This randomized clinical trial assesses whether an intervention for depression prevention provided by lay counselors is effective in older adults from low- and middle-income countries.
Key Points
Question
Is a depression prevention intervention effective when provided by lay counselors in low- and middle-income countries?
Finding
In this randomized clinical trial of 181 participants, a depression prevention intervention was effectively provided by lay counselors to older at-risk adults under circumstances characteristic of low- and middle-income countries.
Meaning
An intervention model of depression prevention is potentially scalable for use in other low- and middle-income countries.
Abstract
Importance
Preventing depression in older adults living in low- and middle-income countries is important because of the scarcity of treatment resources and the risk of disability, suicide, and dementia.
Objective
To assess whether an intervention for depression prevention provided by lay counselors is effective in older adults from low- and middle-income countries.
Design, Setting, and Participants
This parallel-group randomized clinical trial with masked outcome assessment was performed in 181 older adults (≥60 years) with subsyndromal depressive symptoms at rural and urban primary care clinics in Goa, India. The first participant entered the trial on March 31, 2015, and the last exited on June 2, 2017. Data analysis used the intention-to-treat approach.
Interventions
Lay counselors provided problem-solving therapy, brief behavioral treatment for insomnia, education in self-care of common medical disorders such as diabetes, and assistance in accessing medical and social programs.
Main Outcomes and Measures
The main outcome was incidence of major depressive episodes. The study also assessed symptom change during 12 months (12-item General Health Questionnaire [GHQ-12]; score range of 0 to 12, with higher scores indicating greater symptoms of depression and anxiety), functional status (World Health Organization Disability Assessment Schedule 2.0; score range of 12 to 60, with higher scores indicating greater disability), cognition (Hindi Mini-Mental State Examination; score range of 0 to 30, with higher scores indicating better cognitive functioning), blood pressure, and body mass index to provide further clinical context.
Results
The study enrolled 181 participants (mean [SD] age, 69.6 [7.2] years; 114 [63.0%] female): 91 to the intervention arm (depression in later life [DIL] intervention) and 90 to care as usual (CAU). Incident episodes of major depression were lower in the DIL intervention than in the CAU group (4.40% vs 14.44%; log-rank P = .04; number needed to treat, 9.95; 95% CI, 5.12-182.43). The 12-month Kaplan-Meier estimates of percentage of depression-free participants were 95.1% (95% CI, 90.5%-99.9%) in the DIL group vs 87.4% (95% CI, 80.4%-95.1%) in the CAU group. The incidence of depressive symptoms (GHQ-12) was also less (12-month mean difference, −1.18; 95% CI, −2.03 to −0.31; group × time interaction P < .001). There were no changes in measures of disability or cognition. The DIL intervention was associated with a significantly greater lowering of systolic blood pressure (12-month mean difference, −6.98; 95% CI, −11.96 to −2.01; group × time interaction P < .001) and change in body mass index (12-month mean difference, 0.23; 95% CI, −0.97 to 1.43; P = .04).
Conclusions and Relevance
The DIL intervention is effective for preventing episodes of major depression in older persons with subsyndromal symptoms. If replicated, the DIL intervention may be effective in older adults living in low- and middle-income countries.
Trial Registration
ClinicalTrials.gov Identifier: NCT02145429
Introduction
Depression in older adults is an increasing challenge in low- and middle-income countries (LMICs), reflecting demographic shifts, scarcity of depression treatment resources, and limited effect of treatment in reducing years lived with disability.1 Thus, developing effective strategies for prevention of depression in LMICs is important.2,3 The mean prevalence of late-life depression in Indian communities is estimated at 18%, higher than the global median rate of 5.4%.4 The characteristics of late-life depression, such as medical and social comorbidities, as well as risk of relapse and chronicity, exacerbate the treatment gap. The social changes facing older adults, such as India’s elderly population, further underscore the importance of concern about mental health.5 Finally, prevention of depression in older adults may lower the risk of Alzheimer disease and vascular dementia.6,7
The Evaluating the Benefits and Affordability of a Program to Improve the Care of Common Mental Disorders in Primary Care (MANAS) trial8 and the Dementia Home Care project in India9 demonstrated the effectiveness of using lay counselors (LCs) to address common mental disorders and dementia, respectively, in primary care and community settings. Considering the treatment gap and mental health specialty workforce shortage in LMICs, task sharing, a process that ensures the transfer of specific tasks from highly qualified specialists to other health workers with less expertise in a specified area of competence, has become increasingly important.10
This study aimed to develop and test a potentially scalable intervention to prevent later-life episodes of major depression by using LCs to provide an intervention called depression in later life (DIL).11 The DIL intervention is grounded in problem-solving therapy for primary care (PST-PC)12,13 and brief behavioral treatment for insomnia (BBTI).14 We hypothesized that the DIL intervention would be effective in preventing onset of major depressive episodes in older adults living with subsyndromal symptoms. The DIL intervention is an example of indicated prevention,15 which is prevention that targets persons with subsyndromal symptoms at risk for developing major depression. Focusing depression prevention on mildly symptomatic persons may have the greatest efficiency from a public health perspective, with a lower number needed to treat to prevent one incident case2,3,16 compared with selective or universal prevention.
