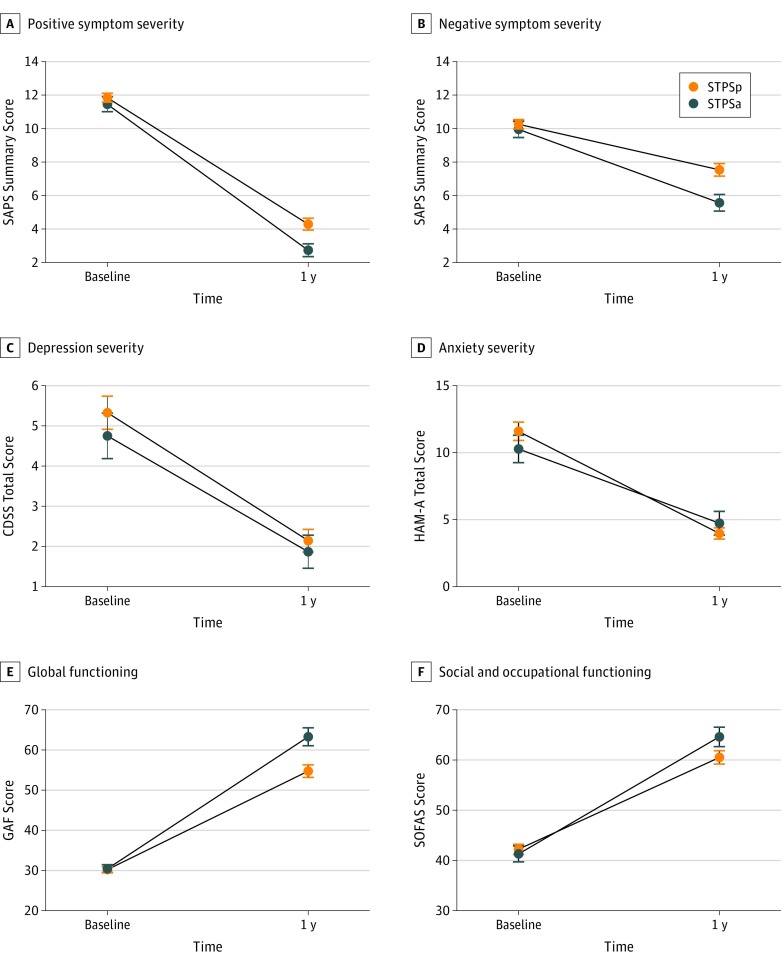
Mixed ANCOVA of SAPS scores revealed main effects of time with decreasing scores (F1,192 = 464; P < .001) and of group (F1,192 = 5.31; P = .02), whereby patients with STPSp exhibited higher SAPS scores. Analyses of SANS scores also revealed a significant group-by-time interaction (F1,192 = 6.17; P = .01). Post hoc simple effects tests comparing STPSp and STPSa groups at each time point revealed no differences at baseline, but showed that patients with STPSp exhibited higher SAPS and SANS scores after 1 year of treatment (Table 3 and Figure).
Table 3. Mean Clinical Scores by Group and Time Point.
