Key Points
Questions
Can digital cognitive behavioral therapy for insomnia improve functional health, psychological well-being, and sleep-related quality of life, and does a reduction in insomnia symptoms mediate these potential improvements?
Findings
In a 2-arm, parallel-group randomized clinical trial that included 1711 persons, digital cognitive behavioral therapy significantly improved insomnia symptoms, functional health, psychological well-being, and sleep-related quality of life at 4, 8, and 24 weeks after initiation of treatment. Improvements at 8 and 24 weeks were mediated by improvements in insomnia at week 4 and 8, respectively.
Meaning
Treating insomnia with digital cognitive behavioral therapy could be a therapeutic pathway for addressing self-reported health, well-being, and quality of life.
Abstract
Importance
Digital cognitive behavioral therapy (dCBT) is a scalable and effective intervention for treating insomnia. Most people with insomnia, however, seek help because of the daytime consequences of poor sleep, which adversely affects quality of life.
Objectives
To investigate the effect of dCBT for insomnia on functional health, psychological well-being, and sleep-related quality of life and to determine whether a reduction in insomnia symptoms was a mediating factor.
Design, Setting, and Participants
This online, 2-arm, parallel-group randomized trial comparing dCBT for insomnia with sleep hygiene education (SHE) evaluated 1711 participants with self-reported symptoms of insomnia. Participants were recruited between December 1, 2015, and December 1, 2016, and dCBT was delivered using web and/or mobile channels plus treatment as usual; SHE comprised a website and a downloadable booklet plus treatment as usual. Online assessments took place at 0 (baseline), 4 (midtreatment), 8 (posttreatment), and 24 (follow-up) weeks. Programs were completed within 12 weeks after inclusion.
Main Outcomes and Measures
Primary outcomes were scores on self-reported measures of functional health (Patient-Reported Outcomes Measurement Information System: Global Health Scale; range, 10-50; higher scores indicate better health); psychological well-being (Warwick-Edinburgh Mental Well-being Scale; range, 14-70; higher scores indicate greater well-being); and sleep-related quality of life (Glasgow Sleep Impact Index; range, 1-100; higher scores indicate greater impairment). Secondary outcomes comprised mood, fatigue, sleepiness, cognitive failures, work productivity, and relationship satisfaction. Insomnia was assessed with the Sleep Condition Indicator (range: 0-32; higher scores indicate better sleep).
Results
Of the 1711 participants included in the intention-to-treat analysis, 1329 (77.7%) were female, mean (SD) age was 48.0 (13.8) years, and 1558 (91.1%) were white. Use of dCBT was associated with a small improvement in functional health compared with SHE (adjusted difference [95% CI] at week 4, 0.90 [0.40-1.40]; week 8, 1.76 [1.24-2.28]; week 24, 1.76 [1.22-2.30]) and psychological well-being (adjusted difference [95% CI] at week 4, 1.04 [0.28-1.80]; week 8, 2.68 [1.89-3.47]; week 24, 2.95 [2.13-3.76]), and with a large improvement in sleep-related quality of life (at week 4, −8.76 [−11.83 to −5.69]; week 8, –17.60 [−20.81 to −14.39]; week 24, −18.72 [−22.04 to −15.41]) (all P < .01). A large improvement in insomnia mediated these outcomes (range mediated, 45.5%-84.0%).
Conclusions and Relevance
Use of dCBT is effective in improving functional health, psychological well-being, and sleep-related quality of life in people reporting insomnia symptoms. A reduction in insomnia symptoms mediates these improvements. These results confirm that dCBT improves both daytime and nighttime aspects of insomnia, strengthening existing recommendations of CBT as the treatment of choice for insomnia.
Trial Registration
isrctn.org identifier: ISRCTN60530898
This randomized clinical trial with mediation analysis compares self-reported health and sleep-related quality-of-life outcomes of a digital cognitive behavioral therapy intervention with sleep hygiene education in persons who reported insomnia.
Introduction
Insomnia disorder, comprising reports of poor sleep with associated daytime effects occurring 3 or more nights per week for 3 or more months,1 presents in 10% to 12% of adults.2,3,4 In addition, insomnia is associated with mental health disorders,5 cardiovascular disease,6 and type 2 diabetes.7,8 Increased fatigue, impaired work productivity, reduced quality of life, and relationship dissatisfaction are also common in those with insomnia.9,10,11 Such impaired functioning is an important driver for help-seeking behavior.12
The recommended intervention for insomnia is cognitive behavioral therapy (CBT),13,14,15,16 a psychological treatment designed to break patterns of maladaptive thinking and behavior. Cognitive behavioral therapy comprises a behavioral component (stimulus control, sleep restriction, and relaxation) combined with a cognitive (managing sleep-related worries, racing mind, and intrusive thoughts) and an educational (sleep hygiene) component. Meta-analyses indicate that CBT has moderate to large and durable effects on sleep quality, sleep efficiency, sleep-onset latency, and wake time after sleep onset.17,18,19 Moreover, recent meta-analyses indicate that digital CBT (dCBT), delivered using automated web platforms or a mobile app,20 is also efficacious.21,22 The effects of CBT and dCBT on the nighttime symptoms of insomnia, therefore, appear robust. However, daytime symptoms are a core part of insomnia disorder, integral to its clinical presentation. Improving constructs such as functional health, psychological well-being, and quality of life may therefore be crucial to treating insomnia satisfactorily.
We investigated the attributable effect of a reduction in insomnia symptoms after receiving dCBT for insomnia on 3 key areas of quality of life: functional health status, psychological well-being, and patient-generated, sleep-related quality of life. Although there is evidence that CBT may yield generalized benefits in both the general population and patient groups23,24,25,26,27,28,29 and some primary data that CBT for insomnia may reduce depressive or anxiety symptoms,30,31,32,33,34 evidence is mixed,35,36 and an adequately powered, definitive trial investigating functional health status, psychological well-being, and a patient-generated assessment of quality of life has not yet been conducted. Moreover, we wanted to conduct a formal test of the mediating effect of improved insomnia symptoms on these outcomes.
Our primary hypotheses were that dCBT for insomnia would improve functional health status and psychological well-being and would reduce sleep-related quality-of-life impairment at weeks 4, 8, and 24 (research question [RQ] 1) and that the effect of dCBT on these outcomes at weeks 8 and 24 would be mediated by a reduction in insomnia symptoms measured at weeks 4 and 8, respectively (RQ 2). Our secondary hypotheses were that dCBT would also improve domains of personal functioning (negative mood, fatigue, and relationship dysfunction) and daytime performance (sleepiness, concentration, and productivity) at weeks 4 and 8 (RQ 3), improvements would be maintained at the week 24 follow-up, and the effect of dCBT at weeks 8 and 24 would be mediated by a reduction in insomnia complaints at weeks 4 and 8, respectively (RQ 4).