Methods
We implemented a parallel-group randomized clinical trial with masked outcome assessment. The study took place in Goa, India, where approximately 11% of the population is 60 years or older. We recruited from rural and urban primary health care clinics and the broader community to reduce selection bias. To reduce contamination effects, we did not enroll more than 1 participant from any household. The first participant entered the trial on March 31, 2015, and the last exited on June 2, 2017. The trial protocol can be found in the Supplement. Written and oral informed consent was obtained per research ethics board procedures at Sangath, Goa, India; Goa Medical College, Goa; and the University of Pittsburgh, Pittsburgh, Pennsylvania. Data were deidentified. The protocol was approved by the institutional review boards at Sangath (our collaborating nongovernmental organization in Goa), the University of Pittsburgh, the Goa Medical College, and the London School of Hygiene and Tropical Medicine.
Enrollment Criteria
Consistent with indicated depression prevention, we enrolled mildly symptomatic persons without current mental illness that warranted other active treatment (eg, antidepressant medication). Participants were 60 years or older (as determined from government identification cards and medical records) with scores of 4 or higher on the rater-administered, 12-item General Health Questionnaire (GHQ-12), with scores ranging from 0 to 12 (higher scores indicating greater symptoms of depression and anxiety),17 and not in a current episode of major depression. Other inclusion criteria included ability to speak Konkani, Hindi, or English and residence in the same locality for the subsequent 12 months. We excluded persons with major depression or anxiety disorders within the past 12 months as determined by the Mini International Neuropsychiatric Interview (MINI) 6.0,18 with moderate to high suicide risk (ie, intent or plan to attempt suicide in the near future), with a history of psychiatric disorders other than nonpsychotic unipolar major depression or anxiety disorder, with low cognitive scores (<24 on the Hindi Mini-Mental State Examination [HMMSE]; score range, 0-30, with higher scores indicating better cognitive functioning),19 currently taking antidepressants, and living with unstable or acute medical illness that would interfere with trial participation (Figure 1).
Figure 1. CONSORT Flowchart for the Depression in Later Life (DIL) Intervention Study.
All data from participants (91 in the DIL intervention group and 90 in the care as usual [CAU] group) were used in the final intention-to-treat analysis. The 12-item General Health Quesionnaire (GHQ-12) and Hindi Mini-Mental State Examination (HMMSE) scores are explained in the Enrollment Criteria subsection of the Methods section. Ten individuals were approached for screening but were deemed ineligible, 157 individuals were eligible for screening but were not screened for eligibility because of consent refusal, 63 were screened for eligibility but did not complete screening, and 38 were eligible but not randomized because of consent refusal.
DIL Intervention
The DIL intervention is a behaviorally activating, learning-based approach grounded in PST-PC and BBTI. The DIL intervention also included help in accessing government-sponsored medical and social programs and education in self-management of common chronic diseases. The DIL intervention’s design was based on input from key stakeholders gathered during a year of formative research and from an open-case series of 21 participants addressing the feasibility, acceptability, and symptom-lowering effects of the DIL intervention.5
The goals of PST-PC are to inculcate a positive problem orientation and to teach active problem-solving skills in place of avoidant coping.12,13 The PST-PC treats or prevents depression in older adults with a variety of medical and neurologic problems.20,21,22,23,24 The PST-PC administered through task shifting has also been used to treat depression successfully in a primary care setting in Zimbabwe.25
Strategies to overcome sleep problems were based on the BBTI, which was previously developed for use in primary care.14 The BBTI teaches participants strategies to improve sleep quality and enhance daytime alertness. The DIL intervention thus dealt with 2 potentially modifiable risk factors for major depression: avoidant or passive coping and insomnia.