| Scale | SAPS | SANS | CDSS | HAM-A | GAF | SOFAS |
|---|---|---|---|---|---|---|
| Patients, No. (STPSp/STPSa) | 135/60 | 135/60 | 135/60 | 124/56 | 132/59 | 135/60 |
| Baseline, Mean (SD) | ||||||
| STPSp | 11.8 (3.1) | 10.3 (3.2) | 5.3 (4.8) | 11.6 (7.6) | 30.2 (8.2) | 42.2 (11.8) |
| STPSa | 11.5 (3.5) | 10.0 (3.8) | 4.8 (4.4) | 10.3 (7.7) | 30.4 (7.7) | 41.3 (12.3) |
| After 1 y of Treatment, Mean (SD) | ||||||
| STPSp | 4.3 (4.1) | 7.5 (4.3) | 2.1 (3.3) | 4.0 (4.7) | 54.8 (17.9) | 60.5 (15.3) |
| STPSa | 2.7 (3.0) | 5.6 (3.9) | 1.9 (3.2) | 4.7 (6.6) | 63.3 (17.2) | 64.6 (15.0) |
| Statistics | ||||||
| Group by time | ||||||
| F value | 2.83 | 6.17 | 0.084 | 2.79 | 7.54 | 3.79 |
| P value | .09 | .01a | .77 | .10 | .006a | .05 |
| Group effect | ||||||
| F value | 5.31 | NA | 0.503 | 0.025 | NA | 0.634 |
| P value | .02a | NA | .48 | .88 | NA | .43 |
| Time effect | ||||||
| F value | 464 | NA | 44.8 | 95.5 | NA | 241 |
| P value | <.001 | NA | <.001 | <.001 | NA | <.001 |
| Post hoc | ||||||
| Baseline mean difference | 0.393 | 0.278 | NA | NA | 0.311 | NA |
| P value | .43 | .60 | NA | NA | .81 | NA |
| Post hoc | ||||||
| Mean difference after 1 y of treatment | 1.57 | 1.94 | NA | NA | 8.36 | NA |
| P value | .009b | .003b | NA | NA | .003b | NA |
Abbreviations: CDSS, Calgary Depression Scale for Schizophrenia, total score; GAF, Global Assessment of Functioning, total score; HAM-A, Hamilton Anxiety Scale, total score; NA, not applicable; SANS, Scale for the Assessment of Negative Symptoms, summary score; SAPS, Scale for the Assessment of Positive Symptoms, summary score; SOFAS, Social and Occupational Functioning Assessment Scale, total score; STPSa, subthreshold psychotic symptoms absent; STPSp, subthreshold psychotic symptoms present.
Significant main effect, Bonferroni corrected (P < .025).
Significant pairwise comparison, Bonferroni corrected (P < .025).
Figure. Symptom Severities and Functioning Over Time by Group.
A, Positive symptom severity. Significant group difference in the Scale for the Assessment of Positive Symptoms (SAPS) summary score at 1 year (P = .009). B, Negative symptom severity. Significant group difference in the Scale for the Assessment of Negative Symptoms (SANS) summary score at 1 year (P = .003). C, Depression severity. D, Anxiety severity. E, Global functioning. Significant group difference in the Global Assessment of Functioning (GAF) total score at 1 year (P = .003). F, Social and occupational functioning. Error bars indicate SE. CDSS indicates Calgary Depression Scale for Schizophrenia, total score; HAM-A, Hamilton Anxiety Scale, total score; SOFAS, Social and Occupational Functioning Assessment Scale, total score; STPSa, subthreshold psychotic symptoms absent; and STPSp, subthreshold psychotic symptoms present.
Nonpsychotic Symptoms
Mixed ANCOVA of CDSS scores revealed significant main effects of time only, showing score reductions over time (F1,192 = 44.8; P < .001; Table 3 and Figure). Mixed ANCOVA of HAM-A scores also revealed score reductions over time (F1,182 = 91.8; P < .001).
Functioning
Mixed ANCOVA on scales of functioning revealed a significant group-by-time interaction for GAF scores (F1,188 = 7.54; P = .006). For SOFAS scores (F1,192 = 3.79; P = .05), the trend toward significance disappeared after correction for multiple comparisons, and a significant effect of time only remained (F1,192 = 241; P < .001). Post hoc simple effects tests comparing groups at time points revealed that only after 1 year of treatment, the STPSp group exhibited significantly lower scores than the STPSa group on the GAF (Table 3 and Figure).
Discussion
The present study investigated symptom severity and functioning at baseline and after 1 year of specialized early intervention in patients with FEP with or without a history of STPS. These groups exhibited few differences at baseline,30 and both demonstrated substantial recovery over time for all outcomes examined (Figure); however, notable differences emerged in the severity of positive and negative psychotic symptoms as well as functioning after 1 year. The divergent trajectories were not explained by depression or anxiety, nor by treatment delays reflected in DUP. All significant and trending findings suggest, to our knowledge for the first time, that a history of pre-onset STPS (consistent with a CHR state) is associated with poorer symptomatic and functional recovery during treatment for FEP, with implications for prognosis and early intervention across stages of early psychosis.