Methods
Research Design
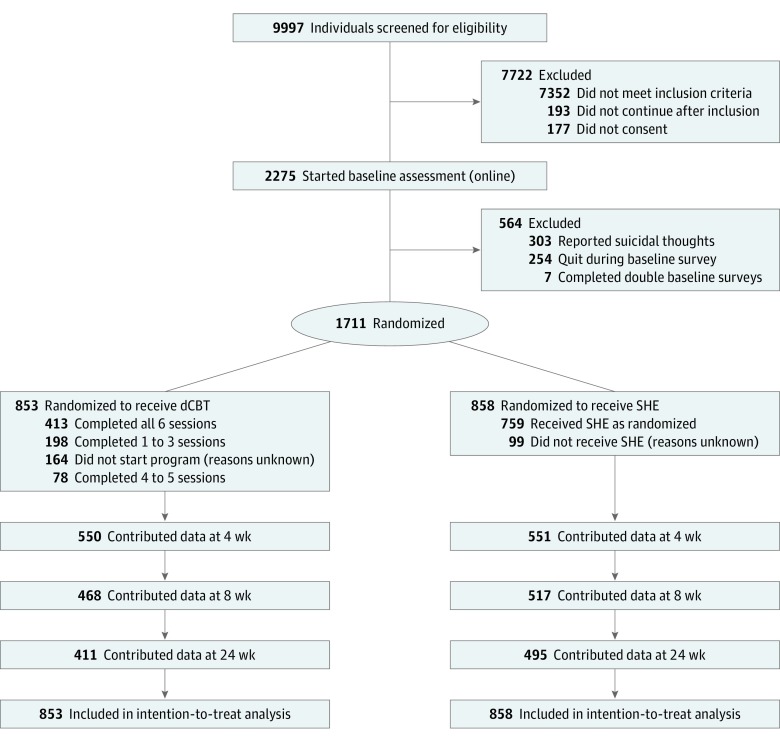
The study was an online, 2-arm, single-blind, parallel-group, superiority randomized clinical trial of dCBT (Digital Insomnia Therapy to Assist Your Life as Well as Your Sleep [DIALS]) in addition to any treatment the participant was previously receiving (treatment as usual [TAU]) vs sleep hygiene education (SHE) in addition to TAU. We used simple randomization with an allocation ratio of 1:1 as recommended for large clinical trials.37 Randomization was carried out by an outside automated online system (surveygizmo.eu), ensuring that allocation could not be influenced by the research team. The trial design and progress of participants through the trial are summarized in the Figure. Screening, informed consent, assessments, allocation to condition, and delivery of the interventions were carried out entirely online. Participants were recruited between December 1, 2015, and December 1, 2016; programs were completed within 12 weeks after inclusion. The study received ethical approval from the University of Oxford Medical Sciences Inter-Divisional Ethics Committee (ref MS-IDREC-C2-2015-024), and participants provided online informed consent. The DIALS trial has been registered at http://www.isrctn.com (ISRCTN60530898); the protocol has been published and is available in Supplement 1.38
Figure. Trial Design for the Digital Insomnia Therapy to Assist Your Life as Well as Your Sleep (DIALS) Study.
dCBT indicates digital cognitive behavioral therapy; SHE, sleep hygiene education.
Participants
According to the original protocol, a sample size of 433 participants per treatment group was required to detect a standardized effect size of 0.25 with 90% power, assuming a significance level of P < .01667 (corrected for 3 primary outcomes), and to detect a large mediation effect with more than 80% power. To account for 13% attrition, we increased the sample size to 500 per treatment arm. During the trial, we further extended recruitment because the attrition rate was larger than expected. A total of 1718 participants enrolled, and the final sample comprised 1711 participants because 7 participants entered the trial twice. For each of these 7 participants, a single entry contributed to the analyses. When both entries were randomized to the same condition, the response with the most completed data was selected; when entries were randomized to different groups, the first response was selected unless the participant accessed treatment; if treatment was accessed, the response corresponding with the treatment allocation was used. Inclusion and exclusion criteria have been reported earlier and are available in Supplement 1.38 Briefly, inclusion criteria comprised the following: a positive screening results based on the DSM-5 criteria for insomnia disorder; a score of 16 or less on the 8-item Sleep Condition Indicator (SCI; scale, 0-4; range: 0-32, with higher scores indicating better sleep)39; aged 18 years or older; reliable internet access; and ability to read and understand English. We screened for comorbid conditions and medication use at baseline but excluded only those people whose health was expected to necessitate hospital admission or who had a life expectancy of less than 6 months, who currently received psychological treatment for insomnia or were expecting treatment within 6 months, and who reported suicidal thoughts. We did not exclude participants taking medication for sleep problems or for any other physical or mental health problem. Several methods were used to direct people to the online recruitment page. Persons who had completed previous sleep surveys (ie, Great British Sleep Survey40; World Sleep Survey41) were contacted by email, a recruitment button was placed on an insomnia intervention website,42 advertisements were placed on Facebook and announcements on Twitter, and information about the study was presented via broadcast media.
Intervention
Digital cognitive behavioral therapy was delivered using the Sleepio program (Big Health Ltd)43 and an associated iOS app (Big Health Ltd). The program is fully automated, and its underlying algorithms feed the delivery of information, support, and advice in a personally tailored manner. Delivery is structured into 6 sessions typically lasting 20 minutes each, and participants had access to the intervention for up to 12 weeks. Treatment content is based on CBT manuals44,45,46 and includes behavioral, cognitive, and educational components. A more extensive description can be found in the study protocol (Supplement 1).38 The program has been evaluated in multiple randomized clinical trials.31,33,43,47,48,49,50
SHE was selected for the control arm because this is the behavioral comparator that people with insomnia are most typically offered in routine care. SHE therefore was based on recognized sleep hygiene advice, for example, recommendations about bed routines and use of alcohol and caffeine.46,51 To ensure consistency of approach and content, SHE was delivered on a dedicated study webpage where materials could be viewed and downloaded in a single session.
Measurements
Assessment Points
Assessments took place at weeks 0 (baseline), 4 (midtreatment), 8 (posttreatment), and 24 (follow-up). At week 25, all participants in the control group (SHE) were offered dCBT, which finished the controlled element of the trial. Uncontrolled follow-up data were collected at week 36 and week 48; these data are not presented herein.