Because our formative work indicated that the most common source of tension or worry in older adults related to chronic illnesses, such as diabetes, hypertension, osteoarthritis, and limited mobility because of somatic distress (aches and pains), the LCs were trained to provide information regarding these common chronic ailments and to provide basic nonpharmacologic self-management and encouragement to seek medical care. We prepared pictorial flip charts illustrating PST-PC and BBTI to aid the LC with engaging participants in the DIL intervention, especially those with limited or no literacy. Finally, guided by formative work, the LCs provided case management to facilitate access to medical clinics and programs dealing with social challenges that face older adults in Goa.5,11,14
Provision, Content, Frequency, Duration, and Quality Control of the DIL Intervention
The 4 LCs (2 women and 2 men) for the DIL intervention were members of the local community, older than 30 years, and graduates of any non–health-related field (including counseling). The LCs were trained in workshops conducted by 2 of us (M.S. and J.Q.M.) and were required to demonstrate proficiency in 2 practice cases. Weekly supervision locally and biweekly via Skype from the United States was performed for therapy quality assurance. Assessment of quality used the Therapy Quality Assessment Form based on selected audio recordings of sessions followed by a round of peer-led feedback. The Therapy Quality Assessment Form addresses such tasks as setting an agenda, identifying a problem and a realistic goal, brainstorming for possible solutions, discussing pros and cons, and setting an action plan. The overall mean (SD) intervention quality rating was 1.60 (0.39) (n = 76) on a scale of 0 to 2, with 0 indicating very good and 2 indicating excellent quality. In addition, the therapists’ conduct was also rated with respect to active listening, positive feedback, empathy, and collaboration. The overall score on therapist conduct was similarly high (mean [SD], 1.69 [0.25] [n = 76]).
The DIL intervention sessions, 30 to 40 minutes in length, were provided at places convenient to participants (usually their home or in social or religious centers) for 6 sessions that spanned 6 to 10 weeks. We included 2 booster sessions, 1 each at months 7 and 10, to encourage practice and maintenance of skills for dealing adaptively with future problems.
Regarding the components of the DIL intervention actually provided, of the 91 participants, 78 received PST-PC (11 participants did not start the intervention but were included in the intention-to-treat analysis, and data were missing for 1 participant), 58 received BBTI, 75 received instruction in self-care for chronic medical diseases and encouragement to seek medical care, 9 received help in accessing social and government resources, and 55 received all 4 DIL intervention components. The most common problems reported dealt with health concerns, family conflicts, and future safety and well-being.
Care as Usual
The control group received care as usual (CAU) together with the same schedule of outcome assessments used in the DIL intervention. The results of additional assessments among CAU participants were made known to them; if participants had developed major depression, they were referred to primary care physicians for treatment.
Outcomes
The primary outcome was the proportion of participants in whom incident episodes of major depression developed during 12 months, as ascertained by administration of the MINI 6.0. To provide additional clinical context for the primary outcome, we monitored changes in the levels of psychological distress on the GHQ-12. Secondary outcomes were longitudinally measured functional status (World Health Organization Disability Assessment Schedule [WHODAS] 2.0; score range, 12-60, with higher scores indicating greater disability) and cognition (HMMSE). Exporatory outcomes were changes in blood pressure and body mass index.
Randomization and Masking
The data manager randomized participants equally to CAU or the DIL intervention via permuted block randomization (block size of 8), using a computer-generated random-number table and stratifying by urban or rural status, sex, and the site of recruitment (community or health care facility). All study personnel, including assessors, were masked to random assignment until outcomes analysis was complete and reviewed by the Data Safety Monitoring Board. Participants were asked not to disclose their assignment to masked assessors.
Power Analysis
We initially set out to enroll 120 participants (60 in each arm) as part of intervention development (following the first year of formative research). As the trial progressed, however, with a faster rate of enrollment than anticipated, we embarked on a fully powered confirmatory randomized clinical trial by increasing the sample size to 180 (90 per arm) after approval by the National Institute of Mental Health and the Data Safety Monitoring Board. With respect to the primary outcome measure, incidence of major depressive episodes, we calculated that 90 participants per study arm would allow detection of a reduction in incidence from 20% to 6% with 82% power, whereas the same reduction would be detected with only 64% power with 60 participants per arm. We based these estimates of incident rate reduction on an earlier meta-analysis of psychological and behavioral interventions for preventing the onset of major depressive disorder.26 With respect to continuous measures of symptom burden (GHQ-12 scores), assuming an α of .05, with 2-sided tests, and with the original sample of 60 per group, we could detect with 81% power a standardized (pre-post) mean difference in GHQ-12 scores of 0.52 SD, which is considered to be a medium to large effect size. Increasing to 90 participants per arm allows detection of a more realistic mean difference of 0.42 SD.
Statistical Analysis
Data were analyzed at the University of Pittsburgh Graduate School of Public Health, before the trial was unmasked. There were no protocol deviations. Following the principles of intention to treat (where comparisons were made according to assigned intervention groups and using all information collected from participants), analyses addressed 3 goals: (1) comparison of participant characteristics at the time of randomization (baseline characteristics), (2) comparison of incident rates of major depressive episodes on the MINI 6.0, and (3) comparison of the trajectories of blood pressure, body mass index, and GHQ-12, WHODAS 2.0, and HMMSE scores during the 12-month period of observation. The WHODAS 2.0 and HMMSE scores were specified by the protocol to be recorded at baseline and 12 months, whereas the GHQ-12 scores were recorded at baseline, 3 months, 6 months, and 12 months. There were small variations on the times of the actual measurements because scheduling issues often prevented measurements to be taken exactly at the protocol-specified times. Kaplan-Meier plots were used to summarize major depressive episode–free survival, and subsequent inferences about differences between the DIL intervention and CAU in incident rates were made via log-rank tests, Cox proportional hazards regression models, and simple χ2 tests. Per our protocol specifications, we compared the trajectories between the DIL intervention and CAU groups on continuous measures at the actual observation times by using a random-effects model approach proposed by Laird and Ware27 and by Hedeker and Gibbons,28 using group, time (months), and group × time interactions. Higher powers of time using quadratic or cubic terms were fitted when group trajectories had nonlinear trends. Although mixed models are robust to nonnormality of outcomes, we inspected the outcome data for normality and any outcome variable that was highly skewed was transformed by square root transformation.
