Key Points
Question
Is there an association between prescription of a renin-angiotensin system (RAS) inhibitor at the time of hospital discharge after transcatheter aortic valve replacement (TAVR) and subsequent mortality or readmission for heart failure?
Findings
In this registry-based retrospective cohort study that included 15 896 propensity-matched patients who underwent TAVR, receiving a prescription for a RAS inhibitor at hospital discharge vs no prescription was associated with a risk for mortality of 12.5% vs 14.9%, respectively, and a risk for heart failure readmission of 12.0% vs 13.8%; both differences were statistically significant.
Meaning
Use of a RAS inhibitor after TAVR may be associated with lower mortality and risk for heart failure readmission, but the potential for selection bias requires further investigation.
Abstract
Importance
Data are lacking on the effect of a renin-angiotensin system (RAS) inhibitor prescribed after transcatheter aortic valve replacement (TAVR). Treatment with a RAS inhibitor may reverse left ventricular remodeling and improve function.
Objective
To investigate the association of prescription of a RAS inhibitor and outcomes after TAVR.
Design, Setting, and Participants
Retrospective cohort study of TAVR procedures performed in the United States (using the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry) between July 2014 and January 2016 that were linked to Medicare claims data (final date of follow-up: March 31, 2017). To account for differences in demographics, echocardiographic findings, and in-hospital complications, 1:1 propensity matching was performed.
Exposures
Initial hospital discharge prescription of a RAS inhibitor after TAVR.
Main Outcomes and Measures
Primary outcomes were all-cause death and readmission due to heart failure at 1 year after discharge, which were considered separately. The secondary outcome was health status assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ; score range: 0-100, with a higher score indicating less symptom burden and better quality of life; a small effect size was defined as 5 points) at 1 year.
Results
Among 21 312 patients who underwent TAVR at 417 US sites, 8468 patients (39.7%) were prescribed a RAS inhibitor at hospital discharge. After propensity matching, 15 896 patients were included (mean [SD] age, 82.4 [6.8] years; 48.1% were women; mean [SD] left ventricular ejection fraction [LVEF], 51.9% [11.5%]). Patients with a prescription for a RAS inhibitor vs those with no prescription had lower mortality rates at 1 year (12.5% vs 14.9%, respectively; absolute risk difference [ARD], −2.4% [95% CI, −3.5% to −1.4%]; hazard ratio [HR], 0.82 [95% CI, 0.76 to 0.90]) and lower heart failure readmission rates at 1 year (12.0% vs 13.8%; ARD, −1.8% [95% CI, −2.8% to −0.7%]; HR, 0.86 [95% CI, 0.79 to 0.95]). When stratified by LVEF, having a prescription for a RAS inhibitor vs no prescription was associated with lower 1-year mortality among patients with preserved LVEF (11.1% vs 13.9%, respectively; ARD, −2.81% [95% CI, −3.95% to −1.67%]; HR, 0.78 [95% CI, 0.71 to 0.86]), but not among those with reduced LVEF (18.8% vs 19.5%; ARD, −0.68% [95% CI, −3.52% to 2.20%]; HR, 0.95 [95% CI, 0.81 to 1.12]) (P = .04 for interaction). Of 15 896 matched patients, 4837 (30.4%) were included in the KCCQ score analysis and improvements at 1 year were greater in patients with a prescription for a RAS inhibitor vs those with no prescription (median, 33.3 [interquartile range, 14.2 to 51.0] vs 31.3 [interquartile range, 13.5 to 51.1], respectively; difference in improvement, 2.10 [95% CI, 0.10 to 4.06]; P < .001), but the effect size was not clinically meaningful.
Conclusions and Relevance
Among patients who underwent TAVR, receiving a prescription for a RAS inhibitor at hospital discharge compared with no prescription was significantly associated with a lower risk of mortality and heart failure readmission. However, due to potential selection bias, this finding requires further investigation in randomized trials.
This cohort study uses registry and Medicare claims data to investigate associations between prescription for a renin-angiotensin system (RAS) inhibitor at hospital discharge after transcatheter aortic valve replacement (TAVR) and 1-year all-cause mortality and heart failure readmissions.
Introduction
Aortic stenosis is a common valve disease in the aging population and comprises one of the major cardiovascular morbidities among older patients.1 Chronic pressure overload due to aortic stenosis promotes left ventricular remodeling through muscle fiber hypertrophy and abnormalities of the collagen network, and results in diastolic dysfunction and increased risk of heart failure.2
Inhibition of the renin-angiotensin system (RAS) with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) is associated with modulation of adverse left ventricular remodeling and reduction in myocardial hypertrophy and fibrosis, leading to clinical improvement in patients with heart failure and reduced left ventricular ejection fraction (LVEF).3,4,5 Some observational studies support the association of the use of a RAS inhibitor and improvement in clinical outcomes among patients with aortic stenosis or surgical aortic valve replacement (SAVR).6,7 There is no specific recommendation for the use of RAS inhibitor therapy in patients with aortic stenosis. However, most patients with aortic stenosis, who have a high prevalence of hypertension, coronary artery disease, and renal dysfunction, are eligible for a prescription for a RAS inhibitor.
Transcatheter aortic valve replacement (TAVR) is a proven treatment option for patients with aortic stenosis considered to have an intermediate, high, or extreme risk for morbidity and mortality with SAVR.8,9,10,11 Despite improving outcomes and devices, among patients in a registry who underwent TAVR from November 2011 through June 2013, 14.3% were readmitted to the hospital due to heart failure and 23.7% died within 1 year after the procedure.12 Given the biological mechanisms of RAS inhibitors that modulate adverse left ventricular remodeling and regression of myocardial hypertrophy, RAS inhibitor therapy may potentially improve clinical outcomes in patients undergoing TAVR; however, little is known about the role of RAS inhibitor therapy after TAVR. Therefore, the aim of this study was to evaluate the association between prescription of a RAS inhibitor and clinical outcomes, including mortality, heart failure readmission, and health status after TAVR.
Methods
The Chesapeake Research Review Incorporated institutional review board and the Duke University institutional review board granted a waiver of informed consent for the Transcatheter Valve Therapy (TVT) Registry protocol. This study was approved by the institutional review board of Duke University.
Data Source
The cohort for this analysis was derived from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. The rationale, design, and methods of the TVT Registry have been published.13 Briefly, the TVT Registry was launched in December 2011 by the Society of Thoracic Surgeons and the American College of Cardiology, and facilities are required to participate in the TVT Registry as part of the Centers for Medicare & Medicaid Services national coverage decision for TAVR. As a result, the TVT Registry contains the majority of TAVR procedures performed in the United States regardless of the type of insurance; however, TAVR procedures performed during clinical trials are not included in the registry.
For this study, we restricted the patient population to those older than 65 years with Medicare to ensure complete 1-year follow-up. The registry data elements characterize the patients, the procedure, and the subsequent outcomes. Details of the elements in the TVT Registry data collection form and definitions appear on the TVT Registry website (https://www.ncdr.com/webncdr/tvt/home/data-collection). To optimize data completeness and accuracy, quality checks were performed at the American College of Cardiology National Cardiovascular Data Registry data warehouse and at the Duke Clinical Research Institute.