Primary Outcomes
The 3 primary measures used to index physical health, psychological well-being, and sleep-related quality of life were the Patient-Reported Outcomes Measurement Information System: Global Health scale52 for physical health (PROMIS-10; 10 items scored 1 to 5; range: 10-50, with higher scores indicating better health), the Warwick-Edinburgh Mental Well-being Scale53 for psychological well-being (WEMWBS, 14 items scored 1 to 5; range: 14-70, with higher scores indicating better well-being), and the Glasgow Sleep Impact Index54 (GSII), a patient-generated outcome rating in which participants rate self-defined sleep-related impairments (range: 0-100, with higher scores indicating greater impairment).
Secondary Outcomes
Secondary outcomes related to specific measurement of 6 areas of daytime consequences typically associated with the clinical diagnosis of insomnia disorder.1,10 These outcomes were mood (9-item Patient Health Questionnaire55 [PHQ-9]; scale, 0-3; range: 0-27, and the 7-item Generalized Anxiety Disorder56 [GAD-7]; scale, 0-3; range: 0-21), energy (7-item Flinders Fatigue Scale57 [FFS]; scale, 0-4; range: 0-28), relationship satisfaction (7-item Relationship Assessment Scale58 [RAS]; scale, 1-5; range: 7-35), cognitive functioning (25-item Cognitive Failures Questionnaire59 [CFQ]; scale, 0-4; range: 0-100), work performance and satisfaction (Work Productivity and Activity Impairment questionnaire: Specific Health Problem60 [WPAI:SHP] and 1 item on job satisfaction61), and sleepiness (8-item Epworth Sleepiness Scale62 [ESS]; scale, 0-3; range: 0-24]). As an exploratory measure, participants also completed 1 item about their general life satisfaction.63 To appraise the mediating effects of improvement of insomnia symptoms per se, we used the SCI.34,55 At week 8, potential adverse effects were assessed by asking participants to rate a number of potential adverse effects for frequency and severity.64
Statistical Analysis
All analyses were performed as intention to treat and blinded using Stata version 14 (StataCorp). In accordance with the Consolidated Standards of Reporting Trials guidelines, all participant flow is reported, including descriptive statistics of recruitment, dropout, and completeness of interventions.
The main efficacy analysis was based on randomized allocation including all participants with an outcome recorded at the relevant time point. No interim analyses for efficacy or futility were conducted.
Each primary outcome was analyzed using a linear mixed-effects model to account for the repeated measures at 4, 8, and 24 weeks.65 This method implicitly accounts for missing-at-random outcome data. The baseline outcome measure, treatment assignment, and categorical time point were included as fixed effects, with a random participant-level effect. Adjusted, absolute between-group changes in outcome at each time point were obtained by including an interaction term between time point and treatment assignment in each model. Results are presented with 95% CIs and 2-sided P values. Cohen d standardized effect sizes were estimated by dividing the adjusted between-group difference by the baseline pooled SD of the corresponding outcome. Assumptions of normality were assessed graphically using histograms. The robustness of the assumptions regarding missing outcome data were examined in a series of sensitivity analyses (pattern mixture models, inclusion of baseline characteristics associated with having a missing outcome, and last observation carried forward). Secondary outcomes were analyzed using similar mixed-effects models as the primary outcomes.
A series of linear mixed-effects models were fitted to assess the extent to which the effect of the intervention on each outcome at 8 weeks was mediated by changes in insomnia at 4 weeks. The total effect of the intervention on outcome at 8 weeks was estimated from a model adjusting for baseline SCI but not the baseline outcome. The direct effect of the intervention at 8 weeks was estimated from a model adjusting for baseline SCI and SCI at 4 weeks. The effect of SCI at 4 weeks was also extracted from this model and multiplied by the estimated effect of the intervention on SCI at 4 weeks to obtain the indirect effect. This approach is similar to that described by Baron and Kenny66 but uses linear mixed-effects models.67 The percentage mediated, estimated as the indirect effect divided by the total effect, was obtained. The extent of mediation of the outcome effects at 24 weeks by insomnia at 8 weeks was evaluated in the same way.
Results
The final sample comprised 1711 adults, of whom 1329 (77.7%) were female and the mean (SD) age was 48.0 (13.8) years; 1558 (91.1%) were white, 45 (0.3%) were Asian, 19 (0.1%) were black, 36 (0.2%) were of mixed race/ethnicity, 35 (0.2%) were of another race/ethnicity, and 17 (0.1%) did not wish to state race/ethnicity. Participants were recruited between December 1, 2015, and December 1, 2016, and allocated to either dCBT plus TAU (n = 853) or SHE plus TAU (n = 858). An overview of sample descriptive statistics and baseline scores for the primary and secondary outcomes can be found in Table 1. Full details of sample characteristics and missing data can be found in eTable 1 in Supplement 2. Dropout from study assessments was greater in the treatment group than in the control group (Figure).
Table 1. Descriptive Characteristics.
| Characteristic | SHE + TAU (n = 858) | dCBT + TAU (n = 853) |
|---|---|---|
| Demographic | ||
| Age, mean (SD), y | 47.7 (13.6) | 48.4 (13.9) |
| Sex, No. (%) | ||
| Women | 675 (78.7) | 654 (76.7) |
| Men | 183 (21.3) | 199 (23.3) |
| Ethnic origin, No. (%) | ||
| Asian | 24 (2.8) | 21 (2.5) |
| Black | 12 (1.4) | 7 (0.8) |
| Mixed | 16 (1.9) | 20 (2.3) |
| Other | 23 (2.7) | 12 (1.4) |
| White | 773 (90.1) | 785 (92.0) |
| Do not wish to state | 9 (1.0) | 8 (0.9) |
| Continuous full education, mean (SD), y | 16.6 (3.5) | 16.5 (3.9) |
| Employment, No. (%) | ||
| Full-time employed | 411 (47.9) | 393 (46.2) |
| Part-time employed | 187 (21.8) | 161 (18.9) |
| Unemployed | 34 (4.0) | 40 (4.7) |
| Retired | 149 (16.2) | 152 (17.7) |
| Full-time student | 32 (3.7) | 46 (5.4) |
| Full-time homemaker or carer | 52 (6.0) | 56 (6.6) |
| Partner, No. (%) | ||
| No | 240 (28.0) | 213 (25.0) |
| Yes, living apart | 64 (7.4) | 76 (8.9) |
| Yes, living together | 553 (64.5) | 560 (65.7) |
| Lifestyle | ||
| Caffeine consumption, No. (%) | ||
| Never | 106 (12.1) | 81 (9.5) |
| Less than once per day | 114 (13.3) | 111 (13.0) |
| Once per day | 197 (23.0) | 204 (23.9) |
| 2-3 Times per day | 305 (35.6) | 330 (38.7) |
| ≥4 Times per day | 134 (15.6) | 124 (14.5) |
| Alcohol consumption, No. (%) | ||
| Never | 200 (23.3) | 205 (24.0) |
| Less than once per week | 183 (21.3) | 154 (18.1) |
| Once per week | 116 (13.5) | 127 (14.9) |
| 2-3 Times per week | 223 (26.0) | 221 (25.9) |
| ≥4 Times per week | 135 (15.7) | 145 (17.0) |
| Smoking, No. (%) | ||
| Never | 483 (54.9) | 481 (56.6) |
| Previously | 309 (36.0) | 297 (34.8) |
| Rarely | 29 (3.4) | 31 (3.6) |
| 1-10 per day | 19 (2.2) | 28 (3.3) |
| 11-20 per day | 19 (2.2) | 13 (1.5) |
| ≥21 per day | 8 (0.9) | 0 |
| Exercising, No. (%) | ||
| Never | 85 (9.9) | 77 (9.0) |
| Less than once per week | 111 (12.9) | 85 (10.0) |
| Once per week | 134 (15.6) | 136 (15.9) |
| 2-3 Times per week | 279 (32.5) | 317 (37.2) |
| ≥4 Times per week | 247 (28.8) | 237 (27.8) |
| Health | ||
| BMI, mean (SD) | 25.3 (6.0) | 25.1 (5.1) |
| Medical diagnosis, No. (%) | ||
| Heart disease or high blood pressure | 106 (12.4) | 106 (12.4) |
| Diabetes | 18 (2.1) | 18 (2.1) |
| Stroke or other neurological problems | 8 (0.9) | 16 (1.9) |
| Cancer | 41 (4.8) | 39 (4.6) |
| Arthritis/other joint problems | 90 (10.5) | 87 (10.2) |
| Digestive disorders | 121 (13.9) | 123 (14.4) |
| Depression or anxiety | 333 (38.8) | 317 (37.2) |
| Hormonal problems | 57 (6.6) | 70 (8.2) |
| Other comorbidity | 115 (13.4) | 127 (14.9) |
| Any diagnosis | 570 (66.4) | 561 (65.8) |
| Prescribed sleep medication, mean (SD), No. of nights per 14 d | 1.6 (3.4) | 1.6 (3.7) |
| Nonprescribed sleeping medication, mean (SD), No. of nights per 14 d | 2.3 (3.9) | 2.2 (3.9) |
| Outcomes at baseline, mean (SD)a | ||
| Functional health (PROMIS-10) | ||
| Total | 31.8 (5.6) | 31.8 (5.8) |
| Physical | 14.3 (2.2) | 14.4 (2.3) |
| Mental | 11.4 (3.0) | 11.2 (3.0) |
| Mental well-being (WEMWBS) | 43.2 (7.9) | 43.1 (7.7) |
| Sleep-related quality of life (GSII) | ||
| Most important concern | 87.3 (12.7) | 87.8 (12.8) |
| Second most important concern | 75.4 (16.4) | 76.3 (17.3) |
| Third most important concern | 60.2 (21.3) | 60.9 (21.4) |
| Combined score | 222.9 (44.5) | 224.9 (45.9) |
| Insomnia (SCI) | 6.6 (3.3) | 6.5 (3.2) |
| Depressive symptoms (PHQ-9) | 9.7 (4.2) | 9.8 (4.1) |
| Anxiety symptoms (GAD-7) | 7.4 (4.7) | 7.4 (4.7) |
| Sleepiness (ESS) | 6.2 (4.5) | 6.1 (4.4) |
| Fatigue (FFS) | 19.1 (5.4) | 19.0 (5.5) |
| Relationship satisfaction (RAS) | 27.6 (5.8) | 27.8 (5.8) |
| Cognitive functioning (CFQ) | 42.5 (16.8) | 43.1 (15.4) |
| Work productivity and impairment, mean (SD), WPAI | ||
| Presenteeism | 41.0 (23.2) | 42.2 (24.0) |
| Absenteeism | 8.03 (16.9) | 7.38 (16.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CFQ, Cognitive Failures Questionnaire; dCBT, digital cognitive behavioral therapy; ESS, Epworth Sleepiness Scale; FFS, Flinders Fatigue Scale; GAD-7, 7-item Generalized Anxiety Disorder; GSII, Glasgow Sleep Impact Index; PHQ-9, 9-item Patient Health Questionnaire; PROMIS-10, 10-item Patient-Reported Outcomes Measurement Information System; RAS, Relationship Assessment Scale; SCI, 8-item Sleep Condition Indicator; SHE, sleep hygiene education; TAU, treatment as usual; WEMWBS, Warwick-Edinburgh Mental Well-being Scale; WPAI, Work Productivity and Activity Impairment questionnaire.
Outcome assessment scales are explained in the Measurements subsection of the Methods section.
In the dCBT group, 689 of the 853 participants (80.8%) logged on for at least 1 session, 491 participants (57.6%) completed at least 4 sessions, and 413 participants (48.4%) completed all 6 sessions (Figure). Sleep hygiene education was accessed at least once by 759 of 858 participants (88.5%).
Treatment Effects on Primary Outcomes
At weeks 4, 8, and 24, dCBT was associated with significant improvement in global health (Cohen d for week 4, 0.16; week 8, 0.31; and week 24, 0.31) and mental well-being (Cohen d for week 4, 0.13; week 8, 0.35; and week 24, 0.38), and a significant reduction in sleep-related impairment to quality of life (Cohen d for week 4, –0.69; week 8, –1.38; and week 24, –1.46; RQ 1 [Table 2]). Effects were robust across sensitivity analyses investigating assumptions regarding missingness of the outcome.
Table 2. Effects of Digital Cognitive Behavioral Therapy vs Sleep Hygiene Education on Primary Outcomes: Physical Health, Psychological Well-being, Sleep-Related Quality of Life, and Insomnia.
| Assessmenta | Unadjusted, Mean (SD) | Adjusted Difference (95% CI) | Cohen d | P Value | |
|---|---|---|---|---|---|
| SHE + TAU | dCBT + TAU | ||||
| PROMIS-10 | |||||
| Week 4 | 32.52 (6.05) | 33.84 (6.49) | 0.90 (0.40 to 1.40) | 0.16 | <.001 |
| Week 8 | 32.92 (6.18) | 35.08 (6.65) | 1.76 (1.24 to 2.28) | 0.31 | <.001 |
| Week 24 | 33.10 (6.10) | 35.24 (6.88) | 1.76 (1.22 to 2.30) | 0.31 | <.001 |
| WEMWBS | |||||
| Week 4 | 44.72 (8.21) | 46.03 (8.55) | 1.04 (0.28 to 1.80) | 0.13 | .007 |
| Week 8 | 45.16 (8.77) | 48.12 (8.82) | 2.68 (1.89 to 3.47) | 0.35 | <.001 |
| Week 24 | 45.31 (8.89) | 48.62 (9.02) | 2.95 (2.13 to 3.76) | 0.38 | <.001 |
| GSIIb | |||||
| Week 4 | 69.80 (23.64) | 60.69 (26.20) | −8.76 (−11.83 to −5.69) | −0.69 | <.001 |
| Week 8 | 65.68 (25.86) | 46.78 (29.90) | −17.60 (−20.81 to −14.39) | −1.38 | <.001 |
| Week 24 | 63.33 (27.26) | 43.78 (31.25) | −18.72 (−22.04 to −15.41) | −1.46 | <.001 |
Abbreviations: dCBT, digital cognitive behavioral therapy; GSII, Glasgow Sleep Impact Index; PROMIS-10, 10-item Patient-Reported Outcomes Measure; SHE, sleep hygiene education; TAU, treatment as usual; WEMWBS, Warwick-Edinburgh Mental Well-being Scale.
Outcome assessment scales are explained in the Measurements subsection of the Methods section.
Highest-ranked impairment.
Mediation Analysis
The results of the mediation analyses can be found in Table 3 (indirect effects, RQ 2). The mediator (SCI) demonstrated an improvement compared with SHE (Cohen d for week 4, 0.89; week 8, 1.51; and week 24, 1.51; all P < .01 Table 4). When we considered functional health on PROMIS-10 as the outcome, the proportion of the effect of dCBT on PROMIS-10 score at 8 weeks that was mediated by changes in insomnia symptoms at 4 weeks was 50.5%, and the proportion of the effect of the intervention on PROMIS-10 scores at 24 weeks that was mediated by changes in insomnia symptoms at 8 weeks was 83.8%. For psychological well-being, these values were 47.0% at 8 weeks and 74.9% at 24 weeks. When we considered sleep-related quality of life, with GSII (rank 1: the most important impairment) as the outcome, the proportion of the effect of the intervention on GSII at 8 weeks that was mediated by changes in insomnia symptoms was 45.5% and increased to 65.9% at 24 weeks. eTable 2 in Supplement 2 provides mediation analyses on PROMIS subscales and the GSII total score and second- and third-ranked reported impairments.
Table 3. Mediation Effects of Insomnia Improvement on Primary Outcomes: Physical Health, Mental Well-being, Sleep-Related Impact, and Insomnia.
| Asssessmenta | Mediation Tested | Total Effect | Direct Effect | Indirect Effect | Mediation, % | |||
|---|---|---|---|---|---|---|---|---|
| Effect Size (SE) | P Value | Effect Size (SE) | P Value | Effect Size (SE) | P Value | |||
| PROMIS-10 | ||||||||
| Week 8 | SCI week 4 | 1.76 (0.26) | <.001 | 0.65 (0.27) | .02 | 0.89 (0.12) | <.001 | 50.5 |
| Week 24 | SCI week 8 | 1.75 (0.27) | <.001 | 0.13 (0.29) | .66 | 1.47 (0.14) | <.001 | 83.8 |
| WEMWBS | ||||||||
| Week 8 | SCI week 4 | 2.67 (0.40) | <.001 | 1.21 (0.42) | .004 | 1.26 (0.17) | <.001 | 47.0 |
| Week 24 | SCI week 8 | 2.93 (0.41) | <.001 | 0.76 (0.45) | .09 | 2.17 (0.20) | <.001 | 74.9 |
| GSIIb | ||||||||
| Week 8 | SCI week 4 | −17.54 (1.63) | <.001 | −8.69 (1.60) | <.001 | −7.98 (0.93) | <.001 | 45.5 |
| Week 24 | SCI week 8 | −18.63 (1.68) | <.001 | −7.84 (1.68) | <.001 | −12.27 (0.84) | <.001 | 65.9 |
Abbreviations: GSII, Glasgow Sleep Impact Index; PROMIS-10, 10-item Patient-Reported Outcomes Measure; SCI, Sleep Condition Indicator; WEMWBS, Warwick-Edinburgh Mental Well-being Scale.
Outcome assessment scales are explained in the Measurements subsection of the Methods section.
Highest-ranked impairment.
Table 4. Effects of dCBT vs SHE on Mediator and Secondary Outcomes of Mood, Fatigue, Relationship, Cognition, Work Performance, and Sleepiness.
| Assessmenta | Unadjusted, Mean (SE) | Adjusted Difference (95% CI) | Cohen d | P Value | |
|---|---|---|---|---|---|
| SHE + TAU | dCBT + TAU | ||||
| SCIb | |||||
| Week 4 | 9.96 (4.70) | 13.00 (5.01) | 2.88 (2.28 to 3.48) | 0.89 | <.001 |
| Week 8 | 11.05 (5.32) | 16.29 (6.17) | 4.90 (4.28 to 5.53) | 1.51 | <.001 |
| Week 24 | 11.66 (5.84) | 16.89 (6.91) | 4.91 (4.27 to 5.56) | 1.51 | <.001 |
| PHQ-9 | |||||
| Week 4 | 8.36 (4.38) | 7.47 (4.26) | −0.72 (−1.15 to −0.29) | −0.17 | .001 |
| Week 8 | 8.16 (4.90) | 6.22 (4.40) | −1.59 (−2.04 to −1.14) | −0.38 | <.001 |
| Week 24 | 7.94 (4.58) | 6.13 (4.59) | −1.58 (−2.05 to −1.12) | −0.38 | <.001 |
| GAD-7 | |||||
| Week 4 | 6.23 (4.52) | 5.51 (4.18) | −0.49 (−0.91 to −0.06) | −0.10 | .03 |
| Week 8 | 6.10 (4.69) | 4.68 (4.21) | −1.19 (−1.63 to −0.74) | −0.25 | <.001 |
| Week 24 | 6.05 (4.50) | 4.70 (4.21) | −1.10 (−1.56 to −0.64) | −0.24 | <.001 |
| ESS | |||||
| Week 4 | 6.41 (4.64) | 5.55 (4.34) | −0.52 (−0.88 to −0.17) | −0.12 | .003 |
| Week 8 | 6.14 (4.62) | 4.81 (3.94) | −1.01 (−1.38 to −0.64) | −0.23 | <.001 |
| Week 24 | 6.24 (4.61) | 4.67 (3.97) | −1.41 (−1.79 to −1.03) | −0.32 | <.001 |
| FFS | |||||
| Week 4 | 16.93 (5.87) | 14.82 (5.96) | −2.01 (−2.63 to −1.39) | −0.37 | <.001 |
| Week 8 | 15.91 (6.08) | 11.84 (6.54) | −3.83 (−4.48 to −3.19) | −0.71 | <.001 |
