Abstract
In the last 10 years, basic and clinical research in orthopaedics has developed rapidly. Understanding of orthopaedic disorders involves not only routine diagnosis, but also the pursuit of highly efficient and accurate three‐dimensional imaging of the intra‐ and extra‐medullary distribution, form and structure of orthopaedic disorders, thus allowing scientific evaluation of the indications for surgery, drawing up of the best surgical plan, minimization of operative trauma and the earliest possible restoration of limb function. Meanwhile, the most important type of basic research, which was previously biomechanical research, has gradually become computational biomechanics based on in vitro cadaver experiments. This review aims to summarize the research status and application prospects of digital technology in orthopaedics, including virtual reality technology, reverse engineering and rapid prototyping techniques, computational biomechanics, computer navigation technology and management of digitization of medical records.
Keywords: Computer‐assisted surgery, Finite element analysis, Orthopedics, Radiology
In the current era of rapidly developing electronic information technology, digital technology has not only brought about great changes in life and work generally, but has also led to profound changes in the pattern of medical practice. In the last 10 years, basic and clinical research in orthopaedics has developed rapidly. Understanding of orthopaedic disorders involves not only routine diagnosis, but also the pursuit of highly efficient and accurate three‐dimensional (3‐D) imaging of the intra‐ and extra‐medullary distribution, form and structure of orthopaedic disorders, thus allowing scientific evaluation of the indications for surgery, drawing up of the best surgical plan, minimization of operative trauma and the earliest possible restoration of limb function. Meanwhile, the most important type of basic research, which was previously biomechanical research, has gradually become computational biomechanics based on in vitro cadaver experiments. This review aims to summarize the research status and application prospects of digital technology in orthopaedics.
The Birth of the Visible Human and Digital Orthopaedics
In 1989, under the direction of the Board of Regents of the National Library of Medicine (NLM), an ad hoc planning panel was convened to provide the library with in‐depth guidance as to its proper role in the rapidly changing field of digital imaging1. In August 1991, the NLM contracted with Victor Spitzer and David Whitlock of the University of Colorado School of Medicine (Boulder, CO, USA) to acquire appropriate cadavers and capture the required images. On 28 November 1994, the NLM announced the availability of a digital data set of human male anatomy. The data set, about 15 gigabytes in size, consists of frontal radiographs, MR and CT images, and images of anatomic serial sections of a single “normal” male cadaver. Within only one year, this data set had been wildly used by hundreds of groups and tens of countries2. South Korea was the second country after the USA to develop visible human data. The first data set of VKH (Visible Korean Human), which has the characteristics of Eastern human males, exceeded the VHP (Visible Human Project) of the USA in both resolution and accuracy3. A China Visible Human (CVH) male was created by October 2002 and a CVH female by February 2003, making China the third country in the world to develop a VH (Visible Human) data set and the second country to complete a visible human male and female pair4. In the field of orthopaedics, a computer‐assisted orthopaedics symposium (CAOS‐Symposium) was held by Bern University (Bern, Switzerland) in 1995. Computer‐assisted orthopaedics surgery international (CAOS‐International) was established in 2000 and the first international annual symposium held in 2001. Subsequently, many countries gradually began to develop all kinds of digital technology in the field of orthopaedics and achieved various objectives.
Digital orthopaedics is an advanced interdisciplinary subject combining computer information, image processing, and medical physics technology; medical education; and clinical and research needs. It includes digital orthopaedic anatomy, orthopaedic simulating education, orthopaedic surgical science, information storage; and remote interaction. Digitization, the underpinning technique of the information society, is causing an overarching and significant industrial revolution. The inexorable trend toward blending digital technology with medicine will certainly push orthopaedic clinical research to a new level. For presenting the research status of digital orthopaedics, we have divided this subject into two parts: intraoperative and preoperative/postoperative status. The intraoperative status primarily includes computer navigation, which we have bundled with specific hardware packages and background software. The preoperative/postoperative status consists of preoperative digital design, postoperative follow‐up visits, remote consultation, digital anatomy and computational biomechanical research that rely on software.
The Application and Research Methods of Digital Technology in Assessment of Preoperative/Postoperative Status
Virtual Reality Technology
Almost two decades ago, Satava proposed early adoption of virtual reality (VR) as a training tool5. Computer‐based training in technical skills has the potential to solve many of the educational, economic, ethical, and patient safety issues related to learning to perform surgery. Although full virtual reality systems are still in development, there has been early progress that should encourage surgeons to incorporate computer simulation into the surgical curriculum6. In a study designed by Andersen et al., VR training was proved to be a possible way for young and inexperienced surgeons to achieve the basic navigation skills necessary for performing arthroscopic surgery7. Vankipuram et al. reported a virtual orthopedic drilling simulator that produces a learning effect that transfers to real‐world drilling8.
Digital preoperative planning, assisted by post‐processing of CT or MRI images, is another kind of virtual reality application. For doctors and patients, every second on the operation table counts. In order to achieve the most appropriate approach, adequate exposure, precise replacement, suitable implant selection, and quick and reliable implantation, we have to design our procedures preoperatively. For decades, orthopaedic experts the world over have made every effort to conduct preoperative planning. They originally based this on attentive reading of X‐ray films, manual drawing and clipping, and more recently on digital photography, printing, clipping, comparison of internal fixators and so on. However, these methods cannot produce accurate preoperative designs because of the zoom in/out phenomenon with imaging films, pincushion distortion or barrel distortion of digital photos and manual errors.
Modern preoperative planning consists of stereoscopic views and surgical simulation. Although multislice CT has been updated quickly because of its popularity, bundling CT image post‐processing software that can output HD‐images still runs only in the workstation. In addition, the two‐dimensional (2‐D) CT images that clinicians still observe on films do not provide optimal views and cannot be edited freely. 3‐D CT reconstruction images, which generally greatly surpass X‐ray films, are limited by the differing requirements of clinicians and imaging specialists. In 2009, Chen Yan‐xi et al.9 set up a digital orthopaedic clinical research platform (SuperImage Orthopaedics edition 1.0, Cybermed, Shanghai, China) which can provide 3‐D reconstruction images with plotting scales, 3‐D preoperative free observation, 3‐D measurement and virtual operation by means of reconstructing initial CT data (Digital Imaging and Communications in Medicine [DICOM]) (Fig 1). These researchers established a set of variables for assessing normal ankle anatomical structure with the SuperImage System that involves measuring a combination of four elements, namely spot, wire, plane and curve, in 3‐D. This study provided some data that is relevant to planning of standard anatomical reduction of injured ankles and repeat surgery after malunion10. The measurement methods are reliable, reproducible, and easy to apply in practice.
Figure 1.
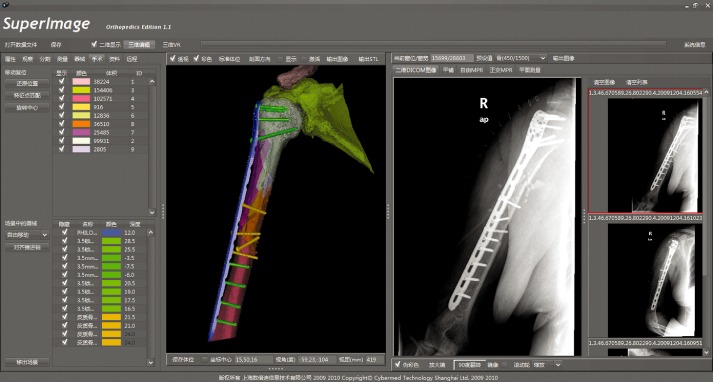
Reproduction (with permission) of a screenshot of SuperImage orthopaedics created by Yan‐xi Chen et al. showing a case with complex middle‐proximal humeral shaft fractures (OTA Type 12‐C3) in which the surgical procedure implemented was strictly according to computer‐assisted preoperative planning. The postoperative X‐ray images show a high consistency between the surgical procedure and the preoperative planning.
Hu Yong‐cheng et al. integrated a method for using the SuperImage System to measure the volume of cavitary bone tumors by 3‐D image segmentation, free profile selection, regional filling and 3‐D combined measuring techniques (Fig. 2)11. Using these key techniques, they also established a new clinical gating system for giant cell tumors according to treatment protocols and prognostic factors. The gating system is an effective, reliable method that guides doctors in clinical selection of appropriate excision and reconstruction methods.
Figure 2.
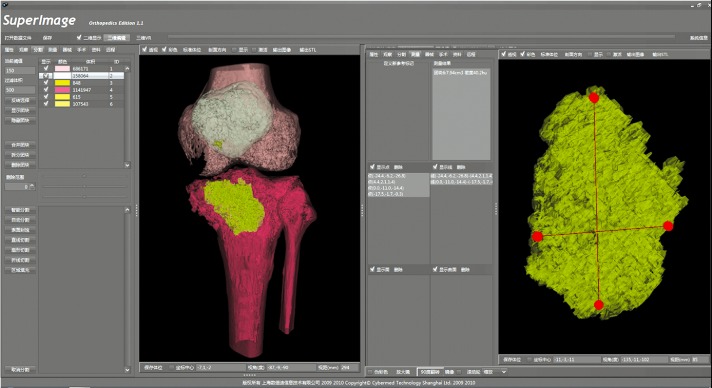
Screenshot showing measurement of the volume of a cavitating bone tumor by a 3‐D image segmentation technique, reproduced from Yong‐cheng Hu et al.11
Reverse Engineering and Rapid Prototyping Techniques
Reverse engineering originated in the 1960s. It is a type of computer‐aided design (CAD) that is based on physical measurements and has been widely used in medical model structuring using CT image data. Unlike other image models, the data format of this kind of CAD model provides not only a stereo model, but can also be used in machining and manufacturing. Rapid prototyping originated in the 1980s. This molding technique involves integration of computer, digital control, and laser techniques, new material technology and so on, and is based on the principles of discreteness and deposition. In recent years, rapid prototyping (RP) has been widely used in medicine, especially combined with reverse engineering in computer‐assisted surgery.