Study Population
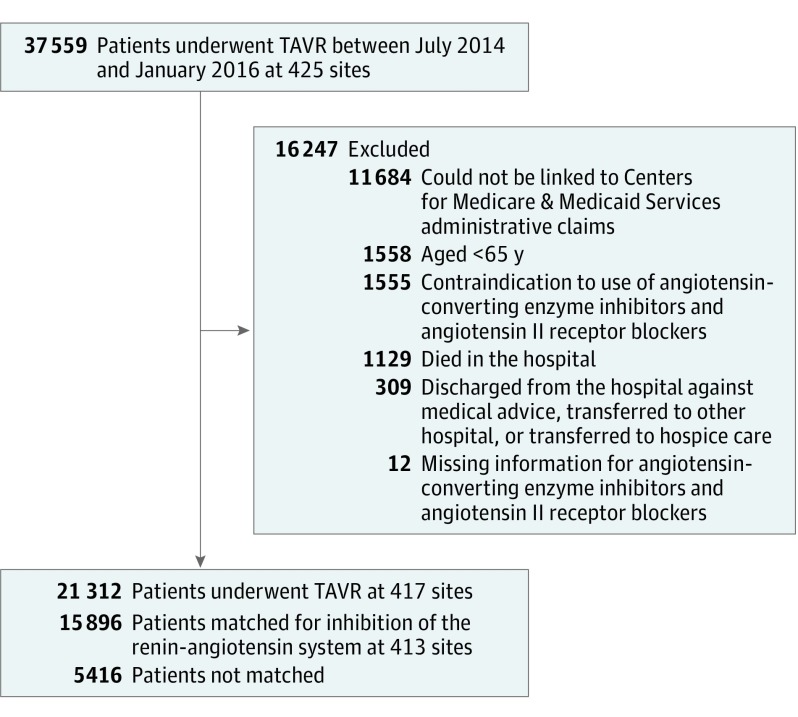
For the purpose of this analysis, we included all consecutive patients older than 65 years with Medicare who underwent TAVR between July 2014 and January 2016 (Figure 1). The rationale for restricting the enrollment period was to reflect a more contemporary TAVR practice and to increase data completeness.
Figure 1. Cohort Creation for Study of Renin-Angiotensin Inhibitor Treatment After TAVR.
Additional exclusion criteria were applied to create a quality-of-care analysis cohort (eFigure 4 in the Supplement). TAVR indicates transcatheter aortic valve replacement.
We excluded patients who (1) died during the index hospitalization, (2) were discharged against medical advice, transferred to another acute care hospital, or transferred to hospice care, (3) had a contraindication to the use of both ACE inhibitors and ARBs or had missing data for prescription of both ACE inhibitors and ARBs, (4) were younger than 65 years, or (5) could not be linked to Centers for Medicare & Medicaid Services administrative claims.
Data on race/ethnicity were included as a variable in this study because prior studies have suggested differences in outcomes after undergoing TAVR related to race/ethnicity. Data on race/ethnicity included the categories of black, Hispanic, Asian, other (American Indian/Alaskan Native, Native Hawaiian/Pacific Islander), and white. Hospital admission staff, medical staff, or both recorded the patient’s self-reported race/ethnicity at the index hospital admission.
Exposure
The exposure was prescription of a RAS inhibitor at hospital discharge. Discharge medications including ACE inhibitors and ARBs were captured on the TVT Registry data collection form; however, data on the specific type and dose were not collected. Patients who were prescribed either ACE inhibitors or ARBs (regardless of type and dose), or both, at hospital discharge after TAVR constituted the group who received a prescription for a RAS inhibitor. Patients who were not prescribed ACE inhibitors or ARBs constituted the control (no prescription) group. Medication adherence was not assessed within this study or within the TVT Registry. The registry database does not include information on medications at hospital admission or preoperative medications.
Primary Outcomes
The primary outcomes were all-cause mortality within 1 year after hospital discharge and readmission due to heart failure within 1 year after discharge. The primary outcomes were evaluated individually and identified using the Medicare Denominator File and in-hospital administrative claims files (eAppendix 1 in the Supplement).
Secondary Outcomes
One-year health status was evaluated as a secondary outcome. In the TVT Registry, the 12-item shortened version of the Kansas City Cardiomyopathy Questionnaire (KCCQ) was collected at baseline, at 30 days, and at 1 year after TAVR to assess health-related quality of life. The KCCQ is a patient-reported, disease-specific health status instrument that was originally developed for patients with heart failure.14 More recently, the KCCQ was validated in patients with severe aortic stenosis.15
The range for the KCCQ overall summary (KCCQ-OS) score is from 0 to 100 (higher scores indicate less symptom burden and better quality of life). A change in the KCCQ-OS score of 5 points corresponds to small clinical improvements; 10 points, moderate improvements; and 20 points, large improvements.16,17 In this analysis, to integrate quality-of-life outcomes with survival, a favorable outcome was defined as survival with a reasonable quality of life (KCCQ-OS score ≥60, roughly equivalent to New York Heart Association class I-II symptoms15,16) without any meaningful worsening (decrease of ≥10 points in the KCCQ-OS score from baseline to 1 year).17
Post Hoc Outcomes
Myocardial infarction at 1 year after hospital discharge was evaluated as a post hoc outcome. In addition, landmark post hoc analyses at 30 days after hospital discharge were performed and any association by type of prescription for a RAS inhibitor (ACE inhibitor vs ARB) and outcome was evaluated.
Statistical Analysis
Baseline characteristics were compared between patients by presence vs absence of a prescription for a RAS inhibitor at hospital discharge. For continuous variables, means and standard deviations were calculated and compared using the Wilcoxon test. Binary variables are reported as counts and percentages and between-group differences were assessed using the χ2 test.
To account for potential treatment bias between those discharged from the hospital with a prescription for a RAS inhibitor vs those discharged from the hospital with no prescription, 1:1 propensity score matching was performed. A logistic regression model was used to estimate the propensity for receipt of a prescription for a RAS inhibitor. Covariates in the model were derived from the list of baseline covariates considered for the TVT Registry in-hospital and 30-day mortality models18,19 and according to clinical expertise. The full list of covariates appears in eAppendix 2 in the Supplement. The distributions of propensity scores appear in eFigure 1 in the Supplement. Greedy matching (on the basis of the estimated propensity score) was performed on the logit of the propensity score with a caliper width of 0.2 times the pooled SD of the logit of the propensity scores for the cohort. Balance between the groups was assessed by calculating standardized differences for which a difference of less than 0.10 was considered to indicate good balance.