Statistical analyses were performed on masked data using SAS statistical software, version 9.4 (SAS Institute Inc) and R software, version 3.3.2 or later (R Foundation for Statistical Computing). Statistical significance was considered to be P < .05, and all tests were 2-sided.
Results
We randomized 181 participants (mean [SD] age, 69.6 [7.2] years; 114 [63.0%] female): 90 to CAU and 91 to the DIL intervention (Figure 1 and Table 1). Attrition was low in both groups: 12.2% in the CAU group and 17.6% in the DIL intervention group (log-rank statistic = 1.047; P = .31). Missingness was attributable to failure to complete evaluations, consent withdrawal, death, or being lost to follow-up. No differences in baseline characteristics (age, sex, or GHQ-12 scores) were found in those with complete vs incomplete data.
Table 1. Characteristics of Study Participantsa.
| Characteristic | Total Participants (N = 181) | CAU (n = 90) | DIL Intervention (n = 91) |
|---|---|---|---|
| Age, mean (SD), y | 69.6 (7.2) | 69.7 (7.3) | 69.6 (7.1) |
| WHODAS 2.0 score, mean (SD)b | 17.38 (5.19) | 17.41 (4.36) | 17.35 (5.92) |
| HMMSE score, mean (SD)c | 27.83 (1.84) | 27.77 (1.85) | 27.90 (1.85) |
| GHQ-12 total score, mean (SD)d | 6.26 (1.88) | 6.29 (1.87) | 6.23 (1.90) |
| Sex | |||
| Male | 67 (37.0) | 33 (36.7) | 34 (37.4) |
| Female | 114 (63.0) | 57 (63.3) | 57 (62.6) |
| Catchment area | |||
| Rural (total) | 126 (69.6) | 63 (70.0) | 63 (69.2) |
| Rural PHC | 46 | 23 | 23 |
| Rural community | 80 | 40 | 40 |
| Urban (total) | 55 (30.4) | 27 (30.0) | 28 (30.8) |
| Urban PHC | 18 | 9 | 9 |
| Urban community | 37 | 18 | 19 |
| Religion | |||
| Hindu | 53 (29.3) | 23 (25.6) | 30 (33.0) |
| Christian | 127 (70.2) | 67 (74.4) | 60 (65.9) |
| Muslim | 1 (0.5) | 0 | 1 (1.1) |
| Marital status | |||
| Never married | 6 (3.3) | 3 (3.3) | 3 (3.3) |
| Married | 77 (42.6) | 38 (42.2) | 39 (42.9) |
| Widowed | 98 (54.1) | 49 (54.5) | 49 (53.8) |
| Educational level | |||
| Illiterate | 41 (22.6) | 19 (21.1) | 22 (24.2) |
| Literate, no formal education | 37 (20.4) | 20 (22.2) | 17 (18.6) |
| Primary school | 57 (31.5) | 25 (27.8) | 32 (35.2) |
| Secondary school | 38 (21.0) | 22 (24.5) | 16 (17.6) |
| Graduate | 7 (3.9) | 3 (3.3) | 4 (4.4) |
| Professional or postgraduate | 1 (0.6) | 1 (1.1) | 0 |
| Self-reported chronic disease | |||
| Diabetes | 86 (47.5) | 45 (50) | 41 (45.1) |
| Hypertension | 132 (73.0) | 68 (75.6) | 64 (70.3) |
| Heart disease | 39 (21.5) | 18 (20) | 21 (23.1) |
| Stroke | 15 (8.3) | 8 (8.9) | 7 (7.7) |
| Kidney disease | 7 (3.9) | 4 (4.4) | 3 (3.3) |
| COPD | 10 (5.5) | 6 (6.7) | 4 (4.4) |
| Asthma | 11 (6.1) | 4 (4.4) | 7 (7.7) |
| Arthritis | 70 (38.7) | 39 (43.3) | 31 (34.1) |
| Tuberculosis | 7 (3.9) | 3 (3.3) | 4 (4.4) |
| Cancer | 2 (1.1) | 2 (2.2) | 0 |
| Mental illnesse | 7 (3.9) | 4 (4.4) | 3 (3.3) |
| Otherf | 14 (7.7) | 9 (10) | 5 (5.5) |
Abbreviations: CAU, care as usual; COPD, chronic obstructive pulmonary disease; DIL, depression in later life; GHQ-12, 12-item General Health Questionnaire; HMMSE, Hindi Mini-Mental State Examination; PHC, primary health care; WHODAS 2.0, World Health Organization Disability Assessment Schedule 2.0.