Tens of RP methods have been produced since its inception10. Some conventional techniques are flawed12. Nowadays, although models made by RP are sufficiently precise, there is still considerable potential error. Available RP processes commonly used in prototyping anatomical modeling have been summarized in published reports as follows: stereolithography apparatus, selective laser sintering (SLS), fused deposited modeling, laminated object manufacturing, multiphase jet solidification, and 3‐D printing13, 14, 15, 16. Winder et al. were the first to present a new method of producing custom titanium plates for repair of cranial defects using RP technology and 3D CT image data17. From 1999 to 2005, more than 40 medical RP applications were implemented in Europe and Asia. Currently, state‐of‐the‐art medical RP is used in diagnosis and treatment in the following medical areas: cranio‐maxillo‐facial and dental surgery, neurosurgery, orthopedics, orthosis and tissue engineering18.
Currently, reverse engineering and RP can be used for morphological study of the skeleton18, surgical planning and its realization15, 19, repair of mandibular defects20, plates for cranioplasty21, total knee arthroplasty22, and bone tissue engineering, all of which have good results23, 24. During the past few years, a combination of medical imaging and rapid manufacturing techniques has proven to be a very important development20. Recently, polycaprolactone scaffolds fabricated by SLS have shown great potential for replacement of skeletal tissues25. Leijnse and Spoor have reported a two dimensional kinematic multi tendon‐string extensor apparatus model of fiber slackness and tautness through interphalangeal motion that was achieved by reverse engineering26. Hsieh et al. have reported using RP models as a component of surgical planning of intra‐articular osteotomy27. Dhakshyani et al. provided an understanding of the use of RP medical models in planning of dysplastic hip orthopaedic surgery28. in Using 30 cadaveric knees and a RP technique, Gan et al. manufactured a navigational template for assisting with knee arthroplasty 29. Schumacher et al. applied RP to manufacturing scaffolds for bone tissue engineering30. Park et al. also applied RP to fabrication of scaffolds and successfully seeded scaffolds with MG63 cells in an in vitro study and implanted them in the tibias of rabbits in an in vivo study31. Chua et al. experimentally verified a functionally graded scaffold model by fabricating a femur bone segment using a SLS system32. Uklejewski et al. presented the main results of a research project concerning a selective laser melted prototype of a new kind of minimally invasive resurfacing hip arthroplasty endoprosthesis incorporating the original multi‐spiked connecting scaffold33.
Computational Biomechanics
Computational biomechanics is a new research mode in the area of biomechanical research. In particular, it includes a series of technologies that use efficient, convenient and fast computational methods to analyze human physiological and pathological mechanics. In the orthopaedic field, the finite element method, kinematic and dynamic analyses are the most widely used computational biomechanical analysis techniques. The basic concept of the finite element method is subdivision of the mathematical model into disjointed (non‐overlapping) components of simple geometry called finite elements, or elements for short. The response of each element is expressed in terms of a finite number of degrees of freedom characterized as the value of an unknown function, or functions, at a set of nodal points. The response of the mathematical model is then considered to be approximated by that of a discrete model obtained by connecting or assembling a collection of all the elements. The finite element method was introduced to orthopedic biomechanics in 1972 for evaluating stresses in human bones34. Since then, this method has been applied with increasing frequency to stress analysis of bone and bone‐prosthesis structures, fracture fixation devices and various kinds of tissues other than bone35.
At present, the main method of orthopaedic finite element analysis is to use an original medical imaging data set to construct a 3‐D geometrical surface model. Next, appropriate material properties are given to the model, after which biomechanical analysis of bone, joint, soft tissue, fracture fixation devices and bone‐prosthesis can be performed using finite element analysis software, such as ANSYS (ANSYS, Canonsburg, PA, USA) and ABAQUS (SIMULIA, Paris, France). In addition, kinematic and dynamic analysis of human bone can establish musculoskeletal models for simulating the coordinated motion of bone and muscle by the use of various systems and software.