While there were no differences in SOFAS scores at baseline, at year 1 the STPSp group achieved SOFAS scores bordering between moderate difficulty (scores, 51-60) and some difficulty (scores, 61-70), whereas the STPSa group crossed this clinical threshold (Table 3). This distinction is relevant because scores of 61 or higher are considered among the criteria for functional remission.58,59 Although this group-by-time interaction was statistically nonsignificant after controlling for multiple comparisons, a longer follow-up period may reveal the association between STPS history and functioning to be meaningful. This is unsurprising when considering that functional outcome improvements (which include vocational attainment such as employment and education) are likely to lag behind remission of symptoms. Analyses of GAF scores after 1 year of treatment also support a clinically significant group effect: the patients with STPSa achieved scores above the threshold for functional remission (≥61),58,60,61 whereas the patients with STPSp were well below this threshold (Table 3).
The overall direction of the results for psychotic symptoms is congruent with those regarding functioning. For example, the decreasing summary SAPS and SANS scores can reliably be interpreted as globally reduced symptoms over time.51 Furthermore, while there was no significant group-by-time interaction for SAPS, our post hoc tests indicate that the STPSp group had relatively higher positive psychotic symptoms after 1 year of FEP services. The larger between-group differences seen in GAF scores compared with SOFAS scores after 1 year of treatment (and the integration of symptoms into the former) suggests that further examination of whether symptomatic differences are more substantive or persistent than functional differences may be warranted.
A range of factors could be invoked to explain the relatively poorer longitudinal outcomes observed for patients with STPSp and/or the improved outcomes seen for patients with STPSa. For example, the experience of pre-onset STPS may demarcate a unique form, or biotype, of psychosis,62 which could be explored by studying diverse variables such as cognition, brain morphologic characteristics, or therapeutic response in a multidimensional data set. Alternatively, examining care pathways and treatment delays may shed light on other variables contributing to our findings. Given the established association between DUP and FEP outcomes, exposure to pre-onset STPS may represent an “extended DUP” (combining both threshold and subtheshold psychotic symptoms). The extended DUP concept, acting through biological and/or psychosocial mechanisms,63,64,65 could further explain the poorer recovery seen in the STPSp group, whereas patients with STPSa may have a relatively acute-onset psychosis with a shorter threshold-level DUP followed by rapid recovery.
The association between STPS and poorer 1-year outcomes is strengthened by the fact that our analyses addressed potential confounders of this association. The STPSp and STPSa groups were similar in symptoms of depression and anxiety at baseline and 1 year (Figure), had equivalent exposure to antipsychotic medications (Table 1), and demonstrated no differences in other clinical or sociodemographic factors at baseline (eg, educational level or affective vs nonaffective psychosis) (Table 1). Furthermore, our analysis controlled for differences in DUP (a known predictor of outcome).7,8,9 For clinicians, this study is the first, to our knowledge, to provide evidence that pre-onset symptoms consistent with the CHR phase (vs lack thereof) have a differential imprint on the clinical course in a subsequent threshold-level (FEP) stage of illness. It implies that inquiring about pre-onset STPS when a patient is seeking help for FEP can reveal important information regarding prognosis, and it suggests that more intensive or tailored efforts during treatment for FEP should be considered to improve functional and not just symptomatic outcomes for patients with prior STPS.
Our approach also offers insights for prevention and early intervention during the CHR stage itself. For example, as in most community settings,28,29 patients with STPSp in this study received no organized early intervention for those symptoms, even though they may have received individual components of evidence-based treatment for the CHR phase (such as cognitive behavioral therapy) in a non–early intervention context.66 While multiple early psychopathologic or other factors can contribute to long-term treatment outcomes, the presence or absence of pre-onset STPS appears to be an important (although not necessarily causal) prognostic marker for patients who transition to FEP. This complements arguments that multicomponent interventions aimed at the CHR stage should attempt to target and improve both symptoms and functioning,67 and raises the possibility that increasing the spread and reach of comprehensive services for the CHR state could ameliorate such difficulties among future patients who transition from a CHR state to FEP.
In addition, viewing STPS and the CHR syndrome through the lens of FEP clinical populations could begin to address recent critiques of the CHR construct and its operationalization,68,69 such as the recognition that transitions to FEP can develop across diagnostic silos rather than solely following a CHR state. This finding, too, has service-level implications: because not all patients with FEP experience pre-onset STPS, CHR services as currently constructed have an upper limit on their ability to identify and prevent transitions to psychosis. Prevention and early intervention efforts may therefore need to begin considering how to identify non-STPS syndromes that could transition to psychosis.
Strengths and Limitations
A strength of this study is that the data were collected from the sole specialized early FEP intervention program within a local catchment area—one that has conducted wide outreach activities70 and is well recognized in the community—and are therefore likely to be epidemiologically representative.71 Furthermore, with the exception of education, no significant differences in baseline clinical and sociodemographic characteristics were found between the sample of participants included in the present study and the sample of participants who were excluded owing to incomplete data (Table 2). This finding suggests that the 195 patients studied (whose STPS status and outcomes were both identified and data were available) were reasonably representative of the total clinical population being served and that the findings are relatively generalizable.
Previous work examining early psychiatric signs and their association with threshold-level psychosishas established that insidious-onset forms of psychosis are (compared with acute-onset forms) associated with poorer outcomes.14,24,72,73,74,75 In such studies, onset is frequently characterized by any noticeable behavioral change (including affective or negative symptoms)13,76,77 and not by STPS or the CHR state specifically. Our investigation complements this literature by focusing on the relevance of pre-onset STPS (reflecting the most common CHR subsyndrome) for outcomes during a subsequent (FEP) stage of illness.
Finally, instead of observing converters who received phase-specific treatment at the CHR and/or FEP stages,27,78 the patients with STPSp in our study received no comprehensive early intervention during their putative CHR phase, which reflects the experience of the vast majority of individuals presenting with FEP.28,29 It also eliminates potential confounding effects of prior interventions for the CHR state that, even if received as individual components elsewhere, are evidence based66 and should therefore contribute to improved outcomes rather than the observed equivalent or poorer outcomes seen in the STPSp group.
Retrospective approaches to identifying STPS are inherently limited by potential recall bias,30,31 leaving room for possible underrepresentation or overrepresentation of STPSp status. For example, the STPS classification is centered around the presence or absence of psychopathologic characteristics; it includes patients with FEP who have any recalled history of STPS (regardless of duration or symptom burden) and also assumes help-seeking behavior that is an important part of the diagnosis of a CHR state. However, in the absence of high-resolution, large-scale, and long-term prospective studies that follow a population for 1 to 2 decades, valid retrospective measures (which are routinely used to document data on DUP and other care pathways) may be the only feasible option for understanding pre-onset psychopathologic characteristics across the entire population of patients who will eventually develop FEP. Our modest sample size of 195 should also be considered in light of the challenges of systematically acquiring and reviewing detailed retrospective information from patients, caretakers, and available records. Finally, because our aim was to specifically examine the association of STPS with outcome, we did not consider other pre-onset factors that might contribute to long-term functioning, such as depression or anxiety prior to FEP, or early childhood adversity.
Conclusions
Building on recent findings that between 30% and 50% of FEP cases develop without pre-onset STPS,30,31 our results provide novel evidence for poorer prognosis in both psychotic symptoms and global functioning during the first year of specialized early intervention in patients who had an FEP with prior STPS (consistent with a CHR state). This implies that the imprint of STPS extends to outcomes in later stages, is not explained by comorbid conditions or treatment delays, and suggests that prevention and early intervention efforts in the CHR and FEP phases should increasingly target functioning alongside psychotic symptoms.
References
- 1.Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172(33)(suppl 1):53-59. doi: 10.1192/S0007125000297663 [DOI] [PubMed] [Google Scholar]
- 2.Eaton WW, Thara R, Federman B, Melton B, Liang KY. Structure and course of positive and negative symptoms in schizophrenia. Arch Gen Psychiatry. 1995;52(2):127-134. doi: 10.1001/archpsyc.1995.03950140045005 [DOI] [PubMed] [Google Scholar]
- 3.Morrison J, Winokur G, Crowe R, Clancy J. The Iowa 500: the first follow-up. Arch Gen Psychiatry. 1973;29(5):678-682. doi: 10.1001/archpsyc.1973.04200050083014 [DOI] [PubMed] [Google Scholar]
- 4.Malla A, Joober R, Iyer S, et al. Comparing three-year extension of early intervention service to regular care following two years of early intervention service in first-episode psychosis: a randomized single blind clinical trial. World Psychiatry. 2017;16(3):278-286. doi: 10.1002/wps.20456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srihari VH, Shah J, Keshavan MS. Is early intervention for psychosis feasible and effective? Psychiatr Clin North Am. 2012;35(3):613-631. doi: 10.1016/j.psc.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565. doi: 10.1001/jamapsychiatry.2018.0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804. doi: 10.1176/appi.ajp.162.10.1785 [DOI] [PubMed] [Google Scholar]
- 8.Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62(9):975-983. doi: 10.1001/archpsyc.62.9.975 [DOI] [PubMed] [Google Scholar]
- 9.Santesteban-Echarri O, Paino M, Rice S, et al. Predictors of functional recovery in first-episode psychosis: a systematic review and meta-analysis of longitudinal studies. Clin Psychol Rev. 2017;58:59-75. doi: 10.1016/j.cpr.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Häfner H, Maurer K, an der Heiden W. ABC Schizophrenia study: an overview of results since 1996. Soc Psychiatry Psychiatr Epidemiol. 2013;48(7):1021-1031. doi: 10.1007/s00127-013-0700-4 [DOI] [PubMed] [Google Scholar]
- 11.Häfner H, Maurer K, Löffler W, et al. The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol. 1998;33(8):380-386. doi: 10.1007/s001270050069 [DOI] [PubMed] [Google Scholar]
- 12.McGorry PD, Nelson B, Goldstone S, Yung AR. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. 2010;55(8):486-497. doi: 10.1177/070674371005500803 [DOI] [PubMed] [Google Scholar]
- 13.Häfner H, Maurer K, Löffler W, an der Heiden W, Hambrecht M, Schultze-Lutter F. Modeling the early course of schizophrenia. Schizophr Bull. 2003;29(2):325-340. doi: 10.1093/oxfordjournals.schbul.a007008 [DOI] [PubMed] [Google Scholar]
- 14.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370. doi: 10.1093/schbul/22.2.353 [DOI] [PubMed] [Google Scholar]
- 15.Fusar-Poli P. The clinical high-risk state for psychosis (CHR-P), version II. Schizophr Bull. 2017;43(1):44-47. doi: 10.1093/schbul/sbw158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGorry PD, Singh BS. Schizophrenia: risk and possibility In: Raphael B, Burrows GD, eds. Handbook of Preventative Psychiatry. New York, NY: Elsevier; 1995:492-514. [Google Scholar]
- 17.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142. doi: 10.1016/S0920-9964(03)00192-0 [DOI] [PubMed] [Google Scholar]
- 18.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60(1):21-32. doi: 10.1016/S0920-9964(02)00167-6 [DOI] [PubMed] [Google Scholar]
- 19.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107-120. doi: 10.1001/jamapsychiatry.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gee DG, Cannon TD. Prediction of conversion to psychosis: review and future directions. Rev Bras Psiquiatr. 2011;33(suppl 2):s129-s142. doi: 10.1590/S1516-44462011000600002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921-928. doi: 10.1001/archpsyc.59.10.921 [DOI] [PubMed] [Google Scholar]
- 22.Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185(4):291-297. doi: 10.1192/bjp.185.4.291 [DOI] [PubMed] [Google Scholar]
- 23.McGorry PD, Nelson B, Amminger GP, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212. doi: 10.4088/JCP.08r04472 [DOI] [PubMed] [Google Scholar]
- 24.Compton MT, Chien VH, Leiner AS, Goulding SM, Weiss PS. Mode of onset of psychosis and family involvement in help-seeking as determinants of duration of untreated psychosis. Soc Psychiatry Psychiatr Epidemiol. 2008;43(12):975-982. doi: 10.1007/s00127-008-0397-y [DOI] [PubMed] [Google Scholar]
- 25.Singh SP, Burns T, Amin S, Jones PB, Harrison G. Acute and transient psychotic disorders: precursors, epidemiology, course and outcome. Br J Psychiatry. 2004;185:452-459. doi: 10.1192/bjp.185.6.452 [DOI] [PubMed] [Google Scholar]
- 26.Renwick L, Lyne J, Donoghue BO, et al. Prodromal symptoms and remission following first episode psychosis. Schizophr Res. 2015;168(1-2):30-36. doi: 10.1016/j.schres.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 27.Valmaggia LR, Byrne M, Day F, et al. Duration of untreated psychosis and need for admission in patients who engage with mental health services in the prodromal phase. Br J Psychiatry. 2015;207(2):130-134. doi: 10.1192/bjp.bp.114.150623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajnakina O, Morgan C, Gayer-Anderson C, et al. Only a small proportion of patients with first episode psychosis come via prodromal services: a retrospective survey of a large UK mental health programme. BMC Psychiatry. 2017;17(1):308. doi: 10.1186/s12888-017-1468-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fusar-Poli P, Rutigliano G, Stahl D, et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry. 2017;74(5):493-500. doi: 10.1001/jamapsychiatry.2017.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah JL, Crawford A, Mustafa SS, Iyer SN, Joober R, Malla AK. Is the clinical high-risk state a valid concept? retrospective examination in a first-episode psychosis sample. Psychiatr Serv. 2017;68(10):1046-1052. doi: 10.1176/appi.ps.201600304 [DOI] [PubMed] [Google Scholar]
- 31.Schultze-Lutter F, Rahman J, Ruhrmann S, et al. Duration of unspecific prodromal and clinical high risk states, and early help-seeking in first-admission psychosis patients. Soc Psychiatry Psychiatr Epidemiol. 2015;50(12):1831-1841. doi: 10.1007/s00127-015-1093-3 [DOI] [PubMed] [Google Scholar]
- 32.Fusar-Poli P. Extending the benefits of indicated prevention to improve outcomes of first-episode psychosis. JAMA Psychiatry. 2017;74(7):667-668. doi: 10.1001/jamapsychiatry.2017.1009 [DOI] [PubMed] [Google Scholar]
- 33.Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449. doi: 10.1176/appi.ajp.162.3.441 [DOI] [PubMed] [Google Scholar]
- 34.Alvarez-Jimenez M, O’Donoghue B, Thompson A, et al. Beyond clinical remission in first episode psychosis: thoughts on antipsychotic maintenance vs. guided discontinuation in the functional recovery era. CNS Drugs. 2016;30(5):357-368. doi: 10.1007/s40263-016-0331-x [DOI] [PubMed] [Google Scholar]
- 35.Penttilä M, Jääskeläinen E, Hirvonen N, Isohanni M, Miettunen J. Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2014;205(2):88-94. doi: 10.1192/bjp.bp.113.127753 [DOI] [PubMed] [Google Scholar]
- 36.Tang JY-M, Chang W-C, Hui CL-M, et al. Prospective relationship between duration of untreated psychosis and 13-year clinical outcome: a first-episode psychosis study. Schizophr Res. 2014;153(1-3):1-8. doi: 10.1016/j.schres.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 37.Albert N, Melau M, Jensen H, Hastrup LH, Hjorthøj C, Nordentoft M. The effect of duration of untreated psychosis and treatment delay on the outcomes of prolonged early intervention in psychotic disorders. NPJ Schizophr. 2017;3(1):34. doi: 10.1038/s41537-017-0034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boonstra N, Klaassen R, Sytema S, et al. Duration of untreated psychosis and negative symptoms—a systematic review and meta-analysis of individual patient data. Schizophr Res. 2012;142(1-3):12-19. doi: 10.1016/j.schres.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 39.Ito S, Nemoto T, Tsujino N, et al. Differential impacts of duration of untreated psychosis (DUP) on cognitive function in first-episode schizophrenia according to mode of onset. Eur Psychiatry. 2015;30(8):995-1001. doi: 10.1016/j.eurpsy.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 40.Clarke M, Whitty P, Browne S, et al. Untreated illness and outcome of psychosis. Br J Psychiatry. 2006;189:235-240. doi: 10.1192/bjp.bp.105.014068 [DOI] [PubMed] [Google Scholar]