Data are presented as number (percentage) of patients unless otherwise indicated.
Scores on the WHODAS 2.0 range from 12 to 60; a higher score indicates greater disability.
Scores on the HMMSE range from 0 to 30; a higher score indicates better cognitive functioning.
Scores on the GHQ-12 range from 0 to 12; a higher score indicates greater symptoms for depression and anxiety.
Patients with mental illness included 2 with anxiety disorder, 2 with insomnia, 1 with hallucinations, 1 with depression, and 1 unknown.
Patients with other self-reported chronic disease included 2 with hernia, 2 with seizure disorder, 3 with hypothyroidism, 1 with gastric ulcer, 1 with spondylitis, 1 with chronic backache, 1 with cholelithiasis, 1 with parkinsonism, 1 with blindness, and 1 with pleural effusion.
Participant Characteristics at Baseline
The DIL and CAU arms were well balanced with respect to sociodemographic and clinical characteristics, including sex, rural vs urban residence, clinic vs community enrollment, GHQ-12 and HMMSE scores, and rates of common medical disorders (diabetes, hypertension, and osteoarthritis). Only 1 participant in each arm reported a history of depression.
Effect on Primary Outcomes
The 12-month Kaplan-Meier estimates (percentage of depression-free participants) were 95.1% (95% CI, 90.5%-99.9%) in the DIL intervention group and 87.4% (95% CI, 80.4%-95.4%) in the CAU group (Figure 2 and Table 2). Seventeen participants (9.4%) experienced an MINI-defined major depressive episode after randomization: 13 of 90 (14.4%) in the CAU group and 4 of 91 (4.4%) in the DIL intervention group (hazard ratio, 0.32; 95% CI, 0.10-0.98; log-rank P = .04). The P values for treatment differences remained similar in all cases to that obtained by the χ2 test (Figure 2 and Table 2).
Figure 2. Kaplan-Meier Plots of Times to First Mini International Neuropsychiatric Interview–Defined Episode of Major Depressive Episode (MDE) by Intervention Arm.
In the group receiving care as usual (CAU), 13 MDEs were recorded; in those receiving the depression in later life (DIL) intervention, 4 MDEs were recorded. One MDE was reported in each group at baseline. The hazard ratio for MDE in the DIL intervention group compared with the CAU group was 0.32 (95% CI, 0.10-0.98; log-rank P = .04).
Table 2. Summary of Results for Primary and Secondary Outcomes.
| Outcome | Mean (SD) | 12-mo Adjusted Mean Differencea (95% CI) | P Value for Difference in Overall Time Effectb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3-mo Visit | 6-mo Visit | 12-mo Visit | |||||||
| CAU (n = 90) | DIL Intervention (n = 91) | CAU (n = 84) | DIL Intervention (n = 80) | CAU (n = 81) | DIL Intervention (n = 79) | CAU (n = 79) | DIL Intervention (n = 75) | |||
| GHQ-12 scorec | 6.29 (1.87) |
6.23 (1.90) |
5.76 (2.84) |
3.45 (2.64) |
5.60 (2.99) |
3.99 (2.95) |
5.67 (3.19) |
3.67 (2.67) |
−1.18 (−2.03 to −0.31) |
<.001 |
| HMMSE scored | 27.77 (1.85) |
27.90 (1.85) |
NR | NR | NR | NR | 27.63 (2.74) |
27.69 (3.02) |
−0.01 (−0.08 to 0.06) |
.70 |
| WHODAS 2.0e | 17.41 (4.37) |
17.35 (5.92) |
NR | NR | HR | NR | 17.73 (6.27) |
16.72 (5.71) |
−0.14 (−1.89 to 1.62) |
.26 |
| Systolic BP, mm Hg | 136.78 (15.21) |
136.27 (14.53) |
129.54 (13.24) |
132.25 (14.01) |
132.25 (14.01) |
128.68 (11.29) |
133.99 (16.84) |
125.55 (20.39) |
−6.98 (−11.96 to −2.01) |
<.001 |
| Diastolic BP, mm Hg | 80.07 (8.43) |
78.28 (9.32) |
79.26 (5.14) |
77.43 (6.92) |
79.10 (6.49) |
78.46 (7.65) |
79.20 (7.82) |
76.44 (8.58) |
0.30 (−2.07 to 2.67) |
.59 |
| BMI | 24.43 (4.14) |
24.56 (4.19) |
NR | NR | NR | NR | 24.76 (4.18) |
24.24 (4.08) |
0.23 (−0.97 to 1.43) |
.04 |
| Mean pulse, /min | 77.78 (7.16) |
77.80 (7.29) |
NR | NR | NR | NR | 78.08 (7.10) |
77.32 (7.01) |
−0.24 (−2.50 to 2.02) |
.57 |
Abbreviations: BP, blood pressure; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAU, care as usual; DIL, depression in later life; GHQ-12, 12-item General Health Questionnaire; HMMSE, Hindi Mini-Mental State Examination; NR, not reported; WHODAS 2.0, World Health Organization Disability Assessment Schedule 2.0.