| Week 24 | 15.67 (6.46) | 11.41 (6.64) | −4.06 (−4.72 to −3.39) | −0.75 | <.001 |
| RAS | |||||
| Week 4 | 24.45 (7.44) | 24.98 (7.78) | 0.12 (−0.38 to 0.62) | 0.02 | .64 |
| Week 8 | 24.36 (7.50) | 25.23 (7.64) | 0.07 (−0.44 to 0.59) | 0.01 | .79 |
| Week 24 | 24.72 (7.42) | 25.45 (7.83) | 0.01 (−0.53 to 0.54) | 0.00 | .98 |
| CFQ | |||||
| Week 4 | 41.79 (16.79) | 39.53 (15.54) | −2.08 (−3.23 to −0.92) | −0.13 | <.001 |
| Week 8 | 41.19 (16.97) | 36.93 (16.44) | −4.18 (−5.38 to −2.99) | −0.26 | <.001 |
| Week 24 | 41.25 (16.49) | 37.47 (15.47) | −3.38 (−4.60 to −2.16) | −0.21 | <.001 |
| WPAI presenteeism | |||||
| Week 4 | 33.61 (23.82) | 31.26 (23.52) | −2.27 (−5.47 to 0.92) | −0.10 | .16 |
| Week 8 | 32.71 (23.32) | 23.56 (21.21) | −9.55 (−12.89 to −6.21) | −0.41 | <.001 |
| Week 24 | 32.08 (23.37) | 20.56 (20.69) | −9.94 (−13.42 to −6.46) | −0.42 | <.001 |
| WPAI absenteeism | |||||
| Week 4 | 2.56 (8.94) | 3.22 (9.87) | 0.39 (−1.31 to 2.10) | 0.02 | .65 |
| Week 8 | 3.54 (11.59) | 2.34 (8.26) | −1.23 (−3.02 to −0.56) | −0.07 | .18 |
| Week 24 | 4.61 (14.01) | 3.41 (12.16) | −2.09 (−3.95 to −0.23) | −0.13 | .03 |
| Job satisfaction | |||||
| Week 4 | 3.48 (2.14) | 3.30 (2.10) | −0.05 (−0.22 to 0.12) | −0.02 | .58 |
| Week 8 | 3.45 (2.07) | 3.43 (2.14) | 0.08 (−0.09 to 0.26) | 0.08 | .36 |
| Week 24 | 3.49 (2.05) | 3.58 (2.16) | 0.27 (0.09 to 0.45) | 0.27 | .004 |
| Life satisfaction | |||||
| Week 4 | 2.84 (0.72) | 2.90 (0.72) | 0.07 (−0.02 to 0.13) | 0.10 | .06 |
| Week 8 | 2.86 (0.70) | 2.96 (0.73) | 0.12 (0.05 to 0.19) | 0.18 | <.001 |
| Week 24 | 2.86 (0.70) | 3.01 (0.74) | 0.16 (0.09 to 0.24) | 0.24 | <.001 |
Abbreviations: CFQ, Cognitive Failures Questionnaire; dCBT, digital cognitive behavioral therapy; ESS, Epworth Sleepiness Scale; FFS, Flinders Fatigue Scale; GAD-7, 7-item Generalized Anxiety Disorder; PHQ-9, 9-item Patient Health Questionnaire; RAS, Relationship Assessment Scale, SCI, Sleep Condition Indicator; SHE, sleep hygiene education; TAU, treatment as usual; WPAI, Work Productivity and Activity Impairment questionnaire.
Outcome assessment scales are explained in the Measurements subsection of the Methods section.
Highest-ranked impairment.
Treatment Effects on Secondary Outcomes
Symptoms of depression (PHQ-9), anxiety (GAD-7), sleepiness (ESS), and cognitive failures (CFQ) all demonstrated significant differences in favor of dCBT at weeks 4, 8, and 24, reflecting small effect sizes (Table 4, RQ 3). There were moderate to large effects observed at weeks 4, 8, and 24 for fatigue (FFS). On the WPAI, productivity at work (presenteeism) that was attributed to sleep problems showed a small to moderate improvement after dCBT relative to control. A significant but small effect in terms of reduced absenteeism attributed to poor sleep and increased job satisfaction was observed at week 24. There were no significant effects at any time point on relationship functioning (RAS). Mediation analyses indicated that changes in insomnia symptoms also accounted for significant and sizeable proportions of effects on these secondary outcomes at both week 8 and week 24 (eTable 3 in Supplement 2, RQ 4).
Reported Adverse Effects
There was 1 serious adverse event reported, which was unrelated to the intervention. The event was reported to the University of Oxford Medical Sciences Inter-Divisional Ethics Committee. Participants who received dCBT reported a higher occurrence of difficulty remembering things, headache and/or migraine, fatigue and/or exhaustion, extreme sleepiness, difficulty with concentration and attention, reduced motivation and/or energy, and irritability attributed to the insomnia improvement program at week 8 (eTable 4 in Supplement 2).
Discussion
It is well established that CBT is the first-line treatment for people with chronic insomnia14,17,18,19,21,22 and that sleep-related outcomes, whether on index measures of insomnia or on derivations from sleep diaries, show sustained improvement.17 The findings from this study confirm large effects on insomnia symptoms. However, we primarily addressed whether CBT for insomnia affects functional health, psychological well-being, and sleep-related quality of life. We used dCBT instead of face-to-face CBT to facilitate an adequately powered scientific inquiry of explanatory processes.
Our findings provide strong support for the hypothesis that dCBT improved participants’ functional health relative to sleep hygiene education. At 24 weeks, effect sizes were small for functional health and psychological well-being (Cohen d, 0.31 and 0.38) and large for sleep-related quality of life (Cohen d, −1.46). The greater effect on sleep-related quality of life, measured with the GSII, likely occurs because the GSII specifically asks participants to define areas of impairment attributed to poor sleep, whereas functional health and well-being were assessed with global measures (PROMIS-10 and WEMWBS). Improvements in all 3 primary outcomes were mediated by insomnia improvements associated with dCBT. These mediation effects were substantial: 51% mediation of functional health, 47% of well-being, and 46% of sleep-related quality of life at 8 weeks, and 84% of functional health, 75% of well-being, and 66% of sleep-related quality of life at 24 weeks’ follow-up. To our knowledge, this is the first large-scale study demonstrating a causal relationship between CBT-mediated reduction in insomnia symptoms and perceived health status and quality of life.
A report of a parallel study (Oxford Access for Students Improving Sleep [OASIS]) exploring mental health symptoms as the outcome variable of interest and likewise demonstrated a causal relationship between insomnia improvement and reductions in mental health symptoms.33 Together, the mediation analyses in these 2 studies (with a total of 5466 participants) provide novel and convincing evidence that insomnia may be a legitimate and important target for mental health and well-being. Consideration of the secondary variables in the present study yields further data on the generalized effects of insomnia improvement on symptoms of depression, anxiety, fatigue, sleepiness, and cognitive failures and productivity, with mediation in substantial part through changes in insomnia.
Limitations
We are mindful of limitations of this research. Although participant demographic characteristics were typical of clinical populations (eg, typically female, middle-aged) and had some similar characteristics (eg, two-thirds reported diagnosed health comorbidities), participants were not drawn from patient groups or health care services and therefore reflect individuals who experience insomnia in the general population. This difference may reduce the generalizability to the insomnia patient group but increases the generalizability to those experiencing insomnia symptoms in the general population. As is typical of digital programs, and indeed of CBT in general, there was a substantial dropout from treatment (58% of participants completed ≥4 dCBT sessions); however, intention-to-treat analyses still identified significant improvements. Sensitivity and missing data analyses did not change the study conclusions. In addition, the instrument used to measure insomnia symptoms includes items around daytime functioning because these items are part of the DSM-5 insomnia disorder diagnosis; these items may have inflated the mediation effects. Finally, we note that attributed adverse effects were more common in the group receiving dCBT than in the control participants. Despite widespread generalized benefits, dCBT can have adverse effects; we hypothesize that these effects might result from the sleep restriction component that is introduced in week 3. Potentially, these adverse effects were short lived; at weeks 8 and 24, sleepiness, fatigue, cognitive failures, and mood symptoms were more common in participants receiving SHE than in those receiving dCBT.
Conclusions
The results of this definitive trial suggest that dCBT not only is effective in improving insomnia symptoms but also demonstrates positive effects around the clock by improving the functional health, psychological well-being, and sleep-related quality of life of people with positive screening results for insomnia disorder. In addition, improved insomnia is a mediator of these benefits. These findings indicate that dCBT improves both daytime and nighttime aspects of insomnia, lending further weight to the clinical guideline recommendation of CBT as the treatment of choice for insomnia.
Trial Protocol.
eTable 1. Descriptives for Participants Who Completed and Who Were Lost to Follow-up.
eTable 2. Mediation Analyses on PROMIS Subscales and GSII Total Score, and 2nd and 3rd Ranked Complaints
eTable 3. Mediation Effects for Secondary Outcomes
eTable 4. Frequency of Adverse Events by Treatment Group
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97-111. doi: 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 3.Lichstein KL. Epidemiology of Sleep: Age, Gender, and Ethnicity. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004. [Google Scholar]
- 4.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30(3):274-280. [PubMed] [Google Scholar]
- 5.Pigeon WR, Bishop TM, Krueger KM. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44. doi: 10.1007/s11920-017-0802-x [DOI] [PubMed] [Google Scholar]
- 6.Khan MS, Aouad R. The effects of insomnia and sleep loss on cardiovascular disease. Sleep Med Clin. 2017;12(2):167-177. doi: 10.1016/j.jsmc.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 7.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32(11):1980-1985. doi: 10.2337/dc09-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414-420. doi: 10.2337/dc09-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14(1):69-82. doi: 10.1016/j.smrv.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Espie CA, Kyle SD, Hames P, Cyhlarova E, Benzeval M. The daytime impact of DSM-5 insomnia disorder: comparative analysis of insomnia subtypes from the Great British Sleep Survey. J Clin Psychiatry. 2012;73(12):e1478-e1484. doi: 10.4088/JCP.12m07954 [DOI] [PubMed] [Google Scholar]
- 11.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey: II. Sleep. 1999;22(suppl 2):S354-S358. [PubMed] [Google Scholar]
- 12.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130. doi: 10.1016/j.sleep.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 13.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians . Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133. doi: 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 14.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700. doi: 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- 15.Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577-1601. doi: 10.1177/0269881110379307 [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1-30. [PubMed] [Google Scholar]
- 17.van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16. doi: 10.1016/j.smrv.2017.02.001 doi: 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13(3):205-214. doi: 10.1016/j.smrv.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Luik AI, Kyle SD, Espie CA. Digital cognitive behavioral therapy (dCBT) for insomnia: a state-of-the-science review. Curr Sleep Med Rep. 2017;3(2):48-56. doi: 10.1007/s40675-017-0065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. doi: 10.1371/journal.pone.0149139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1-10. doi: 10.1016/j.smrv.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Peoples AR, Garland SN, Perlis ML, et al. Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: a randomized placebo-controlled trial. J Cancer Surviv. 2017;11(3):401-409. doi: 10.1007/s11764-017-0597-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morin CM, Beaulieu-Bonneau S, Bélanger L, et al. Cognitive-behavior therapy singly and combined with medication for persistent insomnia: impact on psychological and daytime functioning. Behav Res Ther. 2016;87:109-116. doi: 10.1016/j.brat.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Houdenhove L, Buyse B, Gabriëls L, Van den Bergh O. Treating primary insomnia: clinical effectiveness and predictors of outcomes on sleep, daytime function and health-related quality of life. J Clin Psychol Med Settings. 2011;18(3):312-321. doi: 10.1007/s10880-011-9250-7 [DOI] [PubMed] [Google Scholar]
- 26.Palermo TM, Beals-Erickson S, Bromberg M, Law E, Chen M. A single arm pilot trial of brief cognitive behavioral therapy for insomnia in adolescents with physical and psychiatric comorbidities. J Clin Sleep Med. 2017;13(3):401-410. doi: 10.5664/jcsm.6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conley S, Redeker NS. Cognitive behavioral therapy for insomnia in the context of cardiovascular conditions. Curr Sleep Med Rep. 2015;1(3):157-165. doi: 10.1007/s40675-015-0019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon S, Morgan K, Mathers N, Thompson J, Tomeny M. Impact of cognitive behavior therapy on health-related quality of life among adult hypnotic users with chronic insomnia. Behav Sleep Med. 2006;4(2):71-84. doi: 10.1207/s15402010bsm0402_1 [DOI] [PubMed] [Google Scholar]
- 29.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651-4658. doi: 10.1200/JCO.2007.13.9006 [DOI] [PubMed] [Google Scholar]
- 30.Espie CA, Kyle SD, Miller CB, Ong J, Hames P, Fleming L. Attribution, cognition and psychopathology in persistent insomnia disorder: outcome and mediation analysis from a randomized placebo-controlled trial of online cognitive behavioural therapy. Sleep Med. 2014;15(8):913-917. doi: 10.1016/j.sleep.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 31.Pillai V, Anderson JR, Cheng P, et al. The anxiolytic effects of cognitive behavior therapy for insomnia: preliminary results from a web-delivered protocol. J Sleep Med Disord. 2015;2(2):1017. [PMC free article] [PubMed] [Google Scholar]
- 32.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489-495. doi: 10.1093/sleep/31.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman D, Sheaves B, Goodwin GM, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. 2017;4(10):749-758. doi: 10.1016/S2215-0366(17)30328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333-341. doi: 10.1016/S2215-0366(15)00536-2 [DOI] [PubMed] [Google Scholar]
- 35.Alessi C, Martin JL, Fiorentino L, et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc. 2016;64(9):1830-1838. doi: 10.1111/jgs.14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omvik S, Sivertsen B, Pallesen S, Bjorvatn B, Havik OE, Nordhus IH. Daytime functioning in older patients suffering from chronic insomnia: treatment outcome in a randomized controlled trial comparing CBT with zopiclone. Behav Res Ther. 2008;46(5):623-641. doi: 10.1016/j.brat.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 37.Hewitt CE, Torgerson DJ. Is restricted randomisation necessary? BMJ. 2006;332(7556):1506-1508. doi: 10.1136/bmj.332.7556.1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espie CA, Luik AI, Cape J, et al. Digital cognitive behavioural therapy for insomnia versus sleep hygiene education: the impact of improved sleep on functional health, quality of life and psychological well-being: study protocol for a randomised controlled trial. Trials. 2016;17(1):257. doi: 10.1186/s13063-016-1364-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, Cape J. The Sleep Condition Indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open. 2014;4(3):e004183. doi: 10.1136/bmjopen-2013-004183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Great British Sleep Survey http://www.greatbritishsleepsurvey.com. Accessed September 12, 2018.
- 41.The World Sleep Survey http://www.worldsleepsurvey.com. Accessed September 12, 2018.
- 42.Sleepio https://www.sleepio.com. Accessed September 12, 2018.
- 43.Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769-781. doi: 10.5665/sleep.1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39(1):45-60. doi: 10.1016/S0005-7967(99)00157-6 [DOI] [PubMed] [Google Scholar]
- 45.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30(5):574-584. doi: 10.1093/sleep/30.5.574 [DOI] [PubMed] [Google Scholar]
- 46.Espie CA. Overcoming Insomnia and Sleep Problems: A Self-help Guide Using Cognitive Behavioral Techniques. London, UK: Constable & Robinson Ltd; 2006. [Google Scholar]
- 47.McGrath ER, Espie CA, Power A, et al. Sleep to lower elevated blood pressure: a randomized controlled trial (SLEPT). Am J Hypertens. 2017;30(3):319-327. doi: 10.1093/ajh/hpw132 [DOI] [PubMed] [Google Scholar]
- 48.Bostock S, Luik AI, Espie CA. Sleep and productivity benefits of digital cognitive behavioral therapy for insomnia: a randomized controlled trial conducted in the workplace environment. J Occup Environ Med. 2016;58(7):683-689. doi: 10.1097/JOM.0000000000000778 [DOI] [PubMed] [Google Scholar]
- 49.Barnes CM, Miller JA, Bostock S. Helping employees sleep well: effects of cognitive behavioral therapy for insomnia on work outcomes. J Appl Psychol. 2017; 102(1):104-113. doi: 10.1037/apl0000154 [DOI] [PubMed] [Google Scholar]
- 50.Cheng P, Luik AI, Fellman-Couture C, et al. The efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized controlled trial [published online May 24, 2018]. Psychol Med. doi: 10.1017/S0033291718001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Academy of Sleep Medicine How to Sleep Better. Darien, IL: American Academy of Sleep Medicine; 2012. [Google Scholar]
- 52.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18(7):873-880. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tennant R, Hiller L, Fishwick R, et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5:63. doi: 10.1186/1477-7525-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kyle SD, Crawford MR, Morgan K, Spiegelhalder K, Clark AA, Espie CA. The Glasgow Sleep Impact Index (GSII): a novel patient-centred measure for assessing sleep-related quality of life impairment in insomnia disorder. Sleep Med. 2013;14(6):493-501. doi: 10.1016/j.sleep.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 55.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 57.Gradisar M, Lack L, Richards H, et al. The Flinders Fatigue Scale: preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007;3(7):722-728. [PMC free article] [PubMed] [Google Scholar]
- 58.Hendrick SS, Dicke A, Hendrick C. The Relationship Assessment Scale. J Soc Pers Relat. 1998;15:137-142. doi: 10.1177/0265407598151009 [DOI] [Google Scholar]
- 59.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(pt 1):1-16. doi: 10.1111/j.2044-8260.1982.tb01421.x [DOI] [PubMed] [Google Scholar]
- 60.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353-365. doi: 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 61.Dolbier CL, Webster JA, McCalister KT, Mallon MW, Steinhardt MA. Reliability and validity of a single-item measure of job satisfaction. Am J Health Promot. 2005;19(3):194-198. doi: 10.4278/0890-1171-19.3.194 [DOI] [PubMed] [Google Scholar]
- 62.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 63.Cheung F, Lucas RE. Assessing the validity of single-item life satisfaction measures: results from three large samples. Qual Life Res. 2014;23(10):2809-2818. doi: 10.1007/s11136-014-0726-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kyle SD, Morgan K, Spiegelhalder K, Espie CA. No pain, no gain: an exploratory within-subjects mixed-methods evaluation of the patient experience of sleep restriction therapy (SRT) for insomnia. Sleep Med. 2011;12(8):735-747. doi: 10.1016/j.sleep.2011.03.016 [DOI] [PubMed] [Google Scholar]
- 65.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61(3):310-317. doi: 10.1001/archpsyc.61.3.310 [DOI] [PubMed] [Google Scholar]
- 66.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 67.Dunn G, Emsley R, Liu H, et al. Evaluation and validation of social and psychological markers in randomised trials of complex interventions in mental health: a methodological research programme. Health Technol Assess. 2015;19(93):1-115, v-vi. doi: 10.3310/hta19930 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Descriptives for Participants Who Completed and Who Were Lost to Follow-up.
eTable 2. Mediation Analyses on PROMIS Subscales and GSII Total Score, and 2nd and 3rd Ranked Complaints
eTable 3. Mediation Effects for Secondary Outcomes
eTable 4. Frequency of Adverse Events by Treatment Group