A critical issue encountered in the finite element method is generation of the finite element model. Whereas in other engineering applications models are typically built in a CAD environment and then imported into finite element software, in biomedical engineering a different approach is adopted. Medical image scans are converted into CAD data to generate a finite element model of anatomical sites. In general, CAD models can be generated from CT, micro‐CT or NMR scans by following two distinct approaches: geometry‐based and voxel‐based. The former method defines a geometric model comprised of curves and surfaces that is finally discretized into finite elements36, 37. The strength of the geometry‐based approach lies in its capacity to create smooth surfaces and hence simulate any kind of interface. The voxel‐based approach is more diffuse than the geometry‐based one and relies on the principle that each group of voxels (the base unit of 3‐D imaging) is directly converted into hexahedral elements38, 39. Commonly used finite element modeling software such as Simpleware (Simpleware, Exeter, UK) and MIMICS (Materialise, Leuven, Belgium) are a combination of geometry‐based and voxel‐based approaches. DICOM imaging data are processed digitally to subdivide target tissue, following which the pixel based three‐dimensional target area is filled by a tetrahedral element to generate a 3‐D reconstructed model that can be analyzed by the finite element method. In kinematics and dynamics simulating research, the reconstructed three‐dimensional model is combined with motion capture data that are acquired by an optical tracking system. The two main types of software are ADAMS (MDI, Ann Arbor, MI, USA) and LifeMod (MSC Software, Santa Ana, CA, USA).
The early orthopaedic computational biomechanical models were basically 2‐D and too simple to simulate the asymmetry and irregular shapes of entities such as complex joints. With the development of computational biomechanical technology, it is possible to construct 3‐D models from the simple to the complex. These models are not only precise in geometric structure, but also take cartilage, tendons, ligaments and other soft tissues into account. In recent years, the computational biomechanics research pattern has been widely used in the areas of the spine40, pelvis41, limbs42, ankle10, and so on. Some good research results have been achieved. Computational biomechanics is supplemental to, and an extension of, traditional experimental anatomic research. In addition to difficulties with obtaining suitable cadaver specimens, problems with traditional cadaver study include deformation of cadaver soft tissues and drying out of the cadavers, which create inconvenience and adversely affect the science, reliability and repeatability of such biomechanical research. Computational biomechanics constructs 3‐D models of human bones and joints efficiently and conveniently. The resulting models simulate human physiological characteristics more accurately than do cadaver specimens and provide large samples for various biomechanical dynamic simulating experiments. Computer simulation allows extensive study of the mechanisms of bone and joint trauma, pathogenesis of bone diseases, assessment and selection of optimal fracture fixation devices and the best positions in which to place them. Computational biomechanics will be one of the most important basic research methods in the orthopaedic field in the future.
Application of Digital Technology to Surgical Procedures
The term computer‐assisted surgery (CAS) was proposed by Sohn and Robins 20 years ago5. In the 1990s CAS transformed into CAOS when orthopedists began to implement navigated techniques in spine surgery. During that decade, using a primary navigational framework, stereotactic navigation in neurosurgery had provided clear 3‐D reconstruction images of brain tissue leading to accurate, minimally invasive surgery. The first application of CAS in orthopaedic and trauma surgery was for placement of lumbar pedicle screws43. So far, computer‐assisted navigation technology in orthopaedics has been mainly concentrated in two pioneering fields, namely medical robots and clinical visualization technology. The ROBODOC system, a typical medical robot developed by Professor Taylor in the USA, was first used in 1992 in a total hip arthroplasty.44 Using clinical visualization, surgeons can acquire visual surgical images or real time feedback from the screen.
Systems that involve different kinds of surgical planning methods include volumetric image‐based navigation, fluoroscopic navigation, and imageless navigation. The aims of CAS are to make the surgery more simple, precise and innovative; achieving this necessitates the training of operators45. Currently, most of these systems use standard personal computers or laptops. Already, many systems have been modified to reflect feedback by users. The actual time for placement of trackers and registration of landmarks is of the order of 10 minutes or less for experienced users of ACL (Vancouver, Canada) software46.
Although navigation systems may improve the accuracy of some orthopaedic surgery, our evaluation of it should incorporate an understanding of the systemic errors of navigation software and the accuracy of this mode. In recent years, there have been many reports of navigation in orthopaedics. Benum et al. reported using a computer‐based guiding device in three hip arthroplasties in two patients with osteopetrosis47. Wu et al. presented a series of cases in which they used intraoperative stealth navigation to treat periarticular tumors48. Levine et al. reported on the clinical success of digital templating using the Advanced Case Plan (Stryker Imaging, Flower Mound, TX, USA) system in primary total hip arthroplasty (THA) and total knee arthroplasty (TKA)49. Nakamura et al. compared the results and complications of robotic‐assisted and hand‐rasping stem implantation techniques in 146 primary THAs on 130 patients. They showed that there was significantly more stress shielding of the proximal femur in the hand‐rasping group and that the postoperative limb lengths of the robotic‐milling group had significantly less variance than those of the hand‐rasping group50. Ryan et al. compared the values measured by an imageless computer navigation system with those measured using postoperative CT scans in 26 THAs of 25 patients; they showed that the imageless computer navigation system was more accurate51. Zhu et al. studied 436 patients (477 hips) undergoing primary THA with the aid of an imageless computer navigation system and reported that intraoperative measurement of pelvic tilt improved the accuracy of cup position52. Kumar et al. evaluated the efficacy of the Stryker imageless navigation system in guiding cup placement in 56 patients undergoing primary THAs and found that the navigation system was more accurate than conventional freehand53. Kalteis et al. used intra‐operative computer‐assisted navigation to measure the orientation of the native acetabular plane as defined by the transverse acetabular ligament and the posterior labrum in 39 hips54. Dastane et al. used computer navigation in 82 patients to reconstruct the hip offset and to compare hip offset changes to quantitative changes in the hip cup center of rotation55. Olsen et al. reviewed the first 100 Birmingham hip resurfacings performed in 94 prospectively followed patients and found that the use of imageless computer navigation to reduce technical errors may reduce the incidence of femoral neck fractures in the short‐term56. However, the radiographic sequelae of neck thinning, stem radiolucencies, and stem migration still occurred. Olsen et al. investigated the accuracy of placement of the femoral components using imageless navigation in 100 consecutive Birmingham hip resurfacings and reported that such navigation may afford the surgeon a reliable and accurate method of placement of the femoral components57. Bailey et al. performed 37 hip resurfacing procedures using an imageless computer navigation system. They stated that computer navigation may reduce the risk of component malpositioning and femoral neck notching58. Schnurr et al. retrospectively analyzed 60 hip surface replacements and found that a navigation device improved the implant position with high accuracy; however, the procedure took 15 minutes longer than conventional implantation59. Leung et al. reported that the major obstacles to general and wider applications are the inability to track individual fracture fragments, no navigated real‐time fracture reduction, and the lack of an objective assessment method for cost‐effectiveness60. In 32 femoral hip resurfacing components implanted on embalmed human femora using an image‐free navigation device, Schnurr et al. demonstrated high accuracy concerning the varus‐valgus angle; however, they found that the software calculation of the proposed implant position was inaccurate and needs improvement61.
Linden et al. measured the differences between the intraoperative stored rotation data of the femoral component and the postoperative rotation on CT in 20 navigated TKAs and showed that the (virtual) individual rotational position of the femoral components using a CAOS system is significantly different from the position on a postoperative CT scan62. Zhang et al. compared computer‐assisted‐navigation and conventional total knee arthroplasties in the alignment of knee prostheses. Computer‐assisted navigation consistently provided coronal plane alignment within 3° of the mechanical axis, which was significantly better than the alignment obtained with conventional total knee arthroplasty63. Hernández‐Vaquero et al. studied the accuracy of computer navigation in TKA of knees with severe deformities64. They stated that positioning of the femoral and tibial components was more accurate in the group treated with surgical navigation than in those with a conventional jig‐based technique. Babazadeh et al. assessed 115 patients to define the role of CAS in maintaining the level of the joint in primary knee joint replacement65. They found no significant differences between computer‐assisted and conventional surgery in terms of maintaining the joint line. Kim et al. reported two successful cases of navigation‐assisted TKA for severe right knee osteoarthritis retaining a femoral intramedullary nail, and left knee osteoarthritis retaining a distal femoral plate66. Meuffels et al. assessed the effects of computer‐assisted reconstruction surgery versus conventional operating techniques for anterior or posterior cruciate ligament deficient knees, including four randomized controlled trials (266 participants). They concluded that a favorable effect of CAS for cruciate ligament reconstructions of the knee compared with conventional reconstructions could neither be demonstrated nor refuted67. In conclusion, there is still some controversy about the actual clinical utility of computer navigation technology. With the development and upgrading of software and hardware, it is believed that computer navigation technology will undergo further refinement and achieve wider application.
Management of Digitization Medical Record Material
The High Performance Computing and Communications Program (HPCC) is a multiagency federal initiative under the leadership of the White House Office of Science and Technology Policy, established by the High Performance Computing Act of 1991 in the USA68. The HPCC program, a multiagency federal effort to advance the state of computing and communications and to provide a technologic platform on which to build a National Information Infrastructure, supports the development of high‐speed computers, high‐speed telecommunications, related software and algorithms, education and training, and information infrastructure technology and applications69. Picture archiving and communication systems (PACS) include collection, digitization, storage, management, high‐speed transmission, reappearance, and information integration of medical images. The aim is for PACS to establish regional and cross regional networks and then cover all of society. Because of differences between various medical equipment manufacturers in image formats, it has not been possible to transfer medical information freely among different kinds of systems during the development of PACS and medical imaging informatics. To solve this problem, the American College of Radiology and National Electrical Manufacturers Association have set up a new standard format: DICOM. Provided orthopedists set up their own data base in the DICOM format, they can retrieve, observe and measure digital images in 3‐D and compare preoperative and postoperative images. They can also improve the clinical flow‐ons and their own experience.
Prospects
In the 21st century, developments in digital orthopaedics will make available new opportunities. Digitization in orthopaedics can not only provide more efficient, accurate, scientific and objective methods for understanding disease, but also help surgeons to summarize, plan surgical procedures and realize digital remote interaction of information. Digital techniques in orthopaedics have set a new standard and a novel pathway for scientific and clinical work and provided a technological basis for carrying out large sample, prospective and multicenter randomized controlled trials. Digitalization makes research in orthopaedics more accurate and quantitative, promotes a depth of orthopaedics, and assists in better summarizing and analysis of data. Digital techniques in orthopaedics should be basic skills for good doctors in the 21st century. Further development of digital orthopaedic technology also depends on more extensive and close cooperation between medical and information technology.
Disclosure: The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
References
- 1. Ackerman MJ. The Visible Human Project: a resource for education. Acad Med, 1999, 74: 667–670. [DOI] [PubMed] [Google Scholar]
- 2. Spitzer V, Ackerman MJ, Scherzinger AL, et al The visible human male: a technical report. J Am Med Inform Assoc, 1996, 3: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park JS, Chung MS, Hwang SB, et al Visible Korean human: improved serially sectioned images of the entire body. IEEE Trans Med Imaging, 2005, 24: 352–360. [DOI] [PubMed] [Google Scholar]
- 4. Zhang SX, Heng PA, Liu ZJ, et al Creation of the Chinese visible human data set. Anat Rec B New Anat, 2003, 275: 190–195. [DOI] [PubMed] [Google Scholar]
- 5. Sohn N, Robbins RD. Computer‐assisted surgery. N Engl J Med, 1985, 312: 924. [DOI] [PubMed] [Google Scholar]
- 6. Haluck RS, Krummel TM. Computers and virtual reality for surgical education in the 21st century. Arch Surg, 2000, 135: 786–792. [DOI] [PubMed] [Google Scholar]
- 7. Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop, 2011, 82: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vankipuram M, Kahol K, McLaren A, et al A virtual reality simulator for orthopedic basic skills: a design and validation study. J Biomed Inform, 2010, 43: 661–668. [DOI] [PubMed] [Google Scholar]
- 9. Chen YX, Shao ZM. Design and application of a digital orthopaedics platform for clinical research. Zhonghua Gu Ke Za Zhi (Chin), 2009, 29: 993–999. [Google Scholar]
- 10. Chen YX, Lu XL, Bi G, et al Three‐dimensional morphological characteristics measurement of ankle joint based on computed tomography image post‐processing. Chin Med J (Engl), 2011, 124: 3912–3918. [PubMed] [Google Scholar]
- 11. Hu YC, Chen YX, Lun DX. Construction of clinical score system of giant cell tumors and clinical verification. Zhonghua Gu Ke Za Zhi (Chin), 2011, 31: 105–112. [Google Scholar]
- 12. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol, 2000, 24: 149–153. [DOI] [PubMed] [Google Scholar]
- 13. Potamianos P, Amis AA, Forester AJ, et al Rapid prototyping for orthopaedic surgery. Proc Inst Mech Eng [H], 1998, 212: 383–393. [DOI] [PubMed] [Google Scholar]
- 14. Yeong WY, Chua CK, Leong KF, et al Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol, 2004, 22: 643–652. [DOI] [PubMed] [Google Scholar]
- 15. Petzold R, Zeilhofer HF, Kalender WA. Rapid protyping technology in medicine–basics and applications. Comput Med Imaging Graph, 1999, 23: 277–284. [DOI] [PubMed] [Google Scholar]
- 16. Sun W, Lal P. Recent development on computer aided tissue engineering–a review. Comput Methods Programs Biomed, 2002, 67: 85–103. [DOI] [PubMed] [Google Scholar]
- 17. Winder J, Cooke RS, Gray J, et al Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J Med Eng Technol, 1999, 23: 26–28. [DOI] [PubMed] [Google Scholar]
- 18. Hieu LC, Zlatov N, Sloten J, et al Medical rapid prototyping applications and methods. Assembly Autom, 2005, 25: 284–292. [Google Scholar]
- 19. Borah B, Gross GJ, Dufresne TE, et al Three‐dimensional microimaging (MRmicroI and microCT), finite element modeling, and rapid prototyping provide unique insights into bone architecture in osteoporosis. Anat Rec, 2001, 265: 101–110. [DOI] [PubMed] [Google Scholar]
- 20. Singare S, Dichen L, Bingheng L, et al Design and fabrication of custom mandible titanium tray based on rapid prototyping. Med Eng Phys, 2004, 26: 671–676. [DOI] [PubMed] [Google Scholar]
- 21. Joffe JM, Nicoll SR, Richards R, et al Validation of computer‐assisted manufacture of titanium plates for cranioplasty. Int J Oral Maxillofac Surg, 1999, 28: 309–313. [PubMed] [Google Scholar]
- 22. Minns RJ, Bibb R, Banks R, et al The use of a reconstructed three‐dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys, 2003, 25: 523–526. [DOI] [PubMed] [Google Scholar]
- 23. Hutmacher DW, Cool S. Concepts of scaffold‐based tissue engineering–the rationale to use solid free‐form fabrication techniques. J Cell Mol Med, 2007, 11: 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevens B, Yang Y, Mohandas A, et al A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater, 2008, 85: 573–582. [DOI] [PubMed] [Google Scholar]
- 25. Williams JM, Adewunmi A, Schek RM, et al Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials, 2005, 26: 4817–4827. [DOI] [PubMed] [Google Scholar]
- 26. Leijnse JN, Spoor CW. Reverse engineering finger extensor apparatus morphology from measured coupled interphalangeal joint angle trajectories–a generic 2D kinematic model. J Biomech, 2012, 45: 569–578. [DOI] [PubMed] [Google Scholar]
- 27. Hsieh MK, Chen AC, Cheng CY, et al Repositioning osteotomy for intra‐articular malunion of distal radius with radiocarpal and/or distal radioulnar joint subluxation. J Trauma, 2010, 69: 418–422. [DOI] [PubMed] [Google Scholar]
- 28. Dhakshyani R, Nukman Y, Abu Osman NA, et al Rapid prototyping medical models for dysplastic hip orthopaedic surgery. P I Mech Eng B‐J Eng, 2010, 224: 769–776. [Google Scholar]
- 29. Gan Y, Xu D, Lu S, et al Novel patient‐specific navigational template for total knee arthroplasty. Comput Aided Surg, 2011, 16: 288–297. [DOI] [PubMed] [Google Scholar]
- 30. Schumacher M, Uhl F, Detsch R, et al Static and dynamic cultivation of bone marrow stromal cells on biphasic calcium phosphate scaffolds derived from an indirect rapid prototyping technique. J Mater Sci Mater Med, 2010, 21: 3039–3048. [DOI] [PubMed] [Google Scholar]
- 31. Park SA, Kim HJ, Lee SH, et al Fabrication of nano/microfiber scaffolds using a combination of rapid prototyping and electrospinning systems. Polym Eng Sci, 2011, 51: 1883–1890. [Google Scholar]
- 32. Chua CK, Leong KF, Sudarmadji N, et al Selective laser sintering of functionally graded tissue scaffolds. MRS Bull, 2011, 36: 1006–1014. [Google Scholar]
- 33. Uklejewski R, Winiecki M, Rogala P, et al Selective laser melted prototype of original minimally invasive resurfacing hip endoprosthesis. Rapid Prototyping J, 2011, 17: 76–85. [Google Scholar]
- 34. Brekelmans WA, Poort HW, Slooff TJA. A new method to analyse the mechanical behaviour of skeletal parts. Acta Orthop Scand, 1972, 43: 301–317. [DOI] [PubMed] [Google Scholar]
- 35. Huiskes R, Chao EY. A survey of finite element analysis in orthopedic biomechanics: the first decade. J Biomech, 1983, 16: 385–409. [DOI] [PubMed] [Google Scholar]
- 36. Lengsfeld M, Schmitt J, Alter P, et al Comparison of geometry‐based and CT voxel‐based finite element modelling and experimental validation. Med Eng Phys, 1998, 20: 515–522. [DOI] [PubMed] [Google Scholar]
- 37. Holzapfel GA, Stadler M, Schulze‐Bauer CA. A layer‐specific three‐dimensional model for the simulation of balloon angioplasty using magnetic resonance imaging and mechanical testing. Ann Biomed Eng, 2002, 30: 753–767. [DOI] [PubMed] [Google Scholar]
- 38. Guldberg RE, Hollister SJ, Charras GT. The accuracy of digital image‐based finite element models. J Biomech Eng, 1998, 120: 289–195. [DOI] [PubMed] [Google Scholar]
- 39. Keyak JH, Rossi SA, Jones KA, et al Prediction of femoral fracture load using automated finite element modeling. J Biomech, 1998, 31: 125–133. [DOI] [PubMed] [Google Scholar]
- 40. Hussain M, Natarajan RN, An HS, et al Reduction in segmental flexibility because of disc degeneration is accompanied by higher changes in facet loads than changes in disc pressure: a poroelastic C5‐C6 finite element investigation. Spine J, 2010, 10: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 41. Shim V, Böhme J, Vaitl P, et al Finite element analysis of acetabular fractures–development and validation with a synthetic pelvis. J Biomech, 2010, 43: 1635–1639. [DOI] [PubMed] [Google Scholar]
- 42. Otake Y, Suzuki N, Hattori A, et al Four‐dimensional model of the lower extremity after total hip arthroplasty. J Biomech, 2005, 38: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 43. Schep NW, van der Broeders IA, Werken C. Computer assisted orthopaedic and trauma surgery. State of the art and future perspectives. Injury, 2003, 34: 299–306. [DOI] [PubMed] [Google Scholar]
- 44. Paul HA, Bargar WL, Mittlestadt B, et al Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res, 1992, 285: 57–66. [PubMed] [Google Scholar]
- 45. Sugano N. Computer‐assisted orthopedic surgery. J Orthop Sci, 2003, 8: 442–448. [DOI] [PubMed] [Google Scholar]
- 46. Koh JL. The future of computer‐assisted surgery (CAS) in sports medicine. Sports Med Arthrosc, 2008, 16: 108–110. [DOI] [PubMed] [Google Scholar]
- 47. Benum P, Aamodt A, Nordsletten L. Customised femoral stems in osteopetrosis and the development of a guiding system for the preparation of an intramedullary cavity: a report of two cases. J Bone Joint Surg Br, 2010, 92: 1303–1305. [DOI] [PubMed] [Google Scholar]
- 48. Wu K, Webber NP, Ward RA, et al Intraoperative navigation for minimally invasive resection of periarticular and pelvic tumors. Orthopedics, 2011, 34: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Levine B, Fabi D, Deirmengian C. Digital templating in primary total hip and knee arthroplasty. Orthopedics, 2010, 33: 797. [DOI] [PubMed] [Google Scholar]
- 50. Nakamura N, Sugano N, Nishii T, et al A comparison between robotic‐assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res, 2010, 468: 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ryan JA, Jamali AA, Bargar WL. Accuracy of computer navigation for acetabular component placement in THA. Clin Orthop Relat Res, 2010, 468: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu J, Wan Z, Dorr LD. Quantification of pelvic tilt in total hip arthroplasty. Clin Orthop Relat Res, 2010, 468: 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumar MA, Shetty MS, Kiran KG, et al Validation of navigation assisted cup placement in total hip arthroplasty. Int Orthop, 2012, 36: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kalteis T, Sendtner E, Beverland D, et al The role of the transverse acetabular ligament for acetabular component orientation in total hip replacement: an analysis of acetabular component position and range of movement using navigation software. J Bone Joint Surg Br, 2011, 93: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 55. Dastane M, Dorr LD, Tarwala R, et al Hip offset in total hip arthroplasty: quantitative measurement with navigation. Clin Orthop Relat Res, 2011, 469: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Olsen M, Schemitsch EH. Avoiding short‐term femoral neck fracture with imageless computer navigation for hip resurfacing. Clin Orthop Relat Res, 2011, 469: 1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Olsen M, Davis ET, Waddell JP, et al Imageless computer navigation for placement of the femoral component in resurfacing arthroplasty of the hip. J Bone Joint Surg Br, 2009, 91: 310–315. [DOI] [PubMed] [Google Scholar]
- 58. Bailey C, Gul R, Falworth M, et al Component alignment in hip resurfacing using computer navigation. Clin Orthop Relat Res, 2009, 467: 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schnurr C, Michael JW, Eysel P, et al Imageless navigation of hip resurfacing arthroplasty increases the implant accuracy. Int Orthop, 2009, 33: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leung KS, Tang N, Cheung LW, et al Image‐guided navigation in orthopaedic trauma. J Bone Joint Surg Br, 2010, 92: 1332–1337. [DOI] [PubMed] [Google Scholar]
- 61. Schnurr C, Nessler J, Meyer C, et al How accurate is image‐free computer navigation for hip resurfacing arthroplasty? An anatomical investigation. J Orthop Sci, 2009, 14: 497–504. [DOI] [PubMed] [Google Scholar]
- 62. van der Linden‐van der Zwaag HM, van der Bos J, Heide HJ, et al A computed tomography based study on rotational alignment accuracy of the femoral component in total knee arthroplasty using computer‐assisted orthopaedic surgery. Int Orthop, 2011, 35: 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang GQ, Chen JY, Chai W, et al Comparison between computer‐assisted‐navigation and conventional total knee arthroplasties in patients undergoing simultaneous bilateral procedures: a randomized clinical trial. J Bone Joint Surg Am, 2011, 93: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 64. Hernández‐Vaquero D, Suarez‐Vazquez A, Sandoval‐Garcia MA, et al Computer assistance increases precision of component placement in total knee arthroplasty with articular deformity. Clin Orthop Relat Res, 2010, 468: 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Babazadeh S, Dowsey MM, Swan JD, et al Joint line position correlates with function after primary total knee replacement: a randomised controlled trial comparing conventional and computer‐assisted surgery. J Bone Joint Surg Br, 2011, 93: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 66. Kim KK, Heo YM, Won YY, et al Navigation‐assisted total knee arthroplasty for the knee retaining femoral intramedullary nail, and distal femoral plate and screws. Clin. Orthop Surg, 2011, 3: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meuffels DE, Reijman M, Scholten RJ, et al Computer assisted surgery for knee ligament reconstruction. Cochrane Database Syst Rev, 2011, (6): CD007601. [DOI] [PubMed] [Google Scholar]
- 68. Lindberg DA. Global information infrastructure. Int J Biomed Comput, 1994, 34: 13–19. [DOI] [PubMed] [Google Scholar]
- 69. Lindberg DA, Humphreys BL. The High‐Performance Computing and Communications program, the national information infrastructure and health care. J Am Med Inform Assoc, 1995, 2: 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]