Values based on 12-month determination for mixed-model effect fits with time included.
P values based on comparing differences between groups for all time effects fitted in the mixed-effect models.
Scores on the WHODAS 2.0 range from 12 to 60; a higher score indicates greater disability. Scores on the GHQ-12 range from 0 to 12; a higher score indicates greater symptoms for depression and anxiety.
Scores on the HMMSE range from 0 to 30; a higher score indicates better cognitive functioning.
Scores on the WHODAS 2.0 range from 12 to 60; a higher score indicates greater disability.
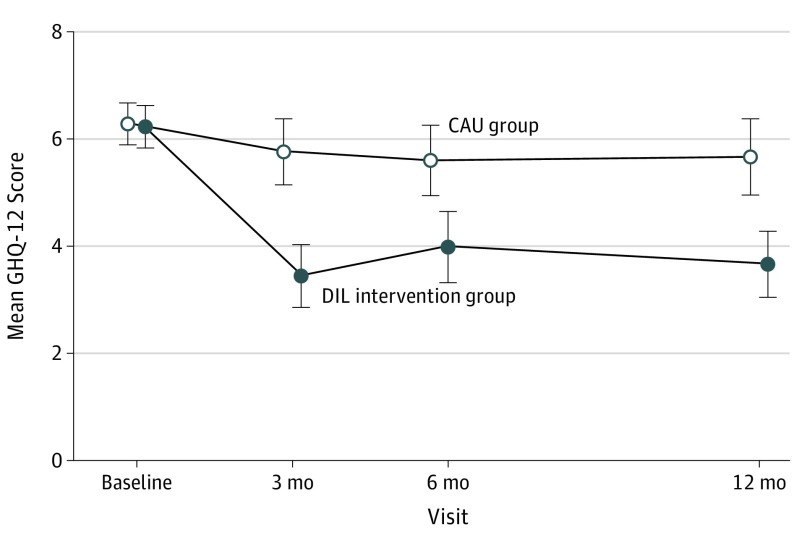
To provide additional clinical context for the primary outcome, we examined mean GHQ-12 scores (95% pointwise CIs) in the DIL intervention and CAU arms by visit during the observation period (Figure 3). A visual inspection of mean GHQ-12 scores indicated that the trajectories were nonlinear over time, reflecting a pattern of marked decrease in GHQ-12 scores after exposure to the DIL intervention (more so than in the CAU group) followed by a period of slower decline. On the basis of the Bayesian Information Criterion, a cubic model best fit the trajectory of GHQ-12 scores over the span, indicating that the DIL intervention and CAU groups showed improvement over time but that the individuals in the DIL intervention had a significantly greater mean improvement (overall group × time interaction F value = 10.78; F3346,0.999>5.54; P < .001).
Figure 3. Mean 12-Item General Health Questionnaire (GHQ-12) Scores (Pointwise 95% CIs) by Visit and Treatment.
Mean GHQ-12 scores over time improved to a significantly greater extent in the depression later in life (DIL) intervention group than in the care as usual (CAU) group (overall group × time interaction P < .001). Scores for the GHQ-12 can range from 0 to 12; a higher score indicates greater symptoms of depression and anxiety. Error bars indicate 95% CIs.
Effects on Secondary and Exploratory Outcomes
No significant differences were found for HMMSE scores or WHODAS 2.0 scores (HMMSE: 12-month mean difference, 0.07; 95% CI, −0.54 to 0.68; overall P = .72; WHODAS 2.0: 12-month mean difference, −0.49; 95% CI, −2.01 to 1.02; overall P = .27) (Table 2). The DIL intervention was associated with greater lowering of systolic, but not diastolic, blood pressure. Body mass index also changed to a greater extent in the DIL intervention group than in the CAU group (Table 2).
We observed 24 medical or surgical hospitalizations (19 in the DIL intervention group [20.9% of participants] and 5 in the CAU group [5.6%]) and 9 deaths (4 in the DIL intervention group and 5 in the CAU group; 6 deaths attributable to myocardial infarction, 1 to respiratory failure while receiving ventilatory support, 1 to decreased intake of food, and 1 to sepsis secondary to urinary tract disease). No participants attempted or completed suicide. All hospitalizations and deaths were evaluated by an independent physician, monitored by the Data Safety Monitoring Board, and not adjudged to warrant a change in protocol.