The log-rank test and Cox proportional hazards models were used to evaluate the association of prescription of a RAS inhibitor with all-cause mortality in the matched cohort. For heart failure readmission, the proportional subdistribution hazards model of Fine and Gray was used to account for the competing risk of death. Furthermore, to evaluate the potential for residual confounding, any association between prescription of a RAS inhibitor and any of the falsification end points was tested, including hip fracture, urinary tract infection, and pneumonia (eAppendix 1 in the Supplement). These end points served as negative controls because the rates of hip fracture, urinary tract infection, and pneumonia are not likely to be influenced by the selection of a prescription for a RAS inhibitor and should theoretically be the same in the absence of residual confounding. The marginal model approach was used to account for hospital clustering.20 For all 1-year outcomes, the proportionality assumption was tested and confirmed by including a prescription for a RAS inhibitor × time interaction term.
The relationship between prescription of a RAS inhibitor after TAVR and cardiovascular outcomes was hypothesized to be influenced by LVEF and renal function. Therefore, interactions were tested between a prescription for a RAS inhibitor and LVEF (>40% vs ≤40%) or renal function (not receiving dialysis and estimated glomerular filtration rate [eGFR] ≥60 mL/min/1.73 m2 vs not receiving dialysis and eGFR between 30 and 60 mL/min/1.73 m2 vs receiving dialysis or eGFR <30 mL/min/1.73 m2). The interaction testing was prespecified. For cases in which significant effect modification was found, a subgroup analysis was performed. In the subgroup analysis, predicted probabilities for prescription of a RAS inhibitor were reestimated for each subgroup and propensity-matched cohorts were generated using the same methods as for the entire cohort.
There were missing data for less than 3% of the patients for the baseline variables. There were greater percentages of missing data for albumin (11.6%), left ventricular end systolic diameter (14.1%), left ventricular end diastolic diameter (11.7%), mean aortic gradient (16.3%), aortic regurgitation (15.1%), mitral regurgitation (15.8%), and 5-meter walk time (15.3%). Because a previous study demonstrated that the prognosis of patients who were not given the walk test was similar to that of patients in the slowest category (walk time >10 seconds),21 the following categories for 5-meter walk test were used: walk time of 6 seconds or less (normal), walk time of greater than 6 seconds to 10 seconds (slow), and walk time greater than 10 seconds (slowest) or unable to walk.
For all other variables, missing data in the multivariable models were addressed using multiple imputation. Specifically, missing data for categorical variables were imputed using the fully conditional method with the discriminant function, allowing all continuous and categorical variables to be predictors for imputation. Continuous variables were imputed using the predictive method for mean matching, which generates imputed variables consistent with observed values. A total of 5 data sets were created during the imputation phase. These data sets were analyzed separately and estimates from each imputed data set were pooled into a single set of statistics.
Quality-of-Life Analysis
The rate of missing KCCQ data was 55.2% either at baseline or at 1-year follow-up. Because data for the KCCQ was not missing at random, the analysis was limited to sites with completion rates of at least 50% for the KCCQ at baseline and 1-year follow-up to ensure a representative cohort of patients was examined.17 Furthermore, to reduce the influence of ascertainment bias, the inverse probability weighting framework was used to increase the weight of available patients who were the most similar to those with missing follow-up data.17,22 This process involved constructing a multivariable logistic regression model to determine the probability of having missing follow-up KCCQ data.
In addition to treatment group, the model included the same variables that were used in the model estimating the propensity score for prescription of a RAS inhibitor. To better reflect the overall TAVR population, each of the patients with KCCQ data at baseline and at 1-year follow-up were weighted by the inverse probability of the likelihood of having follow-up KCCQ data. Changes in the KCCQ-OS score at 1 year from baseline were compared using an analysis of covariance to account for the differences in the KCCQ-OS score at baseline. To determine if a favorable outcome had been reached, the adjusted odds ratio of a prescription for a RAS inhibitor was estimated using a logistic regression model that included the same variables used in the model to estimate the propensity score for prescription of a RAS inhibitor.
Post Hoc Analyses
To evaluate the association of a prescription for a RAS inhibitor with myocardial infarction in the matched cohort, the proportional subdistribution hazards model of Fine and Gray was used to account for the competing risk of death. Landmark analyses were conducted and the data were displayed using a 30-day landmark period with 1 set of curves for days 0 to 30 and a second set for days 30 to 365. To evaluate any association by type of RAS inhibitor (ACE inhibitor vs ARB) and outcomes, matched patients (main cohort) were stratified by type of RAS inhibitor and hazard ratios (HRs) for the 2 primary outcomes were calculated using the proportional subdistribution hazards model by Fine and Gray.
The between-group standardized differences (patients discharged from the hospital with a prescription for an ACE inhibitor vs those discharged from the hospital with no prescription or patients discharged from the hospital with a prescription for an ARB vs those discharged from the hospital with no prescription) were checked and further adjustment was made for covariates with standardized differences that were greater than 0.1.
All P values were 2-sided and P < .05 was considered significant for all tests. No adjustment for multiple testing was undertaken. As a result, the secondary and post hoc analyses should be interpreted as exploratory. The deidentified, aggregate data were analyzed by the Duke Clinical Research Institute using SAS software version 9.4 (SAS Institute Inc).
Results
Baseline Characteristics
Between July 2014 and January 2016, a total of 37 559 patients underwent TAVR at 425 US sites. After exclusion criteria were applied, 21 312 eligible patients from 417 US sites were identified and included in the overall analysis (Figure 1). Of these patients, 8468 (39.7%) were prescribed a RAS inhibitor at the time of hospital discharge. The baseline characteristics of patients who were included vs those who were excluded were not different in a clinically meaningful way (eTable 1 in the Supplement).
The baseline characteristics by RAS inhibitor prescription status (yes vs no) appear in Table 1. Patients with a prescription for a RAS inhibitor had a higher prevalence of atherosclerotic comorbidities compared with patients with no prescription. Patients with a prescription for a RAS inhibitor had lower LVEF, were more likely to be prescribed a concomitant β-blocker, and were less likely to have a higher Society of Thoracic Surgeons Predicted Risk of Mortality score compared with patients with no prescription. Among patients with a prescription for a RAS inhibitor at hospital discharge, the rates of moderate or severe mitral regurgitation and new requirement of dialysis after the TAVR procedure were lower than among patients with no prescription.
Table 1. Baseline Characteristics Before and After Propensity Score Matching.
| Characteristics | Before Propensity Score Matching | SMD | After Propensity Score Matchinga | SMD | ||
|---|---|---|---|---|---|---|
| RAS Inhibitor Prescription (n = 8468) |
No Prescription (n = 12 844) |
RAS Inhibitor Prescription (n = 7948) |
No Prescription (n = 7948) |
|||
| Angiotensin-converting enzyme inhibitor, No. (%) | ||||||
| Taking one | 5641 (66.6) | 0 | 5249 (66.0) | 0 | ||
| Had contraindication to use | 85 (1.0) | 467 (3.6) | 79 (1.0) | 264 (3.3) | ||
| Angiotensin II receptor blocker, No. (%) | ||||||
| Taking one | 2956 (34.9) | 0 | 2820 (35.5) | 0 | ||
| Had contraindication to use | 27 (0.3) | 51 (0.4) | 25 (0.3) | 33 (0.4) | ||
| Age, mean (SD), y | 82.3 (6.8) | 82.9 (6.9) | −0.0797 | 82.4 (6.8) | 82.4 (6.9) | 0.004 |
| Body mass index, mean (SD)b | 28.4 (6.6) | 27.8 (6.4) | 0.0848 | 28.3 (6.6) | 28.3 (6.6) | −0.003 |
| Sex, No. (%) | ||||||
| Female | 3983 (47.0) | 6087 (47.4) | −0.0069 | 3792 (47.7) | 3847 (48.4) | 0.378 |
| Male | 4485 (53.0) | 6757 (52.6) | 4156 (52.3) | 4101 (51.6) | ||
| Society of Thoracic Surgeons Predicted Risk of Mortality scorec | ||||||
| Mean (SD), % | 7.4 (5.0) | 8.3 (6.1) | −0.1566 | 7.5 (5.1) | 7.5 (5.0) | 0.004 |
| <4% (low risk), No. (%) | 1935 (22.9) | 2663 (20.7) | 0.1335 | 1792 (22.5) | 1813 (22.8) | 0.02 |
| 4%-<8% (intermediate risk), No. (%) | 3778 (44.6) | 5306 (41.3) | 3528 (44.4) | 3509 (44.1) | ||
| 8%-<15% (high risk), No. (%) | 2108 (24.9) | 3478 (27.1) | 1996 (25.1) | 2040 (25.7) | ||
| ≥15% (prohibitive risk), No. (%) | 647 (7.6) | 1397 (10.9) | 632 (8.0) | 586 (7.4) | ||
| Medical history, No. (%) | ||||||
| CABG surgery | 2515 (29.7) | 3251 (25.3) | 0.0989 | 2286 (28.8) | 2269 (28.5) | 0.005 |
| Aortic valve procedure | 1041 (12.3) | 1737 (13.5) | −0.0368 | 993 (12.5) | 996 (12.5) | −0.001 |
| Permanent pacemaker or ICD | 1539 (18.2) | 2484 (19.3) | −0.0298 | 1415 (17.8) | 1443 (18.2) | −0.009 |
| Atrial fibrillation or flutter | 3358 (39.7) | 5657 (44.0) | −0.0896 | 3180 (40.0) | 3165 (39.8) | 0.003 |
| Stroke | 1052 (12.4) | 1510 (11.8) | 0.0204 | 979 (12.3) | 1004 (12.6) | −0.01 |
| Transient ischemic attack | 759 (9.0) | 1145 (8.9) | 0.0019 | 718 (9.0) | 720 (9.1) | <0.001 |
| Peripheral arterial disease | 2585 (30.5) | 3807 (29.6) | 0.019 | 2424 (30.5) | 2421 (30.5) | <0.001 |
| Hypertension | 7950 (93.9) | 11 289 (87.9) | 0.2091 | 7439 (93.6) | 7403 (93.1) | 0.02 |
| Diabetes mellitus | 3371 (39.8) | 4377 (34.1) | 0.1185 | 3077 (38.7) | 3074 (38.7) | <0.001 |
| Chronic lung disease | 2090 (24.7) | 3516 (27.4) | −0.0629 | 1980 (24.9) | 1959 (24.6) | 0.005 |
| Myocardial infarction | 2104 (24.8) | 2966 (23.1) | 0.0414 | 1920 (24.2) | 1905 (24.0) | 0.005 |
| Triple vessel disease | 2348 (27.7) | 3226 (25.1) | 0.0608 | 2135 (26.9) | 2117 (26.6) | 0.006 |
| Left main disease | 885 (10.5) | 1206 (9.4) | 0.0358 | 799 (10.1) | 800 (10.1) | <0.001 |
| NYHA class within 2 wk prior to hospital admission, No. (%)d | ||||||
| I or II (mild limitation) | 1643 (19.4) | 2402 (18.7) | −0.0175 | 1547 (19.5) | 1559 (19.6) | 0.004 |
| III or IV (severe limitation) | 6766 (79.9) | 10 332 (80.4) | 6349 (79.9) | 6315 (79.5) | ||
| 5-meter walk test, No. (%) | ||||||
| ≤6 s (normal) | 1715 (20.3) | 2445 (19.0) | 0.0481 | 1598 (20.1) | 1551 (19.5) | 0.02 |
| >6-10 s (slow) | 3036 (35.9) | 4454 (34.7) | 2852 (35.9) | 2813 (35.4) | ||
| >10 s (slowest or unable to walk) | 2454 (29.0) | 3922 (30.5) | 2316 (29.1) | 2387 (30.0) | ||
| Walk test not performed | 1253 (14.8) | 2000 (15.6) | 1174 (14.8) | 1178 (14.8) | ||
| Estimated glomerular filtration rate, mL/min/1.73 m2 | ||||||
| Mean (SD) | 64.5 (24.4) | 60.3 (26.5) | 0.165 | 63.8 (24.4) | 63.5 (24.9) | 0.01 |
| <30 (or receiving dialysis), No. (%) | 446 (5.3) | 1365 (10.6) | 0.2206 | 444 (5.6) | 442 (5.6) | 0.01 |
| 30-<60, No. (%) | 3371 (39.8) | 5378 (41.9) | 3266 (41.1) | 3230 (40.6) | ||
| ≥60, No. (%) | 4634 (54.7) | 6081 (47.3) | 4222 (53.1) | 4257 (53.6) | ||
| Hemoglobin, mean (SD), g/dL | 12.0 (1.9) | 11.8 (1.9) | 0.1243 | 12.0 (1.9) | 12.0 (1.9) | 0.001 |
| Albumin, mean (SD), g/dL | 3.7 (0.5) | 3.7 (0.5) | 0.1088 | 3.7 (0.5) | 3.7 (0.5) | 0.01 |
| Preprocedure echocardiogram | ||||||
| LVEF, mean (SD), % | 51.1 (12.1) | 52.6 (10.8) | −0.1306 | 51.9 (11.5) | 52.0 (11.5) | −0.002 |
| LVEF ≤40%, No. (%) | 1795 (21.2) | 2078 (16.2) | 0.1281 | 1477 (18.6) | 1477 (18.6) | <0.001 |
| LV systolic diameter, mean (SD), cm | 3.3 (1.0) | 3.2 (0.9) | 0.1079 | 3.3 (0.9) | 3.3 (0.9) | 0.005 |
| LV diastolic diameter, mean (SD), cm | 4.7 (0.9) | 4.6 (0.9) | 0.1157 | 4.6 (0.9) | 4.6 (0.8) | 0.006 |
| Postprocedure echocardiogram, No./total No. (%) | ||||||
| Moderate or severe aortic regurgitation | 313/7168 (4.4) | 529/11 014 (4.8) | −0.0252 | 292/6744 (4.3) | 289/6801 (4.2) | <0.001 |
| Moderate or severe mitral regurgitation | 1145/7168 (16.0) | 2065/11 014 (18.7) | −0.0767 | 1096/6744 (16.3) | 1063/6801 (15.6) | 0.005 |
| β-Blocker at hospital discharge, No. (%) | 5908 (69.8) | 8725 (67.9) | 0.0399 | 5475 (68.9) | 5502 (69.2) | −0.007 |
| Complication during hospitalization, No. (%) | ||||||
| Myocardial infarction | 18 (0.2) | 25 (0.2) | 0.0041 | 17 (0.2) | 11 (0.1) | 0.02 |
| Stroke | 130 (1.5) | 230 (1.8) | −0.0199 | 124 (1.6) | 135 (1.7) | −0.01 |
| Vasculare | 314 (3.7) | 533 (4.2) | −0.0228 | 304 (3.8) | 309 (3.9) | −0.003 |
| New requirement for dialysis | 6 (0.1) | 96 (0.7) | −0.1056 | 6 (0.1) | 3 (0) | 0.02 |
Abbreviations: CABG, coronary artery bypass graft; ICD, implantable cardioverter-defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RAS, renin-angiotensin system; SMD, standardized mean difference.
To account for differences between those discharged from the hospital with a prescription for a RAS inhibitor vs those discharged from the hospital with no prescription, 1:1 propensity score matching was performed. A logistic regression model was used to estimate the propensity for prescription of a RAS inhibitor. Covariates in the model included baseline characteristics and demographics, coronary anatomical information, echocardiographic findings before and after transcatheter aortic valve replacement, medications at hospital discharge, and in-hospital adverse events. The entire list of covariates appears in eAppendix 2 in the Supplement.
Calculated as weight in kilograms divided by height in meters squared.
Estimates the potential risk for operative mortality (range, 0%-100%); a higher score indicates an increased risk.
Categorizes patients based on how much they are limited during physical activity.
Defined according to the Valve Academic Research Consortium-2 consensus document. The detailed definition appears in eAppendix 4 in the Supplement.
Prescription of a RAS Inhibitor and Outcomes
After propensity matching, a total of 15 896 patients with similar propensity scores for prescription of a RAS inhibitor were identified (main cohort: mean [SD] age, 82.4 [6.8] years; 48.1% were women; mean [SD] LVEF, 51.9% [11.5%]). The characteristics of patients in the matched sets were well balanced and the standardized mean differences were all below 0.1 (Table 1).
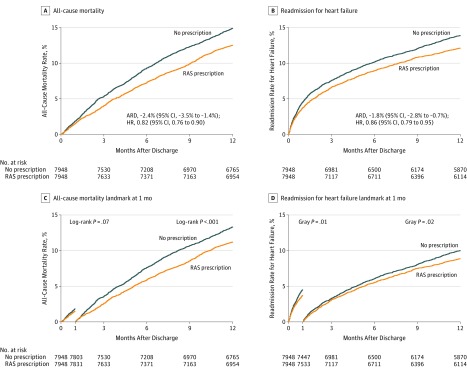
The Kaplan-Meier curves for mortality and the cumulative incidence curves for heart failure readmission appear in Figure 2A and 2B. Among patients with a prescription for a RAS inhibitor, there were significantly lower rates for mortality than among patients with no prescription (12.5% vs 14.9%, respectively; absolute risk difference [ARD], −2.4% [95% CI, −3.5% to −1.4%]; HR, 0.82 [95% CI, 0.76 to 0.90]) and for heart failure readmission (12.0% vs 13.8%; ARD, −1.8% [95% CI, −2.8% to −0.7%]; HR, 0.86 [95% CI, 0.79 to 0.95]) (both outcomes were measured at 1 year after hospital discharge).
Figure 2. Kaplan-Meier Curves and Landmark Analysis for Mortality and Cumulative Incidence Curves for Heart Failure Readmission.
The median observation time in panel A was 4.7 months (95% CI, 4.2-5.1 months) for no prescription and 5.0 months (95% CI, 4.5-5.5 months) for prescription of an renin-angiotensin system (RAS) inhibitor; panel B, 2.4 months (95% CI, 2.0-6.8 months) vs 2.6 months (95% CI, 2.1-8.0 months), respectively; panel C, 5.3 months (95% CI, 4.9-5.7 months) vs 5.6 months (95% CI, 5.2-6.1 months); and panel D, 4.8 months (95% CI, 4.2-5.7 months) vs 4.6 months (95% CI, 4.0-6.7 months). The landmark analyses were conducted post hoc. Only patients without events at 1 month were considered in the analysis after the landmark time point (panels C and D). ARD indicates absolute risk difference; HR, hazard ratio.
The falsification end points of hip fracture, urinary tract infection, and pneumonia demonstrated no statistically significant between-group differences; however, the point estimates for hip fracture and pneumonia were in the same direction as the primary outcomes (all-cause death and readmission due to heart failure; eTable 2 in the Supplement).
Subgroup Analyses
For mortality, there was a significant interaction between having a prescription for a RAS inhibitor and LVEF (P = .04 for interaction), whereas there was no significant interaction between having a prescription for a RAS inhibitor and eGFR (P = .85 for interaction). For heart failure readmission, there was no significant interaction between having a prescription for a RAS inhibitor and either LVEF (P = .84 for interaction) or eGFR (P = .42 for interaction).
Based on these results, a subgroup analysis by LVEF was performed for mortality. Among 21 312 eligible patients, 17 308 (81.2%) had preserved LVEF (>40%), whereas 3873 (18.2%) had reduced LVEF (≤40%). Propensity-score matching resulted in a total of 12 942 patients with preserved LVEF and 2954 patients with reduced LVEF. In each subgroup, the characteristics of the patients in the matched sets were well balanced and the standardized mean differences were all below 0.1 (eFigure 2, eFigure 3, and eTable 3 in the Supplement).
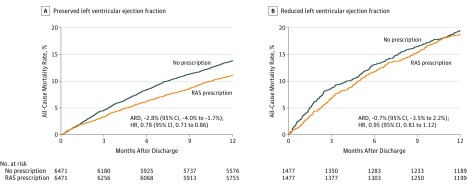
Among patients with preserved LVEF, having a prescription for a RAS inhibitor was associated with lower 1-year mortality compared with no prescription (11.1% vs 13.9%, respectively; ARD, −2.81% [95% CI, −3.95% to −1.67%]; HR, 0.78 [95% CI, 0.71 to 0.86]); however, such an association was not observed in patients with reduced LVEF (18.8% vs 19.5%; ARD, −0.68% [95% CI, −3.52% to 2.20%]; HR, 0.95 [95% CI, 0.81 to 1.12]) (Figure 3).
Figure 3. Kaplan-Meier Curves for Mortality by Left Ventricular Ejection Fraction Status.
The median observation time in panel A was 4.8 months (95% CI, 4.4-5.3 months) for no prescription and 5.1 months (95% CI, 4.6-5.9 months) for prescription of an renin-angiotensin system (RAS) inhibitor; and panel B, 3.9 months (95% CI, 2.6-5.0 months) vs 4.5 months (95% CI, 3.6-5.3 months), respectively. There was a significant interaction between the prescription of a RAS inhibitor and left ventricular ejection fraction (P = .04 for interaction). ARD indicates absolute risk difference; HR, hazard ratio.
Prescription of a RAS Inhibitor and Quality of Life
Among 15 896 patients in the main cohort, after excluding patients from 196 sites with KCCQ completion rates of less than 50%, the KCCQ cohort included 4837 patients (30.4%) who completed the KCCQ at baseline and at 1 year (Table 2 and eFigure 4 and eTable 4 in the Supplement). Of these 4837 patients, 2416 (49.9%) were prescribed a RAS inhibitor at hospital discharge, whereas 2421 (50.1%) had no prescription. Both treatment groups experienced substantial improvement in health status at 1 year.
Table 2. Association of Renin-Angiotensin System (RAS) Inhibitor Therapy and Quality-of-Life Scale in Data Set Resulting From the Propensity Analysisa.
| Kansas City Cardiomyopathy Questionnaire Overall Score (KCCQ-OS)b | RAS Inhibitor Prescription (n = 2416) |
No Prescription (n = 2421) |
Between-Group Differencec | P Value |
|---|---|---|---|---|
| Median score at baseline (interquartile range) | 42.7 (26.0 to 61.5) | 43.1 (25.5 to 61.5) | ||
| Median score at 1 y (interquartile range) | 83.3 (66.2 to 94.4) | 82.3 (64.1 to 93.8) | ||
| Change in score | 33.3 (14.2 to 51.0)d | 31.3 (13.5 to 51.1)d | 2.10 (0.10 to 4.06)e | <.001f |
| Change in score >20 points, No. (%) | 1649 (68.3) | 1591 (65.7) | 2.54 (−0.11 to 5.19)g | .06 |
| Change in score between >20 and ≥60 points at 1 y, No. (%) | 1475 (61.1) | 1412 (58.3) | 2.73 (−0.04 to 5.49)g | .05 |
| Favorable outcome, No. (%)h | 1904 (78.8) | 1862 (76.9) | 1.90 (−0.44 to 4.24)g | .11 |
To reduce the effect of selection bias in the KCCQ cohort, the inverse probability weighting framework was used to increase the weight of available patients who were most like those with missing follow-up data. This process involved constructing a multivariable logistic regression model to determine the probability of having missing KCCQ follow-up data. In addition to treatment group, the model included the same variables listed in eAppendix 2 in the Supplement.
A patient-reported, disease-specific health status instrument (range, 0-100); a higher score indicates less symptom burden and better quality of life. A change in score of 5 points corresponds to small clinical improvements; 10 points, moderate; and 20 points, large.
Expressed as percentages unless otherwise indicated.
Expressed as a median (interquartile range). Formula: (score with RAS inhibitor therapy at 1 year minus score at baseline) minus (score with no prescription at 1 year minus score at baseline).
Expressed as difference in improvement (95% CI). Formula: (RAS inhibitor therapy group) minus (no prescription group).
An analysis of covariance was performed. Change in KCCQ-OS from baseline adjusted for RAS inhibitor therapy in the presence of baseline KCCQ-OS.
Expressed as a risk difference (95% CI). Formula: (RAS inhibitor therapy group) minus (no prescription group).
Defined as survival with a reasonable quality of life (KCCQ-OS score ≥60) without any meaningful worsening (decrease of ≥10 points in the KCCQ-OS score from baseline to 1 year).
Although the extent of improvement was statistically greater among patients with a prescription for a RAS inhibitor than among patients with no prescription (median adjusted change in KCCQ score, 33.3 [interquartile range, 14.2 to 51.0] vs 31.3 [interquartile range, 13.5 to 51.1]; difference in improvement, 2.10 [95% CI, 0.10 to 4.06]; P < .001), the effect size was less than the minimal clinically important difference of 5 points. The rates for a large (>20 points) improvement in the KCCQ-OS score were not significantly different between patients with a prescription for a RAS inhibitor at hospital discharge vs those with no prescription (61.1% vs 58.3%, respectively; P = .05) as well as the rates for reaching a favorable outcome (78.8% vs 76.9%; ARD, 0.3% [95% CI, −0.3% to 0.9%]; adjusted odds ratio, 1.09 [95% CI, 0.94 to 1.27]).
Post Hoc Analyses
There was no between-group difference in the risk of a subsequent myocardial infarction (2.0% in the group with a prescription for a RAS inhibitor vs 1.7% in the no prescription group; ARD, 0.39% [95% CI, −0.04% to 0.81%]; HR, 1.23 [95% CI, 0.98 to 1.56]; P = .08). Landmark analysis revealed that the difference in mortality occurred mainly after 30 days (Figure 2C and 2D). In an analysis examining the association by type of RAS inhibitor (ACE inhibitor vs ARB) and outcomes, any type of prescription for a RAS inhibitor at hospital discharge was consistently associated with reduction in mortality and heart failure readmission at 1 year after hospital discharge (eTable 5 in the Supplement).
Discussion
In this registry-based retrospective cohort study of patients who underwent TAVR, receipt of a prescription for a RAS inhibitor at hospital discharge compared with no prescription was significantly associated with a lower risk for mortality and for hospital readmission at 1 year. The improvement in disease-specific health status (as assessed by the KCCQ but based on data from only 30.4% of the study cohort) was not different in a clinically meaningful way among patients with a prescription for a RAS inhibitor at hospital discharge vs among patients with no prescription.
Although prior studies have demonstrated that receiving a prescription for a RAS inhibitor is associated with improved survival among patients with varying degrees of aortic stenosis and among those undergoing SAVR,6,7 there have been limited data to date regarding the potential benefit of RAS inhibitor therapy on clinical outcomes in patients undergoing TAVR. In a study of 1215 patients, Ochiai et al23 reported a lower 2-year mortality rate among patients treated with a RAS inhibitor after TAVR compared with those not treated with a RAS inhibitor (7.5% vs 12.5%, respectively); however, the difference was not statistically significant after propensity matching.
Unlike previous research, the current study included a large contemporary cohort of patients who underwent TAVR at 417 US hospitals. The landmark analyses revealed that Kaplan-Meier curves for mortality started to separate 30 days or longer after hospital discharge, suggesting the differences in mortality were not biased by complications during hospitalization, but potentially may have been related to the early effect of treatment with a RAS inhibitor. However, these results were based on post hoc testing, and therefore are only hypothesis generating and need to be cautiously interpreted. This phenomenon also was shown in previous studies evaluating the association of RAS inhibitor therapy and mortality in patients with heart failure or who underwent SAVR. In these studies, divergence of cumulative event curves occurred within the first year, and there was preservation of benefit from RAS inhibitor therapy afterward.4,7,24,25 To our knowledge, the present study has, for the first time, demonstrated the association of prescription of a RAS inhibitor at hospital discharge with lower mortality after TAVR and also has demonstrated an association with a lower rate of heart failure readmission.
Several potential mechanisms may explain the higher survival rate and the lower heart failure readmission rate associated with RAS inhibitor therapy among patients who underwent TAVR. First, prescription of a RAS inhibitor may facilitate the regression of left ventricular hypertrophy and myocardial interstitial fibrosis, which may lead to lower mortality. Reflecting chronic pressure overload, left ventricular hypertrophy and myocardial interstitial fibrosis are commonly observed in patients with severe aortic stenosis.26 Both of these changes are not completely reversible and remain after patients undergo SAVR or TAVR, and are associated with increased risk of mortality and heart failure readmission.27,28,29,30 A RAS inhibitor prescription was suspected to have a positive effect on myocardial remodeling through reversal of left ventricular hypertrophy and reduction of myocardial fibrosis.
Second, reduction in sympathetic activity via RAS inhibitor therapy may be related to the improvement in clinical outcomes. Among patients with heart failure, the association of increased sympathetic nerve activity and progression of heart failure is well recognized,31,32 and a potential mechanism of RAS inhibitor therapy in modulating heart failure includes reduction in sympathetic activity.33 Given the high prevalence of heart failure, this potential mechanism could also be applied to patients undergoing TAVR. In addition, patients with aortic stenosis who have coexistent coronary artery disease and myocardial ischemia may benefit from the cardioprotective effects of RAS inhibitor therapy similar to the antiarrhythmic effects associated with higher potassium levels.34
Among patients who underwent SAVR, the use of RAS inhibitor therapy has been associated with higher survival rates independent of LVEF at baseline.7 The present study further investigated the difference in the magnitude of association between RAS inhibitor therapy and mortality after TAVR according to LVEF status. Among patients who underwent TAVR, prescription of a RAS inhibitor compared with no prescription was associated with lower mortality in patients with preserved LVEF, but not in those with reduced LVEF. This finding contrasts with current guidelines recommending the use of RAS inhibitor therapy only for patients with reduced LVEF in the setting of heart failure.35,36
These inconsistent results may raise a signal of unmeasured confounding; however, the contradictory findings may be explained by the different magnitude of effect on regression of left ventricular hypertrophy with use of RAS inhibitor therapy according to LVEF status. Several factors, such as smaller left ventricular systolic diameter at baseline and lower degrees of postprocedure aortic regurgitation, or mitral regurgitation, or both, are known to be associated with greater regression of left ventricular mass after aortic valve replacement.23,29 In the present study, patients with preserved LVEF were more likely to have these features than those with reduced LVEF (eTable 3 in the Supplement), which might result in greater reverse remodeling of left ventricular mass with RAS inhibitor therapy and subsequently lower mortality.
Limitations
This study has several limitations. First, despite the inclusion of a large number of variables and rigorous statistical methods to adjust for potential confounders, residual confounding, unmeasured confounding, or both, may exist. Even though the 95% CIs of the falsification end-point analyses indicated no significant between-group differences, the point estimates of these analyses suggested that perhaps those who were prescribed a RAS inhibitor at hospital discharge had less overall illness severity at baseline than those who were not prescribed a RAS inhibitor. These point estimates may reflect possible residual confounding, unmeasured confounding, or both; therefore, randomized studies are required to corroborate the present findings.
Second, information on the use of RAS inhibitor therapy prior to the TAVR procedure and on adherence to RAS inhibitor therapy during follow-up was unknown; however, patient-reported adherence with long-term use of RAS inhibitor therapy was available (eAppendix 3 in the Supplement). Treatment allocation was determined by the presence or absence of RAS inhibitor therapy at hospital discharge, but initiation or discontinuation during the follow-up period was not assessed because these data were not well captured in the TVT Registry. However, there are data suggesting that the likelihood of cardiovascular medications being initiated after hospital discharge is significantly lower than prior to hospital discharge.37,38 In addition, considering only hospital discharge medication prescriptions without information on medication adherence would tend to bias the results toward the null.
Third, only a few echocardiographic parameters were available and serial trends of clinically important imaging variables (eg, left ventricular mass, LVEF, left atrial volume, and myocardial fibrosis) could not be assessed. Therefore, the potential mechanisms for the effects of RAS inhibitor therapy are speculative.
Fourth, follow-up blood pressures were not available in the TVT Registry, which leaves the possibility that the outcomes associated with RAS inhibitor therapy could be mediated by better blood pressure control. However, previous studies have demonstrated that RAS inhibitor therapy contributes to regression of left ventricular hypertrophy and regression of myocardial fibrosis independent of its antihypertensive effects, a finding which is also consistent with the results from experimental animal models.39,40
Fifth, despite the large sample size in the subgroup analysis by LVEF, statistical power might be insufficient for patients with reduced LVEF, which might result in a statistically nonsignificant difference in mortality.
Sixth, the analysis was conducted among patients with linkage to Medicare claims data to facilitate 1-year outcomes assessment; as a result, the findings may not be generalizable to all patients undergoing TAVR.
Seventh, unmatched patients (n = 5416) were more likely to be of prohibitive risk (Society of Thoracic Surgeons Predicted Risk of Mortality score ≥15) or receiving dialysis and have an eGFR of less than 30 compared with the matched patients (eTable 6 in the Supplement). As a result, the findings of this study may be less generalizable to this group.
Eighth, only a limited number of patients were included in the health status analysis due to missing data at baseline or missing 1-year KCCQ data (55.1% of the study cohort had missing data). This may have resulted in selection bias (eTable 4 in the Supplement) and the findings must be interpreted with caution.
Conclusions
Among patients who underwent TAVR, receiving a prescription for a RAS inhibitor at hospital discharge compared with no prescription was significantly associated with a lower risk of mortality and heart failure readmission. However, due to potential selection bias, this finding requires further investigation in randomized trials.
eAppendix 1. International Classification of Disease 9th and 10th revisions (ICD-9 and ICD-10) codes for each endpoint
eAppendix 2. List of covariates for generating propensity score
eAppendix 3. Patient-reported persistence on an RAS inhibitor at 30 days and 1 year
eAppendix 4. Detailed definition of vascular complication
eReference
eFigure 1. Distributions of propensity scores according to RAS blockade therapy status in entire cohort
eFigure 2. Standardized differences before and after propensity score matching in pLVEF
eFigure 3. Standardized differences before and after propensity score matching in rLVEF
eFigure 4. Quality of life analysis cohort creation
eTable 1. Baseline characteristics of those who were included versus excluded
eTable 2. Falsification endpoints
eTable 3. Baseline characteristics after propensity score matching in sub-groups by LVEF
eTable 4. Baseline characteristics of those who were included versus excluded in KCCQ cohort
eTable 5. Association between the prescription of an RAS inhibitor at discharge and clinical outcomes by the type of an RAS inhibitor
eTable 6. The comparison of baseline characteristics between matched and unmatched patients
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases. Lancet. 2006;368(9540):1005-1011. doi: 10.1016/S0140-6736(06)69208-8 [DOI] [PubMed] [Google Scholar]
- 2.Zaid RR, Barker CM, Little SH, Nagueh SF. Pre- and post-operative diastolic dysfunction in patients with valvular heart disease. J Am Coll Cardiol. 2013;62(21):1922-1930. doi: 10.1016/j.jacc.2013.08.1619 [DOI] [PubMed] [Google Scholar]
- 3.CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316(23):1429-1435. doi: 10.1056/NEJM198706043162301 [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302. doi: 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall Programme. Lancet. 2003;362(9386):759-766. doi: 10.1016/S0140-6736(03)14282-1 [DOI] [PubMed] [Google Scholar]
- 6.Nadir MA, Wei L, Elder DH, et al. Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol. 2011;58(6):570-576. doi: 10.1016/j.jacc.2011.01.063 [DOI] [PubMed] [Google Scholar]
- 7.Goel SS, Aksoy O, Gupta S, et al. Renin-angiotensin system blockade therapy after surgical aortic valve replacement for severe aortic stenosis. Ann Intern Med. 2014;161(10):699-710. doi: 10.7326/M13-1505 [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 9.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790-1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 10.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 11.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 12.Holmes DR Jr, Brennan JM, Rumsfeld JS, et al. ; STS/ACC TVT Registry . Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019-1028. doi: 10.1001/jama.2015.1474 [DOI] [PubMed] [Google Scholar]
- 13.Carroll JD, Edwards FH, Marinac-Dabic D, et al. The STS-ACC transcatheter valve therapy national registry. J Am Coll Cardiol. 2013;62(11):1026-1034. doi: 10.1016/j.jacc.2013.03.060 [DOI] [PubMed] [Google Scholar]
- 14.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire. J Am Coll Cardiol. 2000;35(5):1245-1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 15.Arnold SV, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61-67. doi: 10.1161/CIRCHEARTFAILURE.112.970053 [DOI] [PubMed] [Google Scholar]
- 16.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707-715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 17.Arnold SV, Spertus JA, Vemulapalli S, et al. Quality-of-life outcomes after transcatheter aortic valve replacement in an unselected population. JAMA Cardiol. 2017;2(4):409-416. doi: 10.1001/jamacardio.2016.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards FH, Cohen DJ, O’Brien SM, et al. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1(1):46-52. doi: 10.1001/jamacardio.2015.0326 [DOI] [PubMed] [Google Scholar]
- 19.Arnold SV, O’Brien SM, Vemulapalli S, et al. ; STS/ACC TVT Registry . Inclusion of functional status measures in the risk adjustment of 30-day mortality after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11(6):581-589. doi: 10.1016/j.jcin.2018.01.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992:237–247. [Google Scholar]
- 21.Alfredsson J, Stebbins A, Brennan JM, et al. Gait speed predicts 30-day mortality after transcatheter aortic valve replacement. Circulation. 2016;133(14):1351-1359. doi: 10.1161/CIRCULATIONAHA.115.020279 [DOI] [PubMed] [Google Scholar]
- 22.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 23.Ochiai T, Saito S, Yamanaka F, et al. Renin-angiotensin system blockade therapy after transcatheter aortic valve implantation. Heart. 2018;104(8):644-651. doi: 10.1136/heartjnl-2017-311738 [DOI] [PubMed] [Google Scholar]
- 24.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors. Lancet. 2003;362(9386):772-776. doi: 10.1016/S0140-6736(03)14284-5 [DOI] [PubMed] [Google Scholar]
- 25.Cleland JG, Tendera M, Adamus J, et al. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338-2345. doi: 10.1093/eurheartj/ehl250 [DOI] [PubMed] [Google Scholar]
- 26.Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97(4):301-307. doi: 10.1136/hrt.2010.192997 [DOI] [PubMed] [Google Scholar]
- 27.Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2014;147(1):362-369.e8, e368. doi: 10.1016/j.jtcvs.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 28.Weidemann F, Herrmann S, Störk S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120(7):577-584. doi: 10.1161/CIRCULATIONAHA.108.847772 [DOI] [PubMed] [Google Scholar]
- 29.Une D, Mesana L, Chan V, et al. Clinical impact of changes in left ventricular function after aortic valve replacement: analysis from 3112 patients. Circulation. 2015;132(8):741-747. doi: 10.1161/CIRCULATIONAHA.115.015371 [DOI] [PubMed] [Google Scholar]
- 30.Lindman BR, Stewart WJ, Pibarot P, et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv. 2014;7(6):662-673. doi: 10.1016/j.jcin.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92(11):3206-3211. doi: 10.1161/01.CIR.92.11.3206 [DOI] [PubMed] [Google Scholar]
- 32.Eisenhofer G, Friberg P, Rundqvist B, et al. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93(9):1667-1676. doi: 10.1161/01.CIR.93.9.1667 [DOI] [PubMed] [Google Scholar]
- 33.Patten RD, Kronenberg MW, Benedict CR, et al. Acute and long-term effects of the angiotensin-converting enzyme inhibitor, enalapril, on adrenergic activity and sensitivity during exercise in patients with left ventricular systolic dysfunction. Am Heart J. 1997;134(1):37-43. doi: 10.1016/S0002-8703(97)70104-2 [DOI] [PubMed] [Google Scholar]
- 34.Domanski MJ, Exner DV, Borkowf CB, et al. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. J Am Coll Cardiol. 1999;33(3):598-604. doi: 10.1016/S0735-1097(98)00609-3 [DOI] [PubMed] [Google Scholar]
- 35.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2017;136(6):e137-e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 36.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 37.Arnold SV, Spertus JA, Masoudi FA, et al. Beyond medication prescription as performance measures. J Am Coll Cardiol. 2013;62(19):1791-1801. doi: 10.1016/j.jacc.2013.04.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis LH, Mi X, Qualls LG, et al. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165(6):979-986.e1, e971. doi: 10.1016/j.ahj.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 39.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102(12):1388-1393. doi: 10.1161/01.CIR.102.12.1388 [DOI] [PubMed] [Google Scholar]
- 40.Tyralla K, Adamczak M, Benz K, et al. High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. PLoS One. 2011;6(1):e15287. doi: 10.1371/journal.pone.0015287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. International Classification of Disease 9th and 10th revisions (ICD-9 and ICD-10) codes for each endpoint
eAppendix 2. List of covariates for generating propensity score
eAppendix 3. Patient-reported persistence on an RAS inhibitor at 30 days and 1 year
eAppendix 4. Detailed definition of vascular complication
eReference
eFigure 1. Distributions of propensity scores according to RAS blockade therapy status in entire cohort
eFigure 2. Standardized differences before and after propensity score matching in pLVEF
eFigure 3. Standardized differences before and after propensity score matching in rLVEF
eFigure 4. Quality of life analysis cohort creation
eTable 1. Baseline characteristics of those who were included versus excluded
eTable 2. Falsification endpoints
eTable 3. Baseline characteristics after propensity score matching in sub-groups by LVEF
eTable 4. Baseline characteristics of those who were included versus excluded in KCCQ cohort
eTable 5. Association between the prescription of an RAS inhibitor at discharge and clinical outcomes by the type of an RAS inhibitor
eTable 6. The comparison of baseline characteristics between matched and unmatched patients