Abstract
Objective: Curcumin, an active ingredient derived from the rhizome of the plant, Curcuma longa, has antioxidant, anti‐inflammatory and anti‐cancer activities. The aims of this study were to examine whether curcumin can induce apoptosis in an osteosarcoma cell line.
Methods: Curcumin‐induced apoptosis in human osteosarcoma U2OS cells was investigated using morphological analysis, marked nuclear condensation and fragmentation of chromatin, which were observed by Hoechst 33258 staining and DNA ladder formation. The U2OS cells were treated with or without curcumin. Cell viability was assessed by the 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐Diphenyltetrazolium (MTT) method. Cell‐cycle, apoptosis and apoptosis‐related proteins in U2OS cells were evaluated by flow cytometry and western blotting.
Results: Curcumin showed growth inhibitory effects on U2OS cells in a dose‐and time‐dependent manner, inducing significant G1 arrest and apoptosis in U2OS cells. This curcumin‐induced apoptosis in U2OS cells was accompanied by up‐regulation of Bax, Bak, and p‐Bad and down‐regulation of Bcl‐2, but no effect on the levels of Bcl‐XL or Bad proteins was noted. Moreover, curcumin treatment resulted in a significant reduction of mitochondrial membrane potential and increase in the concentrations of mitochondrial cytochrome C and caspase‐3.
Conclusion: Multiple molecular pathways are involved in curcumin‐induced apoptosis of human U2OS cells. These include pro‐and anti‐apoptotic Bcl‐2 family proteins, mitochondrial membrane potential, mitochondrial cytochrome C and caspase‐3.
Keywords: Apoptosis, bcl‐2 homologous antagonist‐killer protein, Curcumin, Osteosarcoma
Introduction
Osteosarcoma is the most common primary malignant bone tumor and exhibits a peak in manifestation during the second and third decade of life. Adjuvant therapy for osteosarcoma requires the use of systemic chemotherapy, including doxorubicin, cisplatin, ifosfamide, methotrexate, and cyclophosphamide 1 . A previous study has demonstrated that the probability of disease‐free survival for patients with extremity osteosarcoma who receive chemotherapy is 66%, compared to 11% for patients not so treated 2 , indicating the need for further therapeutic improvement. In addition, the frequent acquisition of multidrug resistant phenotypes and the occurrence of ‘second malignancy’ are serious problems often associated with chemotherapy. New therapeutic strategies need to be evaluated to improve osteosarcoma survival. An improved clinical response could be gained by adopting therapies that are particularly effective in activating apoptosis. Thus, searching for other drugs that can cause apoptosis of osteosarcoma cells in vitro may be useful in developing new treatments for osteosarcoma.
Curcumin is a yellow colored phenolic compound derived from the plant, Curcuma longa, and has been used as a type of traditional Asian medicine for centuries. Because of its ability to scavenge free radicals and inhibit inflammation, curcumin has been proved to have some preventive effects in carcinogenesis in vitro 3 , 4 . Curcumin has been shown to have antitumor activity in the colon 5 , lymph node 6 , stomach 7 and breast 8 . Curcumin also inhibits cell proliferation and induces apoptosis in human leukemia 9 , prostate cancer 10 , and lung cancer cell lines 11 . A recently published phase I clinical trial of oral curcumin in colon cancer patients showed high tolerance of the drug in humans and proposed the possibility of developing curcumin as an oral cancer preventive or therapeutic agent 12 .
Apoptosis plays an important role in the maintenance of tissue homeostasis by the selective elimination of excessive cells. Currently, natural and dietary agents able to induce apoptosis in cancer cells have caught the eye of many researchers seeking novel anti‐cancer drugs.
Human osteosarcoma is characterized by rapid cell proliferation and strong expression of antiapoptotic genes, which suggests that its poor prognosis is mainly due to incomplete cell‐cycle arrest and apoptosis‐resistance with therapies in current use. Curcumin has previously been shown to induce cell‐cycle arrest and apoptosis in various cancer cells 3 , 4 , 5 , 6 , 7 , 8 . However, little is known about its effects on human osteosarcoma cells. The aims of this study were to examine whether curcumin can induce apoptosis in the osteosarcoma U2OS cell line, and to elucidate the mechanisms of apoptosis and growth suppression by analysis of cell‐cycle regulation and profiles of proapoptotic and antiapoptotic proteins.