Discussion
The DIL intervention is, to our knowledge, the first randomized clinical trial of indicated depression prevention in older adults living in an LMIC and as such addresses a previously unmet need in global mental health.2,3,29,30 The trial found that LCs could successfully administer the DIL intervention to older adults with subsyndromal symptoms of depression and that, compared with CAU, the DIL intervention led to a reduced incidence of major depressive episodes. The DIL intervention did not affect measures of functional status (WHODAS 2.0) or cognition (HMMSE).
Previous studies20,21,22,23 of depression prevention have been conducted in high-income countries, enrolling mixed-age participants. These studies have used brief behavioral or depression-specific psychotherapies, such as cognitive behavioral therapy, interpersonal psychotherapy, and problem-solving therapy, provided by mental health care professionals in primary care, mental health specialty settings, and community settings. None used LCs. A meta-analysis26 of 38 such trials estimated an incident rate reduction of 21% during 1 to 2 years compared with CAU or wait-list control. The DIL intervention results are consistent with those reported from high-income countries, although we observed somewhat fewer incident cases of major depression than expected.
As is the case with many medical and psychological interventions, the DIL intervention is multicomponent—the result of extensive formative research.11 Judging from retention rates, the DIL intervention proved to be acceptable to older participants and implementable by LCs. Qualitative feedback showed that participants engaged in physical and social activities they found pleasurable and distracting from the tension and worry of their daily lives. Booster sessions reinforced practice and perception of benefit from the use of these strategies. Such behavioral activation may be one mechanism by which the positive effects of the DIL intervention were mediated.31
In addition to improving well-being, prevention of depression in vulnerable older adults may ultimately repay benefits to brain and cognitive health because depression is a risk factor for the subsequent onset of dementing illness.6,7 In addition, greater medical multimorbidity is associated with a higher risk of onset of major depression in older adults with subsyndromal depressive symptoms.20,21,22 Our qualitative data (to be reported separately) suggested that the greater frequency of hospitalization appeared to be attributable to encouragement by LCs to seek medical attention, together with education in self-care for chronic diseases. Thus, it is plausible that the greater rate of medical and surgical hospitalizations in the DIL intervention group vs the CAU group reflected modification of the risk posed by medical multimorbidity for the onset of depression.
Limitations
The limitations of the current study include the following: (1) the exclusion of persons with mild cognitive impairment or dementia, who may require a modified approach to the DIL intervention; (2) the limited follow-up period to determine durability of effect; (3) the absence of data on biomarkers of risk for depression to better target the DIL intervention to higher-risk individuals; and (4) the inability to control for nonspecific effects of time and attention.
Conclusions
The success of the DIL intervention extends the earlier work of the MANAS8 and Program for Effective Mental Health Interventions in Underresourced Health Systems (PREMIUM) trials,32 which showed that LCs can effectively treat prevalent cases of depression and anxiety in primary care practice. If the success of the DIL intervention in depression prevention can be replicated in other LMICs, then its utility and scalability would be further supported.24,33 In addition, the cost-effectiveness of the model in LMICs should be studied.33
Trial Protocol
References
- 1.Andrews G, Issakidis C, Sanderson K, Corry J, Lapsley H. Utilising survey data to inform public policy: comparison of the cost-effectiveness of treatment of ten mental disorders. Br J Psychiatry. 2004;184(6):526-533. doi: 10.1192/bjp.184.6.526 [DOI] [PubMed] [Google Scholar]
- 2.Cuijpers P, Beekman AT, Reynolds CF III. Preventing depression: a global priority. JAMA. 2012;307(10):1033-1034. doi: 10.1001/jama.2012.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds CF III, Cuijpers P, Patel V, et al. Early intervention to reduce the global health and economic burden of major depression in older adults. Annu Rev Public Health. 2012;33:123-135. doi: 10.1146/annurev-publhealth-031811-124544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajkumar AP, Thangadurai P, Senthilkumar P, Gayathri K, Prince M, Jacob KS. Nature, prevalence and factors associated with depression among the elderly in a rural south Indian community. Int Psychogeriatr. 2009;21(2):372-378. doi: 10.1017/S1041610209008527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen A, Dias A, Azariah F, et al. Aging and well-being in Goa, India: a qualitative study. Aging Ment Health. 2016;22(2):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828. doi: 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF III. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329-335. doi: 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel V, Weiss HA, Chowdhary N, et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet. 2010;376(9758):2086-2095. doi: 10.1016/S0140-6736(10)61508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dias A, Dewey ME, D’Souza J, et al. The effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: a randomised controlled trial from Goa, India. PLoS One. 2008;3(6):e2333. doi: 10.1371/journal.pone.0002333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries: a systematic review. PLoS One. 2014;9(8):e103754. doi: 10.1371/journal.pone.0103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias A, Azariah F, Health P, et al. Intervention development for the indicated prevention of depression in later life: the “DIL” protocol in Goa, India. Contemp Clin Trials Commun. 2017;6:131-139. doi: 10.1016/j.conctc.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegel M, Arean PA. Problem-Solving Therapy for Primary Care: A Treatment for Depression. NH, Lebanon: Project IMPACT, Dartmouth College; 2003. [Google Scholar]
- 13.Nezu AM, D’Zurilla TJ. Problem-Solving Therapy: A Positive Approach to Clinical Intervention. New York, NY: Springer Publishing Co; 2006. [Google Scholar]
- 14.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887-895. doi: 10.1001/archinternmed.2010.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz RF, Mrazek PJ, Haggerty RJ. Institute of Medicine report on prevention of mental disorders: summary and commentary. Am Psychol. 1996;51(11):1116-1122. doi: 10.1037/0003-066X.51.11.1116 [DOI] [PubMed] [Google Scholar]
- 16.Offord DR, Kraemer HC, Kazdin AE, Jensen PS, Harrington R. Lowering the burden of suffering from child psychiatric disorder: trade-offs among clinical, targeted, and universal interventions. J Am Acad Child Adolesc Psychiatry. 1998;37(7):686-694. doi: 10.1097/00004583-199807000-00007 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg D. General Health Questionnaire (GHQ-12). Windsor, UK: Nfer-Nelson; 1992. [Google Scholar]
- 18.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI): a short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12(5):224231. doi: 10.1016/S0924-9338(97)83297-X [DOI] [Google Scholar]
- 19.Ganguli M, Ratcliff G, Chandra V, et al. A Hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatry. 1995;10(5):367-377. doi: 10.1002/gps.930100505 [DOI] [Google Scholar]
- 20.Reynolds CF III, Thomas SB, Morse JQ, et al. Early intervention to preempt major depression among older black and white adults. Psychiatr Serv. 2014;65(6):765-773. doi: 10.1176/appi.ps.201300216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rovner BW, Casten RJ, Hegel MT, Leiby BE, Tasman WS. Preventing depression in age-related macular degeneration. Arch Gen Psychiatry. 2007;64(8):886-892. doi: 10.1001/archpsyc.64.8.886 [DOI] [PubMed] [Google Scholar]
- 22.Robinson RG, Jorge RE, Moser DJ, et al. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA. 2008;299(20):2391-2400. doi: 10.1001/jama.299.20.2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van’t Veer-Tazelaar P, Smit F, van Hout H, et al. Cost-effectiveness of a stepped care intervention to prevent depression and anxiety in late life: randomised trial. Br J Psychiatry. 2010;196(4):319-325. doi: 10.1192/bjp.bp.109.069617 [DOI] [PubMed] [Google Scholar]
- 24.Alexopoulos GS, Raue P, Areán P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11(1):46-52. doi: 10.1097/00019442-200301000-00007 [DOI] [PubMed] [Google Scholar]
- 25.Chibanda D, Mesu P, Kajawu L, Cowan F, Araya R, Abas MA. Problem-solving therapy for depression and common mental disorders in Zimbabwe: piloting a task-shifting primary mental health care intervention in a population with a high prevalence of people living with HIV. BMC Public Health. 2011;11(1):828. doi: 10.1186/1471-2458-11-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Zoonen K, Buntrock C, Ebert DD, et al. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol. 2014;43(2):318-329. doi: 10.1093/ije/dyt175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963-974. doi: 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- 28.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: John Wiley & Sons Inc; 2006:47-129. [Google Scholar]
- 29.Demyttenaere K, Bruffaerts R, Posada-Villa J, et al. ; WHO World Mental Health Survey Consortium . Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291(21):2581-2590. doi: 10.1001/jama.291.21.2581 [DOI] [PubMed] [Google Scholar]
- 30.Baldwin RC. Preventing late-life depression: a clinical update. Int Psychogeriatr. 2010;22(8):1216-1224. doi: 10.1017/S1041610210000864 [DOI] [PubMed] [Google Scholar]
- 31.Orgeta V, Brede J, Livingston G. Behavioural activation for depression in older people: systematic review and meta-analysis. Br J Psychiatry. 2017;211(5):274-279. [DOI] [PubMed] [Google Scholar]
- 32.Patel V, Weobong B, Weiss HA, et al. The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. Lancet. 2017;389(10065):176-185. doi: 10.1016/S0140-6736(16)31589-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smit F, Ederveen A, Cuijpers P, Deeg D, Beekman A. Opportunities for cost-effective prevention of late-life depression: an epidemiological approach. Arch Gen Psychiatry. 2006;63(3):290-296. doi: 10.1001/archpsyc.63.3.290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol