Abstract
Objective
Surgical treatment of spinal metastasis is generally a palliative procedure. Although minimally invasive surgical (MIS) techniques are supposedly less morbid than open techniques, there is a lack of stratification of MIS techniques based on anticipated longevity. A simple stratification into three percutaneous surgical techniques based on modified Tokuhashi score is here proposed.
Methods
Patients recommended for spinal surgery for metastatic spinal disease between 2009 and 2012 and operated on by the senior author (RJM) were retrospectively reviewed. One of three MIS techniques was offered based on estimated survival using a modified Tokuhashi score. Technique #1 is suitable for patients with predicted short longevity (<6 months). Using a mini‐open midline or paramedian decompression and percutaneous screw fixation, the goal here is for rapid mobilization and minimization of hospitalization. Technique #2 is suitable for patients with predicted medium longevity (6–12 months). They are suitable for decompression and/or cement vertebral body replacement and a two levels stabilization. Technique #3 is suitable for patients with predicted long term survival survival (>12 months). In these patients, the primary goal of surgery is a wide local or marginal resection of tumor, decompression of the neurological elements and a robust stabilization construct. They are suitable for an open 360°decompression, vertebral body reconstruction and a multilevel stabilization.
Results
The study included eight patients with a mean age of 59 years (range, 36–72 years). Mean modified Tokuhashi score was 10 (range, 7–13) with three patients in the short term, two in the medium term and three in the long term survival category. Mean blood loss was 700 mL (range, 100–1200 mL), mean operating time 280 min (range, 120–360 min) and length of stay in the hospital was on average 13 days (range, 3–30 days).
Conclusion
The authors present three minimally invasive technique options for the management of spinal metastatic disease corresponding to three clinical prognostic categories. In this small series, MIS techniques resulted in speedy recovery, minimal morbidity and no mortality.
Keywords: Metastases, Minimally invasive, Morbidity, Percutaneous, Spine
Introduction
Surgical approaches for the management of metastatic spine disease have long been debated. There is a lack of published reports with class 1 evidence concerning whether certain approaches are superior to others in terms of surgical morbidity. Neoplasms of the spine are typically secondary tumors; the skeletal system is the third most common site of metastases and the spine the most common site within this system1.
In most patients with cancer, spinal metastases are present at autopsy2, 3, approximately 30% of them having experienced symptomatic metastatic spinal disease4. Although only a minority (10%) of these progress to epidural spinal cord, conus or cauda equina compression, because of the large numbers involved, metastatic compression of the neural elements is a common occurrence in clinical practice4. Spinal metastases can cause instability manifesting as neurological symptoms and pain, which impact significantly on the patients' quality of life2, 5. Finkelstein et al. reported a median survival time of 227 days for metastatic spine cancer of all primary types 6.
Surgical treatment of spinal metastases is largely palliative, the exception being solitary metastases with certain favorable histologies7. The aim of palliative surgery in such cases is to reduce or eliminate neurologic deficits and improve pain control, thus improving the patient's quality of life. The role of surgery is adjuvant to radiotherapy and/or chemotherapy as indicated by the primary cancer pathology. Surgery for spinal metastases is a contentious issue related largely to the fact that it involves performing significant surgical procedures on those nearing death. Published reports have shown that laminectomies were no more successful historically than radiotherapy, conversely direct decompressive surgery followed by radiotherapy is superior to radiotherapy alone in patients with common metastatic histologies8.
However, the surgery performed must be tailored to the patient's condition and the degree of palliation varies from patient to patient9, 10, 11. For patients with medium‐term prognoses, such as renal or breast cancer primaries, more aggressive surgical techniques, which may entail more radical resections and definitive stabilization, are recommended11. For patients in whom long‐term control is the goal of surgery, Tomita et al. suggest wide excision such as total en bloc spondylectomy11. Medium‐term control may require intra‐lesional excision or perhaps even wide excision. Short‐term or palliative surgery will generally be limited to spinal decompression and stabilization, followed by palliative radiotherapy.
Because of the poor general condition of many of these patients, open spinal decompression with stabilization surgery may be associated with high morbidity rates12, 13, 14. According to published reports, MIS and open techniques have similar effectiveness (neurological recovery and pain alleviation); however, there are no studies of MIS techniques with class 1 evidence (Table 1). In patients with metastatic spine disease, open surgery and complete vertebrectomy may not be necessary: the goal is surgical decompression and stabilization to relieve pain and improve neurological function. More importantly, MIS techniques are associated with reduced operative times, blood loss, length of hospital stay and complication rates, all of which may lead to lower morbidity rates in patient who are in poor general condition. Unfortunately, no studies directly comparing the two techniques have been published.
Table 1.
Studies reporting MIS techniques for spinal metastatic disease: operative data, outcomes and complication rates19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
| Author | No of patients | MBL (mL) | MOT (min) | LOS (d) | MNI (%) | MPA (%) | MCR (%) |
|---|---|---|---|---|---|---|---|
| Lin et al.22 | 25 | 1047 | 324 | NA | 76 | 68 | 4 |
| Tancioni et al.23 (Prospective) | 25 | NA | NA | 6 | 88 | 96 | 12 |
| Zairi et al.20 (Prospective) | 10 | 400 | 170 | 6 | 100 | 100 | 10 |
| Rosenthal et al.21 | 4 | 1450 | 390 | 7.5 | 100 | 100 | 0 |
| Huang et al.25 | 41 (VAST) | 775 | 190 | NA | NA | NA | 54 |
| Huang et al.24 | 29 (MASS) | 1100 | 179 | — | 70.8 | — | 24 |
| Le Huec et al.28 | 2 | 350 | 156 | NA | 100 | 100 | 50 |
| McLain et al.31 | 8 | 1677 | 360 | 6.5 | 100 | 100 | 0 |
| Mobbs et al.26 | 1 | — | — | — | 100 | 100 | 0 |
| Deutsch et al.34 | 8 | 227 | 132 | 4 | 62.5 | 62.5 | 0 |
| Muhlbauer et al.29 | 5 | 1120 | 360 | NA | 100 | 100 | 0 |
| Kan and Schmidt27 | 5 | 610 | 258 | 6.25 | 100 | 100 | 0 |
| Payer and Sottas30 | 11 | 711 | NA | — | 91 | NA | 18 |
| Taghva et al.32 | 1 | 1200 | 420 | 5 | 100 | 100 | 0 |
LOS, length of stay; MASS: minimal access spine surgery; MBL, mean blood loss; MCR, mean complication rate; MNI, median neurologic improvement; MOT, mean operating time; MPA: median pain alleviation rate; VAST, video‐assisted thoracosopic surgery.
Although there are various scoring systems for predicting prognosis and determining optimal extent of resection5, 11, these systems are generalized and not specific to minimally invasive surgical (MIS) techniques. We here propose a simplified means of guiding surgical decisions in patients with spinal metastases that is based on predicted survival and utilizes percutaneous stabilization techniques. We propose stratifying patients into three groups based on predicted survival according to modified Tokuhashi score5; namely, predicted short term (ST; <6 months); medium term (MT; 6–12 months) and long term survival (LT; >12 months). We argue that simple decompression with one level stabilization is appropriate for those in with ST survival, decompression and/or cement vertebral body replacement and two levels stabilization for those with predicted MT survival and comprehensive decompression/radical resection of the tumor and multi‐level stabilization for those with predicted LT survival. We demonstrate this proposal with a small case series and examples of each survival category.
Patients and Methods
Eight patients who had been recommended for spinal surgery for metastatic spinal disease at neuro‐oncology multidisciplinary meetings between 2009 and 2012 and operated on by the senior author (RJM) were retrospectively reviewed (Table 2). One of three MIS techniques was offered based on estimated survival using a modified Tokuhashi score5. Clinical data, particularly neurological status, were compared pre and post operatively, and operative details such as operating time in minutes (OT), blood loss in mL (BL), complications (C), and length of stay in days (LOS) were documented. Survival (S) in months from the time of surgery was also assessed.
Table 2.
Consecutive cases with spine metastases treated with MIS techniques
| Case | Primary | Age (years) | Preoperative Tokuhashi score | pP | PP | AL | PL | MIS | BL | OT | LOS | C | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lung | 58 | 9 | 2 | 4 | T9 | T7–11 | 2 | 800 | 220 | 5 | Nil | 11 |
| 2 | MM † | 72 | 8 | 2 | 4 | T12 | T11‐L1 | 1 | 20 | 300 | 30 | Nil | 56* |
| 3 | Breast | 62 | 7 | 4 | 4 | T8 | T7–9 | 1 | 20 | 120 | 8 | Nil | 3 |
| 4 | Breast | 46 | 10 | 4 | 4 | T4–6 | T3–7 | 2 | 1000 | 300 | 3 | Nil | 43* |
| 5 | GIST | 72 | 7 | 0 | 4 | T9 | T8–10 | 1 | 800 | 180 | 14 | Nil | 13 |
| 6 | RCC | 68 | 13 | 4 | 4 | T6,7 | T5–8 | 3 | 600 | 360 | 10 | Nil | 30 |
| 7 | HP | 56 | 12 | 0 | 4 | T2–4 | T2–4 | 3 | 1200 | 330 | 20 | Nil | 41 |
| 8 | RCC | 36 | 12 | 2 | 4 | L3 | T12‐L4 | 3 | 1000 | 330 | 11 | Inf | 28 |
Note: AL, affected level; HP, hemangiopericytoma; Inf, wound infection; MM, multiple myeloma; PL, percutaneous level; PP, post‐ operative power; pP, pre‐operative power; RCC, renal cell carcinoma. *, patient still alive; †, patient did not receive chemotherapy and radiotherapy.
We will demonstrate these MIS techniques with case examples for each.
Technique #1
Technique #1 is suitable for patients with predicted short longevity (<6 months). In these patients, the primary goal of surgery is decompression of the neurological elements and mechanical stabilization. Using a mini‐open midline or para‐median decompression and percutaneous screw fixation, the goal here is for rapid mobilization and minimization of hospitalization (Fig. 1).
Figure 1.
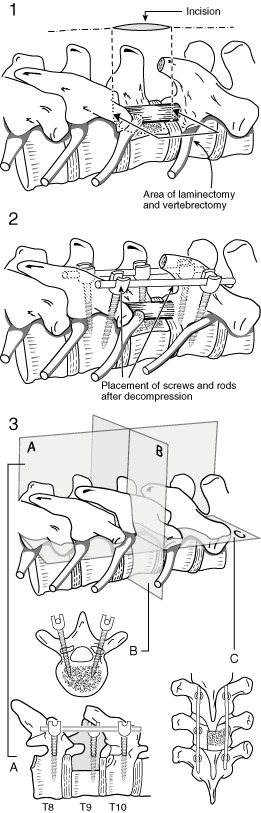
Technique # 1 involves a mini‐open decompression (Part 1) without anterior reconstruction, with a stabilization one level above and below the decompression (Part 2). A, Coronal section; B, Transverse section; C, Sagittal section.
Case #1
A 72‐year‐old man presented with a gastrointestinal stromal tumor (GIST) with multiple metastases, including in the liver, lung and spine. He developed a paraparesis rapidly over 2 weeks (Fig. 2A). According to the oncology team, his likely survival was less than 6 months, based on modified Tokuhashi score5. Surgery was performed at the request of both patient and oncology team. A mini‐open decompression including a unilateral pediculectomy and partial vertebral body resection (Fig. 2B–D) was performed with stabilization one segment above and below the decompression. The patient's neurological status improved and he was able to mobilize with minimal assistance. His length of stay was 3 days. The patient died 13 months post‐procedure from cerebral metastases.
Figure 2.
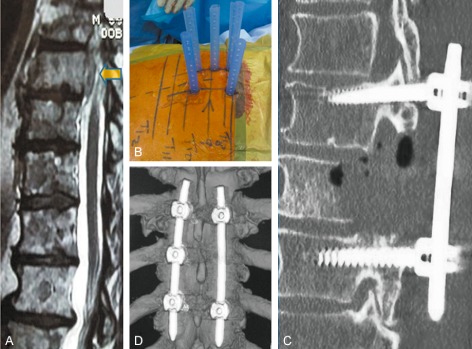
A 72‐year‐old man presented with a gastrointestinal stromal tumor (GIST) with multiple metastases. He developed a paraparesis rapidly over 2 weeks. (A) MRI scan image showing cord compression caused by anterior epidural disease. (B) Intraoperative photograph showing percutaneous sleeves in situ. (C) Post unilateral pediculectomy and decompression. (D) Post‐operative CT scan image.
Technique #2
Technique #2 is suitable for patients with predicted medium longevity (6–12 months). In these patients, the primary goal of surgery is a decompression of the neurological elements and strong stabilization construct. They are suitable for decompression and/or cement vertebral body replacement and a two levels stabilization (Fig. 3).
Figure 3.
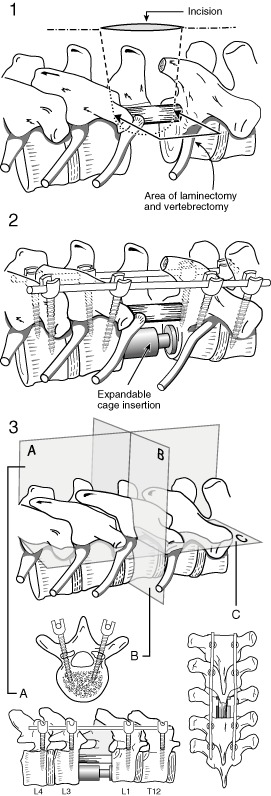
Technique #2 involves a mini‐open decompression and vertebrectomy with or without anterior cement augmented reconstruction (Part 1), with a stabilization two levels above and below the decompression (Part 2). A, Coronal section; B, Transverse section; C, Sagittal section.
Case #2
A 58‐year‐old woman with lung cancer presented with mid‐thoracic pain of 2 months duration, and rapidly developing paraplegia with power 1–2/5 (Medical Research Council [(MRC] grade)14. Investigations revealed a metastatic lesion in T9 with significant cord compression (Fig. 4A,B). Based on discussions with oncologists and assessment of her prognosis5, she was predicted to have MT survival. Therefore the surgical technique aimed at decompressing the affected level, with vertebrectomy plus a two levels stabilization. A mini‐open midline linear incision was made and bilateral pediculectomy and vertebrectomy performed (Fig. 4C). Vertebral reconstruction with cement augmentation was performed (Fig. 4D) and the midline wound closed. Percutaneous pedicle screw stabilization was performed two levels above and below the affected vertebra (Fig. 4E). A postoperative CT scan revealed satisfactory decompression and stabilization (Figs 4F,G). The patient improved to MRC strength 4/5 and was she discharged on postoperative day 5. She underwent radiotherapy and chemotherapy and survived for 11 months.
Figure 4.
A 58‐year‐old woman with lung cancer presented with midthoracic pain of 2 months duration. (A) and (B) MRI images showing circumferential spinal cord compression at T9 level. (C) Intraoperative photograph showing mini‐open midline linear incision with bilateral pediculectomy and vertebrectomy. (D) Post vertebroplasty with cement augmentation. (E) Intraoperative photograph showing percutaneous sleeves in situ. (F) and (G) Post‐operative CT scan images.
Technique #3
Technique #3 is suitable for patients with predicted LT survival (>12 months). In these patients, the primary goal of surgery is a wide local or marginal resection of tumor, decompression of the neurological elements and a robust stabilization construct. They are suitable for an open 360° decompression, vertebral body reconstruction and a multilevel stabilization (Fig. 5).
Figure 5.
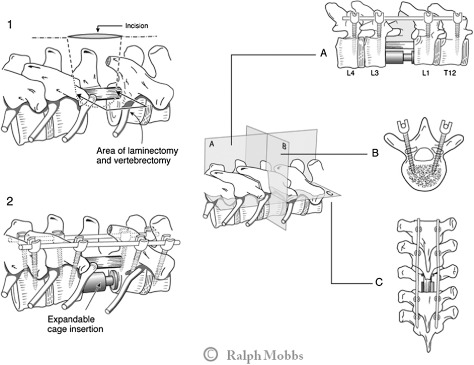
Technique # 3 involves an open decompression and vertebrectomy with anterior vertebral reconstruction (expandable cage) (Part 1), with a stabilization two levels above and below the decompression (Part 2). A, Coronal section; B, Transverse section; C, Sagittal section.
Case 3#
A 32‐year‐old woman presented with mid back pain and leg weakness over 3 weeks. A large renal lesion was identified and removed, the pathological diagnosis being renal cell carcinoma. She had several metastases including in the L3 vertebra and liver. Because of her potential LT survival, a decision was made to perform an L3 vertebrectomy and stabilization. Autologous bone graft and tri‐calcium phosphate bone graft substitute was placed within an L3 expandable cage. Her back pain and leg weakness improved and she returned to independent living. She died of widespread metastatic disease 28 months postoperatively (Fig. 6).
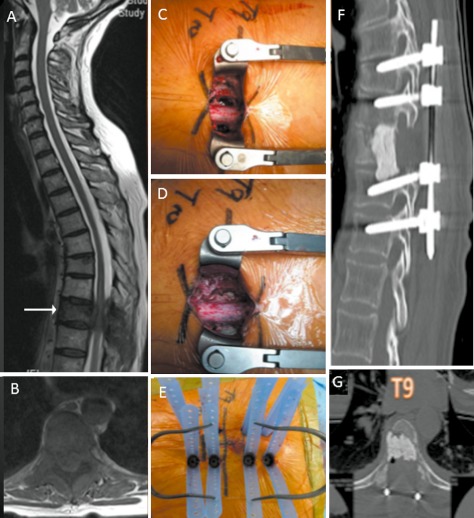
Figure 6.
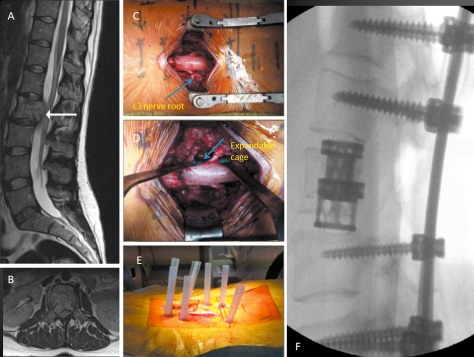
A 32‐year‐old woman presented with mid back pain and leg weakness over 3 weeks. (A) and (B) MRI images showing solitary L2 lesion causing circumferential cauda equina compression. (C) Intraoperative photograph showing midline incision with circumferential decompression and vertebrectomy. Decompressed L3 nerve roots are displayed (arrow). (D) Intraoperative photograph showing expandable cage (arrow) assisted vertebral body reconstruction. (E) Intraoperative photograph showing midline wound closure and percutaneous fixation two levels above and below the vertebrectomy. (F) Post‐operative radiograph.
Results
The study included eight patients (five women and three men) with a mean age of 59 years (range, 36–72 years). Renal cell carcinoma and breast carcinoma were the primary malignancies in two patients each and there was one case each of lung carcinoma, hemangiopericytoma, GIST and malignant melanoma. The mean modified Tokuhashi score was 10 (range, 7–13) with three patients in the ST survival, two in the MT survival and three in the LT survival category. The technique used was type 1 in ST, type 2 in MT and type 3 in LT. The mean blood loss was 700 mL (range, 100–1200 mL), mean operating time 280 min (range, 120–360 min) and mean length of hospital stay 5 days (range, 3–30 days). In the ST group survival was 3 months, 13 months and 56 months, in the MT group 11 and 43 months and in the LT group 28 months, 30 months and 41 months. In three patients preoperative power improved from 2 to 4 (MRC), whereas in one GIST patient it improved from 0 to 4; in the remaining patients it was unchanged at 4 (Table 2).
Discussion
Minimally invasive techniques for spinal fusion have evolved from the original descriptions of lumbar interbody fusion in the 1930's and Cloward's description of posterior lumbar interbody fusion in the 1950's16. Over time innovative procedures such as the transforaminal lumbar interbody fusion and extreme lateral lumbar interbody fusion techniques have aimed to improve access to the lumbar spine while avoiding significant anatomical structures, thus reducing complications. Similarly, the minimally invasive approach aims to reduce the amount of muscle dissection required, therefore reducing post‐operative pain and duration of hospital stay17, 18. In our experience, there is a reduction in blood loss and reduced need for wound drains17. We have also found that the minimally invasive method for percutaneous pedicle screws achieves a higher degree of screw accuracy19. In addition, the duration of exposed and open wounds is shorter, potentially decreasing infection rates, and the wounds are shorter, potentially making radiotherapy safer. All these factors may contribute to an overall improved quality of life, which is particularly important for those with poorer prognoses as described in published reports which lack class I evidence (Table 1)20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33.
Spinal metastases are common among patients with cancer and their consequences can significantly affect the quality of life. Radiotherapy is used to treat almost all patients with symptomatic spinal metastases; the response of any given lesion to radiotherapy being predictable based on histology. However, radiation therapy cannot restore mechanical integrity to a spine compromised by pathologic fracture or impending instability; however, it can ameliorate pain. Once neurologic signs are present, there is level I evidence for the superiority of direct decompressive surgery followed by radiotherapy over radiotherapy alone in patients with common metastatic histologies8. Some authors have reported surgical categorization, particularly in regard to relating aims of surgery to prognosis; the usual surgical categories are palliative, limited excision and wide excision5, 11. However, there is little evidence to support specific surgical techniques. We propose that, because of the associated reduced muscle dissection and subsequent reduced pain and blood loss, greater accuracy, smaller wounds and reduced duration of stay, MIS techniques be prescribed for the treatment of spinal metastases, and that these be placed in three categories based on prognosis.
There are a number of variables relevant to selecting treatment options in patients with symptomatic metastatic spinal disease: diversity of primary tumors, varied metastatic vertebral disease, varied visceral metastasis, a wide range of performance status determined by tumor burden and previous treatments, varied neurological deficits and a wide range of radio‐chemo sensitivity of the tumor itself. Prognostication scores are helpful for guiding surgical treatment5, 11; however, the accuracy of such prediction is around 80% in most series. Hence the decision to operate and the extent of surgery must tailored for each individual patient and their circumstances.
Our preference for MIS techniques is based on our own experience with MIS and open lumbar fusions17 and on published reports concerning MIS for metastatic spine lesions (Table 1). Although there is neither published direct comparisons between MIS and open techniques for metastatic spine disease nor class I evidence for MIS, available reports are biased towards MIS because of the reduction in peri‐operative morbidity and faster recovery.
MIS techniques have several important advantages for spinal decompression and stabilization of epidural spinal cord compression caused by metastases. In such cases, the goals of surgery (open or MIS) are tumor debulking, neuronal decompression and mechanical stabilization, all of which can be achieved readily via MIS procedures. Definitive treatment of these patients relies on chemo‐radiotherapy, which can be started earlier in patients who have undergone MIS because wound and general recovery are quicker than with open procedures. Although circumferential compression is common, metastatic disease usually occurs in the vertebral body. The commonest MIS approach is posterior, however anterior or lateral approaches can be utilized either as a standalone or in combination with percutaneous posterior pedicle screw stabilization. Another advantage of MIS techniques is faster wound healing that is more resilient to radiotherapy‐induced wound breakdown. It is commonly acknowledged that prolonged operative time is associated with increased infection rates and blood loss. In addition, blood transfusion is associated with risk of systemic infection, gastrointestinal complaints, and hemolytic reactions34. Thus, with the minimal exposed surgical corridor in MIS procedures, blood loss is reduced and the need for transfusion lessened. Additionally, although historically a criticism of MIS technique has been longer operating times, because of the minimal exposure operating times are in fact reduced35, 36. Therefore, the morbidity of spine surgery in patients with metastasis‐related neuronal compression can be minimized by using MIS techniques that reduce operating time, blood loss, need for blood transfusion and iatrogenic muscle injury and encourage wound healing, thus allowing patients to resume life‐saving adjuvant therapies earlier than after conventional open surgery.
The “minimization” here is based on the prognosis of the patient (modified Tokuhashi score) and the goal(s) of surgery. Patients with predicted short longevity (<6 months) can be offered simple decompression followed by two‐level stabilization. With predicted medium longevity (6–12 months), we recommend more rigorous decompression and attempts to reconstruct anteriorly with minimal effort but a stable construct. In patients with a reasonably long prognosis (>12 months), we recommend making every effort to resect the tumor and provide a robust stabilization construct. The aims are as follows: for short longevity patients to improve quality of life in terms of pain and mobility while minimizing operative morbidity and mortality, whereas for predicted long‐term survivors surgery is aimed at good local tumor control and robust stability capable of withstanding stresses for a long time.
In this small series there was no perioperative mortality, as compared with open surgical series with surgical mortality of 4%–7.6%8, 12, 13, 15. Morbidity rates in open surgery are reportedly 20%–25% across various studies12, 13, 15: in our series the rate was 1/8 (wound infection). Mean operative times were 280 min and mean blood loss 700 mL, similar to other MIS studies20. Short hospital stays in this series (13 days average) is also similar to other MIS studies20 indicating that patients universally recover rapidly, which is one of the goals of palliative but effective surgery. Modified Tokuhashi scores were accurate in 5/8 patients. One malignant melanoma patient survived longer than 56 months and, at the most recent follow‐up, a patient with metastasis from breast cancer was still alive 43 months after surgery.
Limitations
The proposed stratification can serve as a guide; further validation in terms of effectiveness and outcomes is required. As previously mentioned, the pathology of metastatic spine disease, patients' performance status and sensitivity to adjuvant treatment varies, as do patient preferences. Hence, bias is unavoidable in any stratification involving such a varied cohort unless well powered prospective studies are undertaken. This case series is small and hence too underpowered to make any definitive conclusions.
Conclusions
The authors present several minimally invasive options for managing spinal metastatic disease, using percutaneous fixation techniques combined with mini‐open approaches to decompress neurological structures. We propose a simple stratification into three percutaneous surgical techniques corresponding with three clinical prognostic categories. The techniques are tailored to the expected longevity of the patient and attempt to reach a balance between invasiveness and effectiveness to manage each patient's unique presentation.
Level of Evidence: Therapeutic Level IV.
Disclosure: No financial support was obtained for this work.
References
- 1. Katagiri H, Takahashi M, Inagaki J, et al Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys, 1998, 42: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 2. Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976), 1990, 15: 1–4. [PubMed] [Google Scholar]
- 3. Lenz M, Freid JR. Metastases to the skeleton, brain and spinal cord from cancer of the breast and the effect of radiotherapy. Ann Surg, 1931, 93: 278–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol, 2006, 15: 141–151. [DOI] [PubMed] [Google Scholar]
- 5. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976), 2005, 30: 2186–2191. [DOI] [PubMed] [Google Scholar]
- 6. Finkelstein JA, Zaveri G, Wai E, Vidmar M, Kreder H, Chow E. A population‐based study of surgery for spinal metastases. Survival rates and complications. J Bone Joint Surgery Br, 2003, 85: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 7. Penas‐Prado M, Loghin ME. Spinal cord compression in cancer patients: review of diagnosis and treatment. Curr Oncol Rep, 2008, 10: 78–85. [DOI] [PubMed] [Google Scholar]
- 8. Patchell RA, Tibbs PA, Regine WF, et al Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet, 2005, 366: 643–648. [DOI] [PubMed] [Google Scholar]
- 9. Gasbarrini A, Cappuccio M, Mirabile L, et al Spinal metastases: treatment evaluation algorithm. Eur Rev Med Pharmacol Sci, 2004, 8: 265–274. [PubMed] [Google Scholar]
- 10. Tokuhashi Y, Ajiro Y, Oshima M. Algorithms and planning in metastatic spine tumors. Orthop Clin North Am, 2009, 40: 37–46, v–vi. [DOI] [PubMed] [Google Scholar]
- 11. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976), 2001, 26: 298–306. [DOI] [PubMed] [Google Scholar]
- 12. Ibrahim A, Crockard A, Antonietti P, et al Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine, 2008, 8: 271–278. [DOI] [PubMed] [Google Scholar]
- 13. Wang JC, Boland P, Mitra N, et al Single‐stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine, 2004, 1: 287–298. [DOI] [PubMed] [Google Scholar]
- 14. Dyck PJ, Boes CJ, Mulder D, et al History of standard scoring, notation, and summation of neuromuscular signs. A current survery and recommendation. Peripher Nerv Syst, 2005, 10: 158–173. [DOI] [PubMed] [Google Scholar]
- 15. Quan GM, Vital JM, Aurouer N, et al Surgery improves pain, function and quality of life in patients with spinal metastases: a prospective study on 118 patients. Eur Spine J, 2011, 20: 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen FH, Samartzis D, Khanna AJ, Anderson DG. Minimally invasive techniques for lumbar interbody fusions. Orthop Clin North Am, 2007, 38: 373–386. [DOI] [PubMed] [Google Scholar]
- 17. Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci, 2012, 19: 829–835. [DOI] [PubMed] [Google Scholar]
- 18. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery, 2010, 66: 296–304; discussion. [DOI] [PubMed] [Google Scholar]
- 19. Raley DA, Mobbs RJ. Retrospective computed tomography scan analysis of percutaneously inserted pedicle screws for posterior transpedicular stabilization of the thoracic and lumbar spine: accuracy and complication rates. Spine (Phila Pa 1976), 2012, 37: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 20. Molina CA, Gokaslan ZL, Sciubba DM. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int J Surg Oncol, 2011, 2011: 598148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zairi F, Arikat A, Allaoui M, Marinho P, Assaker R. Minimally invasive decompression and stabilization for the management of thoracolumbar spine metastasis. J Neurosurg Spine, 2012, 17: 19–23. [DOI] [PubMed] [Google Scholar]
- 22. Rosenthal D, Marquardt G, Lorenz R, Nichtweiss M. Anterior decompression and stabilization using a microsurgical endoscopic technique for metastatic tumors of the thoracic spine. J Neurosurg, 1996, 84: 565–572. [DOI] [PubMed] [Google Scholar]
- 23. Lin F, Yamaguchi U, Matsunobu T, et al Minimally invasive solid long segmental fixation combined with direct decompression in patients with spinal metastatic disease. Int J Surg, 2013, 11: 173–177. [DOI] [PubMed] [Google Scholar]
- 24. Tancioni F, Navarria P, Pessina F, et al Early surgical experience with minimally invasive percutaneous approach for patients with metastatic epidural spinal cord compression (MESCC) to poor prognoses. Ann Surg Oncol, 2012, 19: 294–300. [DOI] [PubMed] [Google Scholar]
- 25. Huang TJ, Hsu RW, Li YY, Cheng CC. Minimal access spinal surgery (MASS) in treating thoracic spine metastasis. Spine (Phila Pa 1976), 2006, 31: 1860–1863. [DOI] [PubMed] [Google Scholar]
- 26. Huang TJ, Hsu RW, Sum CW, Liu HP. Complications in thoracoscopic spinal surgery: a study of 90 consecutive patients. Surg Endosc, 1999, 13: 346–350. [DOI] [PubMed] [Google Scholar]
- 27. Mobbs RJ, Nakaji P, Szkandera BJ, Teo C. Endoscopic assisted posterior decompression for spinal neoplasms. J Clin Neurosci, 2002, 9: 437–439. [DOI] [PubMed] [Google Scholar]
- 28. Kan P, Schmidt MH. Minimally invasive thoracoscopic approach for anterior decompression and stabilization of metastatic spine disease. Neurosurg Focus, 2008, 25: E8. [DOI] [PubMed] [Google Scholar]
- 29. Le Huec JC, Lesprit E, Guibaud JP, Gangnet N, Aunoble S. Minimally invasive endoscopic approach to the cervicothoracic junction for vertebral metastases: report of two cases. Eur Spine J, 2001, 10: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mühlbauer M, Pfisterer W, Eyb R, Knosp E. Minimally invasive retroperitoneal approach for lumbar corpectomy and anterior reconstruction. Technical note. J Neurosurg, 2000, 93 (1 Suppl.): S161–S167. [DOI] [PubMed] [Google Scholar]
- 31. Payer M, Sottas C. Mini‐open anterior approach for corpectomy in the thoracolumbar spine. Surg Neurol, 2008, 69: 25–31; discussion 31–32. [DOI] [PubMed] [Google Scholar]
- 32. McLain RF. Spinal cord decompression: an endoscopically assisted approach for metastatic tumors. Spinal Cord, 2001, 39: 482–487. [DOI] [PubMed] [Google Scholar]
- 33. Taghva A, Li KW, Liu JC, Gokaslan ZL, Hsieh PC. Minimally invasive circumferential spinal decompression and stabilization for symptomatic metastatic spine tumor: technical case report. Neurosurgery, 2010, 66: E620–E622. [DOI] [PubMed] [Google Scholar]
- 34. Pull ter Gunne AF, Skolasky RL, Ross H, van Laarhoven CJ, Cohen DB. Influence of perioperative resuscitation status on postoperative spine surgery complications. Spine J, 2010, 10: 129–135. [DOI] [PubMed] [Google Scholar]
- 35. Deutsch H, Boco T, Lobel J. Minimally invasive transpedicular vertebrectomy for metastatic disease to the thoracic spine. J Spinal Disord Tech, 2008, 21: 101–105. [DOI] [PubMed] [Google Scholar]
- 36. Schwab JH, Gasbarrini A, Cappuccio M, et al Minimally invasive posterior stabilization improved ambulation and pain scores in patients with plasmacytomas and/or metastases of the spine. Int J Surg Oncol, 2011, 2011: 239230. [DOI] [PMC free article] [PubMed] [Google Scholar]