Materials and methods
Reagents
Curcumin, bisbenzimide (Hoechst H33258), ethidium bromide (EB), heat‐inactivated fetal bovine serum (FBS) and monoclonal mouse anti‐β‐actin antibody were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Stock solution (10 mM), prepared in dimethyl sulfoxide (DMSO) and protected from light, was diluted to the appropriate concentration with medium. Z‐DEVD‐FMK (Kaimana Biomedical, Seattle, WA, USA) was prepared in 100 mM DMSO stock solution. Propidium iodide (PI) was purchased from Bio Basic, (Toronto, Canada). L‐glutamine (200 mM) and a mix of penicillin/streptomycin (10 000 IU/ml and 10 000 mg/ml, respectively) were purchased from Mediatech (Herdon, VA, USA). Plastic dishes were obtained from Corning‐Costar (Cambridge, MA, USA) and all other tissue culture plastics were from Falcon Plastics (Los Angeles, CA, USA). Protein concentration was assayed using the Bio‐Rad protein assay based on the Bradford dye‐binding procedure obtained from Bio‐Rad Laboratories (Hercules, CA, USA). Anti‐human Bax polyclonal antibody and anti‐human Bcl‐2 polyclonal antibody were purchased from Sigma (St Louis, MO, USA). Secondary horseradish peroxidase‐labeled goat anti‐mouse and anti‐rabbit antibodies were obtained from Santa Cruz Biotechnology, (Santa Cruz, CA, USA). PVDF membrane was purchased from Millipore (Bedford, MA, USA). The enhanced chemiluminescence (ECL) detection system was purchased from Amersham (Piscataway, NJ, USA). All other reagents were of analytical reagent quality.
Cell culture and treatment
The human osteosarcoma cell lines U2OS (ATCC, CRL‐1427, HTB 96, Manassas, VA, USA) 13 , 14 , were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum, 100 µg/ml streptomycin and 100 U/ml penicillin. Cultures were maintained at 37°C in a humidified incubator in an atmosphere of 95% O2 plus 5% CO2. Curcumin was dissolved in DMSO at a concentration of 10 mM and stored in a dark‐colored bottle at 4°C as a stock solution. The stock was diluted with growth media to the required concentration immediately before use. The cells were exposed to curcumin at different concentrations (5, 10, 25, 50, 75, 100 µM) and for different time (6, 12, 24, 36 h). Cells grown in media containing equivalent amount of DMSO without curcumin served as control.
Cell cytotoxicity assay
The viability of the cells was assessed by MTT assay, which is based on the reduction of MTT by the mitochondrial dehydrogenase of intact cells to a purple formazan product. Cells (2 × 104) were plated in a 96‐well plate. After 24 h, they were treated with different concentrations of curcumin for different time intervals. After treatment, media containing curcumin were carefully removed by aspiration. 100 µl of 0.5 mg/ml MTT in cell culture medium was added to each well and incubated for 4 h as described previously 15 . 100 µl of 1% sodium dodecylsulfate (SDS) was added to each well after 4 h. The plates were covered with aluminum foil and kept in an incubator for 12 h for dissolution of the formazan crystals that had formed. An ELISA reader was used to measure the absorbance at 570 nm and the IC50 value was assessed by the Bliss method 11 .
Hoechst 33258 staining
Apoptotic morphology was studied by staining the cells with Hoechst 33258 stain: cells were seeded on coverslips in 6‐well plates and treated with 50 µM curcumin. After 24 h, the coverslips were washed carefully with PBS and stained with 20 µg/ml Hoechst 33258 for 10 min. Thereafter, the cells were washed in PBS, and observed under a fluorescence microscope (Leica Microsystems AG, Wetzlar, Germany).
DNA fragmentation
U2OS cells (2 × 106) were treated with curcumin for various concentrations and collected by centrifugation (500 × g, 5 min) at 4°C. Cells were lysed in ice‐cold lysis buffer (5 mM Tris, pH = 8.0, 0.5% Triton X‐100, 20 mM ethylenediaminetetraacetic acid [EDTA]), incubated on ice for 20 min and centrifuged at 13 000 ×g for 20 min at 4°C to separate the low molecular weight DNA from intact chromatin. The supernatant was extracted by phenol/chloroform and DNA was precipitated by ethanol. The DNA pellet was dissolved in 300 µl TE buffer (10 mM Tris, pH 8.0, 1 mM EDTA), and 5 µl of a 10 mg/ml solution of DNase‐free Rnase A (ICN Chemical, Hercules, CA, USA) was added. After incubation at 37°C for 30 min, phenol/chloroform extraction and ethanol precipitation were repeated, and DNA was dissolved in 15 µl TE buffer and separated by electrophoresis on 2% agarose containing 0.5 mg/ml ethidium bromide. DNA bands were examined using a UV Transilluminator Image System (BioRad, Hercules, CA, USA).
Cell cycle analysis by flow cytometry
U2OS cells were plated in 12‐well culture plates at a density of 1 × 105 cells/ml and treated with curcumin or vehicle for the indicated time. Cells were washed once with phosphate–buffered saline (PBS) and fixed with 70% ice‐cold ethanol at −20°C overnight. Then the cells were resuspended in a staining solution containing 1% Triton X‐100, 0.1 mg/ml RNase and 4 µg/ml PI. The cell suspensions were incubated at 37°C for 30 min in the dark and analyzed on a fluorescence‐activated cell sorter flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA, USA) and all histograms were analyzed by ModFit software.
Assay of mitochondrial membrane potential
After incorporation of the fluorescent probe, the cells were incubated up to 4 h with or without 50 µM curcumin. Initially, 1 × 106 U2OS cells/ml were incubated with 10 µM rhodamine 123 for 10 min at 37°C. At the end of incubation, the cells were washed twice with PBS, harvested by centrifugation, and then resuspended in 1.5 ml PBS. The fluorescent intensity of each cell suspension was measured at an excitation wavelength of 480 nm and an emission wavelength of 530 nm in a Perkin Elmer L15B fluorescence spectrophotometer (Boston, MA, USA). The fluorescence intensity was used as an arbitrary unit to represent the mitochondrial transmembrane potential.
Assay of caspase‐3 activity
After treatment with the indicated agents, U2OS cells were harvested and washed with PBS by centrifugation at 750 ×g for 5 min at 4°C. The cell pellets were resuspended in lysis buffer (caspase colorimetric assay kits; Bivision, San Diego, CA, USA) and left on ice for 30 min. The lysates were centrifuged at 10 000 ×g for 10 min and the supernatant (20 µl) was collected for caspase‐3 activity assay in a lysis buffer containing Asp‐Glu‐Val‐Asp p‐nitroanilide (DEVD‐pNA), a specific substrate for caspase‐3. The concentration of pNA, as the product from enzymatic converting of DEVD‐pNA by caspase‐3, was measured at 405 nm and used as an indicative of caspase‐3 activity.
Mitochondrial cytochrome C release
U2OS cells were seeded in 2 ml fresh medium at an initial density of 1 × 106 cells/ml and incubated up to 4 h with or without 50 µM curcumin. After incubation, the cells were harvested by centrifugation and washed twice with PBS, then suspended in 200 µl lysis buffer (195 mM mannitol; 65 mM sucrose; 2 mM 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid [HEPES], pH = 7.4; 0.05 mM ethylene glycol tetraacetic acid [EGTA]; 0.01 mM MgCl2; 0.5 mg/ml bovine serum albumin [BSA]) and lysed by the addition of 0.01% digitonin. The cytosolic fraction was obtained from 10 000 × g centrifugation for 10 min and was collected for cytochrome c assay in 1 × RD5P calibrator diluent (cytochrome c Immunoassay Kit; R&D Systems, Minneapolis, MN, USA).
Western blot analysis
After exposed to the indicated concentrations of curcumin, U2OS cells were washed with cold PBS. Whole cell extracts were prepared by incubating the cells with cold lysis buffer (20 mM Tris‐HCl; pH = 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM b‐glycerophosphate, 1 mM Na3VO4, 1 mg/ml leupeptin, and 1 mM phenylmethanesulphonylfluoride [PMSF]). The protein content of the lysates was determined using the DC protein assay kit (Bio‐Rad). The cell lysates (25 µg protein/lane) were electrophoresed on 12% SDS‐polyacrylamide gels. The cellular proteins were then transferred to PVDF membranes by electroblotting for 2 h and western blot analysis was carried out as previously described 15 . The protein levels were visualized with an enhanced chemiluminescence detection kit.
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis of data was performed by one‐way ANOVA, followed by Student's t‐test. p‐values <0.05 were considered statistically significant.
Results
Effects of curcumin on human U2OS cells cytotoxicity
To investigate the effect of curcumin on U2OS cell proliferation, the cells were treated for 24 h in medium containing varying concentrations of curcumin up to 100 µM. Cells were counted by the MTT method. In the present study, curcumin showed a potent cytotoxic effect on U2OS cells in a dose‐ and time‐dependent manner, the results being expressed as percentage cell survival (Fig. 1). The survival rate of human U2OS cells treated with 50 µM curcumin started to decrease 6 h after treatment and dropped sharply after 12 h incubation (Fig. 1B). The IC50 at 24 h exposure to curcumin was 56.7 ± 5.6 µM. Thus, 50 µM of curcumin was selected to monitor changes in molecular events for the subsequent experiments.
Figure 1.
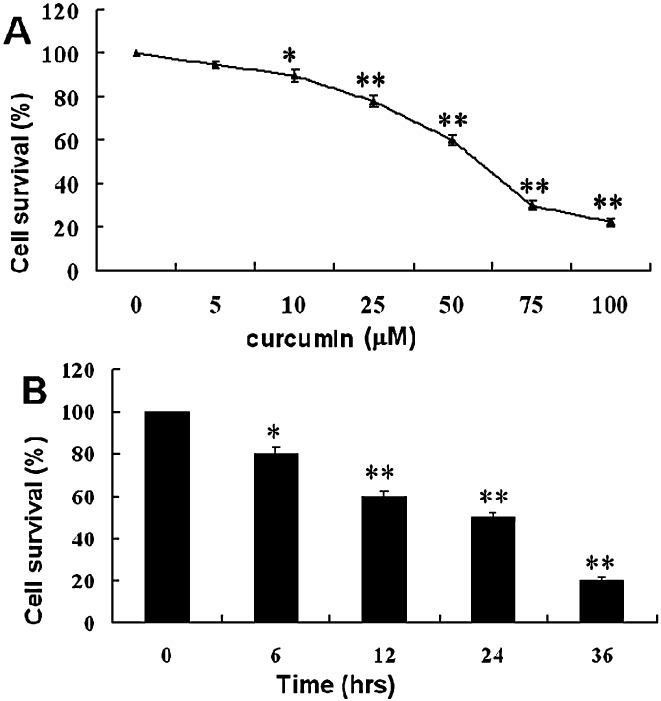
Cytotoxicity of curcumin in human U2OS cells. (A) U2OS cells were incubated with 0, 5, 10, 25, 50, 75 or 100 µM of curcumin for 24 h. (B) U2OS cells were incubated with curcumin (50 µM) for various periods of time. At the end of incubation, cell survival rate was determined by MTT methods. Cytotoxicity is expressed as the percentage of cell survival rate compared to the control. The data are expressed as mean ± SD of 3–5 determinations, *P < 0.01 and **P < 0.001.
Curcumin induced apoptosis of U2OS cells
To characterize curcumin‐induced cell death, several hallmarks of apoptosis were examined, namely, nuclear chromatin condensation and fragmentation of DNA by Hoechst 33258. In contrast to control cells (Fig. 2A), cells exposed to 50 µM curcumin had nuclei with chromatin condensation and fragmentation (Fig. 2B). During morphological examination, curcumin‐treated cells were found to show typical apoptotic morphological changes, such as cell shrinkage, nuclear fragmentation and apoptotic body formation. Furthermore, treatment with curcumin resulted in DNA fragmentation, another hallmark piece of evidence of cell undergoing apoptosis, as shown by formation of DNA ladder on 2% agarose gel (Fig. 3). These results confirmed that curcumin induces apoptosis in human U2OS cells.
Figure 2.
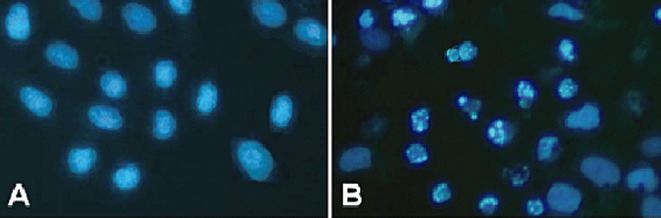
Hoechst 33258 fluorescent staining detection of apoptotic morphology in U2OS cells treated with 50 µM curcumin for 24 h. (A) Untreated cells. (B) Cells treated with 50 µM curcumin for 24 h.
Figure 3.
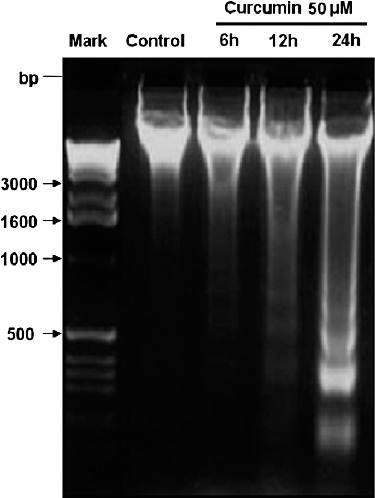
DNA fragmentation formed in U2OS cells after treatment with curcumin. U2OS cells were incubated with 0.1% DMSO as vehicle control and treated with 50 µM curcumin for the indicated time. DNA fragmentation was analyzed by 2% agarose gel electrophoresis (M‐base pair size standards).
Effect of curcumin on U2OS cell cycle distribution
In order to quantify the kinetics of events both during apoptosis and cell cycle phases, we performed flow cytometric analysis. We cultured U2OS cells for various lengths of time with 50 µM curcumin and analyzed DNA content by flow cytometry. Curcumin induced a time‐dependent accumulation of G1 phase in U2OS cells (Fig. 4), and then the cells underwent apoptosis (sub‐G1 peak appeared at 6 h). The curcumin‐induced G1 block peaked at 12 h, with approximately 56.3% of the cells in G1 at this time compared with 33.7% in the control.
Figure 4.
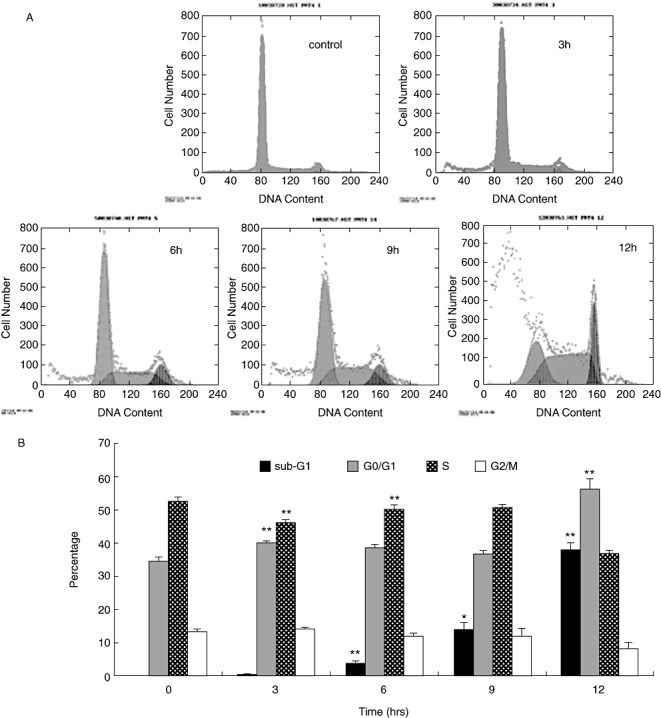
(A) Cells were treated with 50 µM curcumin for the indicated times, then harvested and subjected to analysis of the DNA content by flow cytometry. (B) U2OS cells were incubated with curcumin for various periods of time and the results of independent experiments are presented in figure form showing percentages (± SD) of cells in each phase of the cell cycle. G1 arrested the effects of curcumin. Data from five independent experiments is presented as mean ± SD. *P < 0.01; **P < 0.001; compared with control. FCM, effects of curcurmin; G1, S, G0/G1 and G2/M, phases of the cell cycle.
Determination of involvement of caspase‐3 activation
In order to verify the requirement for caspases on curcumin‐induced apoptosis in U2OS, we used the cell permeable caspase inhibitor Z‐DEVD‐FMK. U2OS cells were pretreated with 50 µM caspase‐3 inhibitors for 2 h, and then induced to undergo apoptosis by treatment with curcumin. The results showed that administration of caspase‐3 inhibitor alone did not affect the cell viability and caspase‐3 activity. However, Z‐DEVD‐FMK significantly inhibited curcumin‐induced cell death and caspase‐3 activation in U2OS cells (Fig. 5).
Figure 5.
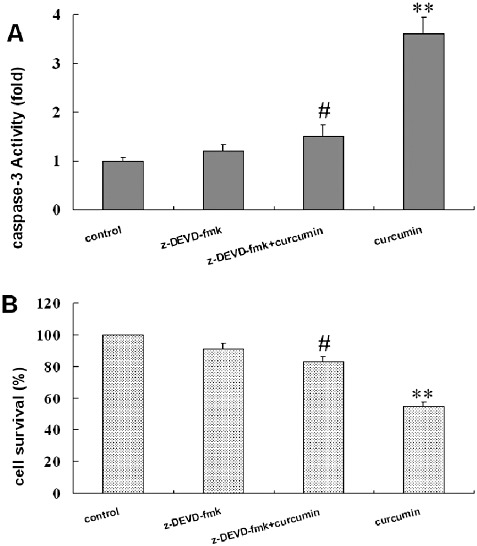
Inhibition of caspase 3 activity and attenuation of curcumin‐induced cell death by Z‐DEVD‐FMK. U2OS cells were treated with 50 µM Z‐DEVD‐FMK 2 h prior to 24 h of 50 µM curcumin treatment. After incubation, (A) caspase‐3 activity and (B) cell survival rate were examined as described in Materials and Methods. All values are mean ± SD of four–five determinations, **P < 0.001 compared with the respective curcumin and Z‐DEVD‐FMK free control and #P < 0.001 comparison between the absence and presence of Z‐DEVD‐FMK in the same curcumin treatment group.
Changes in mitochondrial membrane potential and release of cytochrome C from mitochondria
As shown in Fig. 6, curcumin induced a time‐dependent mitochondrial transmembrane depolarization, represented as a decrease in mitochondrial membrane potential (Fig. 6A). Concomitantly, a time‐dependent curcumin‐induced cytochrome C release was also observed in U2OS cells, represented as a significant increase in cytosolic cytochrome C concentration (Fig. 6B). In this study, the cytochrome C release and mitochondrial membrane potential were analyzed spectrophotometrically. These data suggest that loss of mitochondrial membrane potential may be required for curcumin‐induced release of cytochrome C release into cytosol, which later triggers cleavage, activation of mitochondrial downstream caspases and onset of apoptosis.
Figure 6.
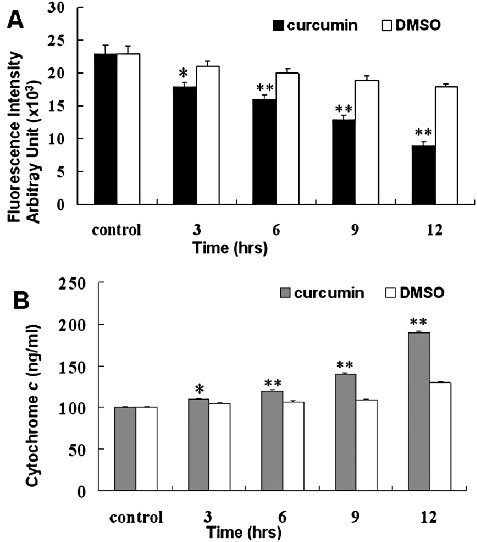
Measurement of mitochondrial membrane potential and cytoplasmic cytochrome c release in U2OS cells during curcumin treatment. (A) U2OS cells were treated with 10 µM rhodamine 123 for 10 min prior to incubation with curcumin and then cells were incubated with or without 50 µM curcumin for various periods of time. At the end of incubation, mitochondrial membrane potential was measured as described in Materials and Methods. (B) U2OS cells were incubated with or without 50 µM of curcumin for the indicated time. Cytoplasmic cytochrome c release was determined by immunoassay as described in Materials and Methods. The data are expressed as mean ± SD of five determinations, *P < 0.01 and **P < 0.001 compared to DMSO treated group.
Effects of curcumin on apoptosis related proteins
We also investigated the expression of several proteins that are involved in curcumin‐induced apoptosis. The expression of several members of the Bcl‐2 family of proteins was examined by western blot analysis. Exposure of U2OS cells to various concentrations of curcumin resulted in a marked decrease of Bcl‐2 protein expression, but a drastic increase in the expression of Bax, Bak and p‐Bad proteins. However, the concentrations of Bcl‐XL and total Bad proteins were not affected by curcumin treatment (Fig. 7).
Figure 7.
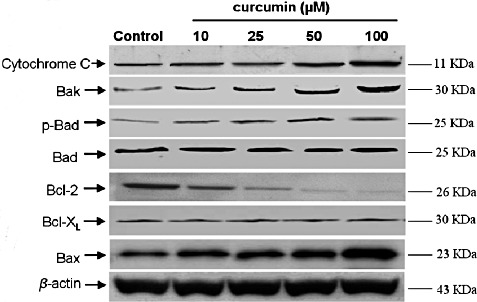
Expression of cytochrome c and Bcl‐2 family proteins in curcumin‐treated U2OS cells. U2OS cells were treated with curcumin for 24 h. After treatment, cell lysates were extracted, and the concentrations of Bcl‐2 family proteins were analyzed by western blot analysis.
Discussion
In the present study, we found that curcumin can induce apoptosis in U2OS cells through increasing mitochondrial membrane permeability by down‐regulation of Bcl‐2 and up‐regulation of Bax, Bak, and p‐Bad, then triggering cytochrome c release from mitochondria into cytosol and activating caspase‐3.
In this study, we demonstrated that curcumin treatment of U2OS cells results in significant cell growth inhibition, G1 phase arrest and apoptosis in a time‐and dose‐dependent manner. U2OS cells treated with curcumin exhibited characteristic morphological features of apoptosis, such as membrane shrinkage, chromosomal condensation and increases in caspase‐3 activity. Moreover, preincubation of cells with Z‐DEVD‐FMK, a specific caspase‐3 inhibitor, effectively inhibited caspase‐3 activity and curcumin‐induced cell death. These data show that curcumin induces U2OS cells apoptosis via a caspase‐dependent apoptotic pathway. Our results were in agreement with previous studies on stomach 7 , prostate 10 and lung 11 cancer cells.
Exposure to curcumin, progressive decrease in mitochondrial membrane potential and release of cytochrome C into the cytosol were also observed in U2OS cells. It has been noticed in many in vitro systems that apoptosis is associated with a loss of mitochondrial membrane potential, which may correspond to the opening of an outer membrane pore. Thus, it has been suggested that this event is responsible for cytochrome c release into the cytosol from mitochondria 16 . In this study, cytosolic cytochrome C accumulation in curcumin‐induced human U2OS cells was probably a consequence of loss of mitochondrial membrane potential, which finally leads to cell death. Although many studies have suggested that caspase‐3 can also be activated through mitochondria‐dependent signaling proteins by releasing cytochrome c from its intermembrane space into the cytoplasm 17,18,23 , it is not clear how cytosolic cytochrome C accumulation and caspase activation are achieved with curcumin treatment.
Bcl‐2 is a member of a family of genes that regulates the apoptosis threshold. The family includes members which act as pro‐apoptotic proteins (such as Bax, Mtd, Bcl‐XS, Bak and Bik) and anti‐apoptotic proteins (such as Bcl‐2, Bcl‐W and Bcl‐XL) 16 . The interactions and relative frequency of occurrence of these proteins appear to modulate the propensity of a cell to undergo apoptotic cell death 18 . Mitochondrial‐mediated apoptosis is facilitated by members of the Bcl‐2 homologous family of proteins 19 , 20 . Reduction of mitochondrial membrane potential leads to release of intermembrane proteins, such as cytochrome c and apoptosis‐inducing factors, into the cytosol and induces apoptotsis 21 .
Several studies have shown that overexpression of Bcl‐2 and Bcl‐XL prevents mitochondrial release of cytochrome c, thereby inhibiting activation of the caspase cascade and apoptosis 22 , 23 . Bcl‐XL is present in the mitochondrial outer membrane and pro‐apoptotic molecules Bax, Bak, and Bad occur in the cytosol in the absence of apoptotic stimuli 24 , 25 . During apoptosis, Bax undergoes homodimerization and translocation to the mitochondrial membrane, which triggers an apoptotic cascade leading to cell death 26 . Moreover, expression of Bax is capable of directly initiating apoptosis 27 . In the present study, curcumin‐induced apoptosis in U2OS cells was accompanied by up‐regulation of Bax, p‐Bad, and Bak and down‐regulation of Bcl‐2, but had no effect on Bcl‐XL and Bad protein expression. Other studies have demonstrated that Bcl‐2, Bcl‐ XL, Bax, and Bak can act as channel proteins within the mitochondrial membrane 28 , 29 . It is conceivable that the channel properties of Bax and Bak may control the mitochondrial permeability transition and other early mitochondrial perturbations. Thus, Bax and Bak may facilitate the passage of some important proteins, such as cytochrome c or other apoptosis inducing factors which trigger activation of the caspase cascade and apoptosis. Previous reports have also documented that the ratio of pro‐ and anti‐apoptotic proteins determines, at least in part, the susceptibility of cells to a death signal 30 , 31 .
Our results showed that expression of Bcl‐2 family proteins Bcl‐2, Bcl‐XL, Bax, Bad, p‐Bad, and Bak can be differently regulated by curcumin, suggesting that curcumin‐induced apoptosis is controlled by balanced expression of those apoptosis‐inducing and apoptosis‐ suppressing molecules. Thus, enforced dimerization of Bax and Bak may result in altered permeability, triggering mitochondrial cytochrome c release into the cytosol, activating the caspase‐3 cascade, and eventually promoting cell death. However, our results did not show involvement of Bcl‐XL and Bad in curcumin‐induced apoptosis in U2OS cells, although other studies have demonstrated that Bcl‐XL is down‐regulated by curcumin in human lung cancer cells 11 and Bad proteins are involved in ischemia‐induced cell death 32 . These results suggest that the molecular events of chemical induced apoptosis may depend on the particular cell types tested or the actions of different chemicals.
In conclusion, the present study demonstrates that curcumin induces apoptosis in U2OS cells. The treatment of U2OS cells with curcumin activates a cell death pathway that regulates mitochondrial membrane permeability by down‐regulation of Bcl‐2 and up‐regulation of Bax, Bak, and p‐Bad and triggers the release of cytochrome c from mitochondria into the cytosol. Subsequently caspase‐3 activation is induced and then specific substrates cleaved leading to the apoptotic process. Overall, our studies indicate that the Bcl‐2 family, cytochrome c and caspase‐3, may play an important role in the regulation and activation of the executioner phase of curcumin induced apoptosis. Curcumin is a promising candidate for an anti‐tumor agent against U2OS cells.
This study was supported by the Science and Technology Foundation of Wuhu (No. 2008722), the Research Fund for the Doctoral Program of Higher Education of China (No. 20070908 and No. 20080715) and the Education Commission Foundation of Anhui (No. 2006KJ406B.)
References
- 1. Bruland OS, Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer, 1997, 33: 1725–1731. [DOI] [PubMed] [Google Scholar]
- 2. Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol, 1994, 12: 423–431. [DOI] [PubMed] [Google Scholar]
- 3. Hendrich AB. Flavonoid‐membrane interactions: possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol Sin, 2006, 27: 27–40. [DOI] [PubMed] [Google Scholar]
- 4. Ruby AJ, Kuttan G, Babu KD, et al. Anti‐tumour and antioxidant activity of natural curcuminoids. Cancer Lett, 1995, 94: 79–83. [DOI] [PubMed] [Google Scholar]
- 5. Kawamori T, Lubet R, Steele VE, et al. Chemopreventive effect of curcumin, a naturally occurring anti‐inflammatory agent, during the promotion/ progression stages of colon cancer. Cancer Res, 1999, 59: 597–601. [PubMed] [Google Scholar]
- 6. Liu HL, Chen Y, Cui GH, et al. Curcumin, a potent anti‐tumor reagent,is a novel histone deacetylase inhibitor regulating B‐NHL cell line Raji proliferation. Acta Pharmacol Sin, 2005, 26: 603–609. [DOI] [PubMed] [Google Scholar]
- 7. Tang XQ, Bi H, Feng JQ, et al. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin, 2005, 26: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 8. Verma SP, Salamone E, Goldin B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF‐7 cells induced by estrogenic pesticides. Biochem Biophys Res Commun, 1997, 233: 692–696. [DOI] [PubMed] [Google Scholar]
- 9. Chakraborty S, Ghosh U, Bhattacharyya NP, et al. Inhibition of telomerase activity and induction of apoptosis by curcumin in K‐562 cells. Mutat Res, 2006, 596: 81–90. [DOI] [PubMed] [Google Scholar]
- 10. Deeb D, Xu YX, Jiang H, et al. Curcumin (Diferuloyl‐Methane) enhances tumor necrosis factor‐related apoptosis‐inducing ligand‐induced apoptosis in LNCaP prostate cancer cells. Mol Cancer Ther, 2003, 2: 95–103. [PubMed] [Google Scholar]
- 11. Lee J, Im YH, Jung HH, et al. Curcumin inhibits interferon‐alpha induced NF‐kappaB and COX‐2 in human A549 non‐small cell lung cancer cells. Biochem Biophys Res Commun, 2005, 334: 313–318. [DOI] [PubMed] [Google Scholar]
- 12. Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res, 2004, 10: 6847–6854. [DOI] [PubMed] [Google Scholar]
- 13. Pontén J, Saksela E. Two established in vitro cell lines from human mesenchymal tumours. Int J Cancer, 1967, 2: 434–447. [DOI] [PubMed] [Google Scholar]
- 14. Heremans H, Billiau A, Cassiman JJ, et al. In vitro cultivation of human tumor tissues. II. Morphological and virological characterization of three cell lines. Oncology, 1978, 35: 246–252. [DOI] [PubMed] [Google Scholar]
- 15. Jow GM, Wu YC, Guh JH, et al. Armepavine oxalate induces cell death on CCRF‐CEM leukemia cell line through an apoptotic pathway. Life Sci, 2004, 75: 549–557. [DOI] [PubMed] [Google Scholar]
- 16. Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post‐translational control of the pro‐apoptotic activity of BH3‐only proteins. Cell Death Differ, 2002, 9: 505–512. [DOI] [PubMed] [Google Scholar]
- 17. Kantrow SP, Piantadosi CA. Release of cytochrome c from liver mitochondria during permeability transition. Biochem Biophys Res Commun, 1997, 232: 669–671. [DOI] [PubMed] [Google Scholar]
- 18. Reed JC. Double identity for proteins of the Bcl‐2 family. Nature, 1997, 387: 773–776. [DOI] [PubMed] [Google Scholar]
- 19. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science, 2004, 305: 626–629. [DOI] [PubMed] [Google Scholar]
- 20. O'Neill J, Manion M, Schwartz P, et al. Promises and challenges of targeting Bcl‐2 anti‐apoptotic proteins for cancer therapy. Biochim Biophys Acta, 2004, 1705: 43–51. [DOI] [PubMed] [Google Scholar]
- 21. Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl‐2 release of cytochrome c from mitochondria blocked. Science, 1997, 275: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 22. Gross A, McDonnell JM, Korsmeyer SJ. Bcl‐2 family members and the mitochondria in apoptosis. Genes Dev, 1999, 13: 1899–1911. [DOI] [PubMed] [Google Scholar]
- 23. Budihardjo I, Oliver H, Lutter M, et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol, 1999, 15: 269–290. [DOI] [PubMed] [Google Scholar]
- 24. Salvesen GS, Dixit VM. Caspase activation: the induced‐proximity model. Proc Natl Acad Sci USA, 1999, 96: 10964–10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fadeel B, Zhivotovsky B, Orrenius S. All along the watchtower: on the regulation of apoptosis regulators. FASEB J, 1999, 13: 1647–1657. [DOI] [PubMed] [Google Scholar]
- 26. Tsujimoto Y. Bcl‐2 family of proteins: life‐or‐death switch in mitochondria. Biosci Rep, 2002, 22: 47–58. [DOI] [PubMed] [Google Scholar]
- 27. Borner C. The Bcl‐2 protein family: sensors and checkpoints for life‐or‐death decisions. Mol Immunol, 2003, 39: 615–647. [DOI] [PubMed] [Google Scholar]
- 28. Eskes R, Desagher S, Antonsson B, et al. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol, 2000, 20: 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res, 2000, 256: 375–382. [DOI] [PubMed] [Google Scholar]
- 30. Vander Heiden MG, Thompson CB. Bcl‐2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol, 1999, 1: E209–E216. [DOI] [PubMed] [Google Scholar]
- 31. Zhang L, Yu J, Park BH, et al. Role of Bax in the apoptotic response to anticancer agents. Science, 2000, 290: 989–992. [DOI] [PubMed] [Google Scholar]
- 32. Radhakrishna Pillai G, Srivastava AS, Hassanein TI, et al. Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett, 2004, 208: 163–170. [DOI] [PubMed] [Google Scholar]