- 41.Canadian Psychiatric Association Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13)(suppl 1):7S-57S. [PubMed] [Google Scholar]
- 42.Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410-472. doi: 10.1177/0004867416641195 [DOI] [PubMed] [Google Scholar]
- 43.First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR, Axis I Disorders, Research Version, Patient Edition. New York, NY: State Psychiatric Institute, Biometric Research; 2002. [Google Scholar]
- 44.Norman RMG, Malla AK. Prevention and Early Intervention for Psychosis Program (PEPP): Course of Onset and Relapse Schedule: Interview and Coding Instruction Guide. London, Ontario, Canada: London Health Sciences Centre; 2002. [Google Scholar]
- 45.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971. doi: 10.1080/j.1440-1614.2005.01714.x [DOI] [PubMed] [Google Scholar]
- 46.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715. doi: 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- 47.Andreasen N. Scale for the Assessment of Positive Symptoms. Iowa City: University of Iowa; 1984. [Google Scholar]
- 48.Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City: University of Iowa; 1984. [Google Scholar]
- 49.Vadhan NP, Serper MR, Harvey PD, Chou JC, Cancro R. Convergent validity and neuropsychological correlates of the schedule for the assessment of negative symptoms (SANS) attention subscale. J Nerv Ment Dis. 2001;189(9):637-641. doi: 10.1097/00005053-200109000-00011 [DOI] [PubMed] [Google Scholar]
- 50.Peralta V, de Leon J, Cuesta MJ. Are there more than two syndromes in schizophrenia? a critique of the positive-negative dichotomy. Br J Psychiatry. 1992;161:335-343. doi: 10.1192/bjp.161.3.335 [DOI] [PubMed] [Google Scholar]
- 51.Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39(7):784-788. doi: 10.1001/archpsyc.1982.04290070020005 [DOI] [PubMed] [Google Scholar]
- 52.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251. doi: 10.1016/0920-9964(90)90005-R [DOI] [PubMed] [Google Scholar]
- 53.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. doi: 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- 54.Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323-329. doi: 10.1034/j.1600-0447.2000.101004323.x [DOI] [PubMed] [Google Scholar]
- 55.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 56.Corp IBM. IBM SPSS Statistics for Windows. Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 57.Hollingshead A. Two-Factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 58.Spellmann I, Riedel M, Schennach R, et al. One-year functional outcomes of naturalistically treated patients with schizophrenia. Psychiatry Res. 2012;198(3):378-385. doi: 10.1016/j.psychres.2011.12.047 [DOI] [PubMed] [Google Scholar]
- 59.Chang WC, Tang JY, Hui CL, et al. Prediction of remission and recovery in young people presenting with first-episode psychosis in Hong Kong: a 3-year follow-up study. Aust N Z J Psychiatry. 2012;46(2):100-108. doi: 10.1177/0004867411428015 [DOI] [PubMed] [Google Scholar]
- 60.Bachmann S, Bottmer C, Schröder J. One-year outcome and its prediction in first-episode schizophrenia—a naturalistic study. Psychopathology. 2008;41(2):115-123. doi: 10.1159/000112027 [DOI] [PubMed] [Google Scholar]
- 61.Verma S, Subramaniam M, Abdin E, Poon LY, Chong SA. Symptomatic and functional remission in patients with first-episode psychosis. Acta Psychiatr Scand. 2012;126(4):282-289. doi: 10.1111/j.1600-0447.2012.01883.x [DOI] [PubMed] [Google Scholar]
- 62.Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373-384. doi: 10.1176/appi.ajp.2015.14091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rund BR. Does active psychosis cause neurobiological pathology? a critical review of the neurotoxicity hypothesis. Psychol Med. 2014;44(8):1577-1590. doi: 10.1017/S0033291713002341 [DOI] [PubMed] [Google Scholar]
- 64.Anderson KK, Voineskos A, Mulsant BH, George TP, Mckenzie KJ. The role of untreated psychosis in neurodegeneration: a review of hypothesized mechanisms of neurotoxicity in first-episode psychosis. Can J Psychiatry. 2014;59(10):513-517. doi: 10.1177/070674371405901003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norman RMG, Malla AK, Manchanda R. Is untreated psychosis socially toxic? Early Interv Psychiatry. 2007;1(3):267-270. doi: 10.1111/j.1751-7893.2007.00038.x [DOI] [Google Scholar]
- 66.Thompson E, Millman ZB, Okuzawa N, et al. Evidence-based early interventions for individuals at clinical high risk for psychosis: a review of treatment components. J Nerv Ment Dis. 2015;203(5):342-351. doi: 10.1097/NMD.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 67.Cotter J, Lin A, Drake RJ, et al. Long-term employment among people at ultra-high risk for psychosis. Schizophr Res. 2017;184:26-31. doi: 10.1016/j.schres.2016.11.033 [DOI] [PubMed] [Google Scholar]
- 68.van Os J, Guloksuz S. A critique of the ‘ultra-high risk’ and ‘transition’ paradigm. World Psychiatry. 2017;16(2):200-206. doi: 10.1002/wps.20423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Os J, Murray RM. Can we identify and treat ‘schizophrenia light’ to prevent true psychotic illness? BMJ. 2013;346:f304. doi: 10.1136/bmj.f304 [DOI] [PubMed] [Google Scholar]
- 70.Malla A, Jordan G, Joober R, et al. A controlled evaluation of a targeted early case detection intervention for reducing delay in treatment of first episode psychosis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1711-1718. doi: 10.1007/s00127-014-0893-1 [DOI] [PubMed] [Google Scholar]
- 71.Iyer S, Jordan G, MacDonald K, Joober R, Malla A. Early intervention for psychosis: a Canadian perspective. J Nerv Ment Dis. 2015;203(5):356-364. doi: 10.1097/NMD.0000000000000288 [DOI] [PubMed] [Google Scholar]
- 72.Häfner H, an der Heiden W. The course of schizophrenia in the light of modern follow-up studies: the ABC and WHO studies. Eur Arch Psychiatry Clin Neurosci. 1999;249(suppl 4):14-26. doi: 10.1007/PL00014180 [DOI] [PubMed] [Google Scholar]
- 73.Kanahara N, Yoshida T, Oda Y, et al. Onset pattern and long-term prognosis in schizophrenia: 10-year longitudinal follow-up study. PLoS One. 2013;8(6):e67273. doi: 10.1371/journal.pone.0067273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalla O, Aaltonen J, Wahlström J, Lehtinen V, García Cabeza I, González de Chávez M. Duration of untreated psychosis and its correlates in first-episode psychosis in Finland and Spain. Acta Psychiatr Scand. 2002;106(4):265-275. doi: 10.1034/j.1600-0447.2002.02302.x [DOI] [PubMed] [Google Scholar]
- 75.Davidson L, McGlashan TH. The varied outcomes of schizophrenia. Can J Psychiatry. 1997;42(1):34-43. doi: 10.1177/070674379704200105 [DOI] [PubMed] [Google Scholar]
- 76.Morgan C, Abdul-Al R, Lappin JM, et al. ; AESOP Study Group . Clinical and social determinants of duration of untreated psychosis in the AESOP first-episode psychosis study. Br J Psychiatry. 2006;189:446-452. doi: 10.1192/bjp.bp.106.021303 [DOI] [PubMed] [Google Scholar]
- 77.Nishii H, Yamazawa R, Shimodera S, Suzuki M, Hasegawa T, Mizuno M. Clinical and social determinants of a longer duration of untreated psychosis of schizophrenia in a Japanese population. Early Interv Psychiatry. 2010;4(2):182-188. doi: 10.1111/j.1751-7893.2010.00179.x [DOI] [PubMed] [Google Scholar]
- 78.Malla A, de Bonneville M, Shah J, et al. Outcome in patients converting to psychosis following a treated clinical high risk state. Early Interv Psychiatry. 2018;12(4):715-719. doi: 10.1111/eip.12431 [DOI] [PubMed] [Google Scholar]