Abstract
This is a systematic review of articles concerning the morbidity, recurrence rate, treatment and treatment complications of pelvic giant cell tumors (GCTs). The key words “giant cell tumor, pelvis” were used to identify articles which included data on patients with pelvic GCTs in English and Chinese databases of published reports from 1949–2012. The articles were filtered by title, abstract and full text. Thirty‐eight articles and 165 patients were identified for this review. Data on all identified patients were studies; data in different articles on the same patients was not used repeatedly. The following patient data were collected where possible and subjected to systematic analysis; age, location of GCT, treatment, follow‐up, complications, recurrence and whether alive or dead. The mean age of onset was 33.2 years (range, 14–73 years), the peak ages of onset being between 21 and 40 years. A pronounced sex difference was identified, the male : female ratio being 1:1.7. The acetabulum was the commonest area for pelvic GCTs. Forty‐eight tumors were primarily located in the iliac, 60 in the acetabular and 31 in the ischiopubic area. Twenty‐seven patients experienced complications of treatment. Patients who had been treated by wide resection had the most complications; these included incisional infection and delayed healing of incisions. Local recurrence was common, having occurred in 39/158 patients (24.6%), comprising 24/72 (33.3%) who had undergone intralesional surgery only; 9/20 (45.0%) who had undergone radiotherapy only; 1/51 (2.0%) who had undergone wide resection; and 5/14 patients (35.7%) who had undergone radiation therapy or cryotherapy plus intralesional surgery. Mortality was low (3.2%, 5/158). Pelvic GCT is not common, the acetabular area appears to the most frequent site and the peak age is the third and fourth decades. Although the recurrence rate is high for all pelvic GCTs, the mortality is low. Treatment has a critical influence on recurrence. In spite of the associated complications, the lower local recurrence rate makes wide resection a reasonable option for patients with extensive and/or aggressive GCTs.
Keywords: Giant cell tumors, Pelvis, Systematic review
Introduction
Giant cell tumors (GCTs) are benign but locally aggressive primary bone neoplasms that are composed of a proliferation of mononuclear cells amongst which are scattered numerous macrophages and large osteoclast‐like giant cells. GCTs typically affect the ends of long bones1, 2, 3. Pelvic GCTs are rare, accounting for only 1.5% to 6.1% of bone GCTs4, 5, 6.
The optimal treatment of pelvic GCTs is a controversial topic in orthopaedic oncology. Treatment options include radiation therapy (RT), surgery with an intralesional margin (S[IL]), surgery with an intralesional margin and RT, surgery with an intralesional margin and adjunctive cryosurgical technique, microwave inactivation of tumor and intralesional curettage, and surgery with a wide margin (S[W]). There are various reconstructive methods for bone defects created by resection or curettage; these include cement filling, autograft, allograft bone transplantation, rod fixation and hemipelvic prostheses5, 7, 8, 9, 10. No consensus has been reached concerning the treatments of pelvis GCTs.
Another problem is that, because of their low incidence, there are few published reports about pelvic GCTs. The resultant lack of clinical data from large samples means that patient characteristics, clinical efficacy of treatment and other important variables concerning pelvic GCTs are unknown. Most of published reports have focused on treatment of a single or a few cases, no series of more than 30 cases have been reported. Offset error cannot be avoided in studies that simple analyze a small series of clinical data in one paper. There are currently no published multi‐center clinical studies of pelvic GCTs. High‐level clinical data about pelvic GCTs would provide an important and useful reference for treatment of this condition. In this study, we searched multiple databases for case reports, discussion of relevant experience, case analyses, research and other aspects of the efficacy of surgery for pelvic GCTs. We have systematically reviewed these data to examine some aspects of pelvic GCTs, including their epidemiological characteristics, treatment and prognosis. Such reviews are recognized to provide objective evaluation of available evidence and are the best way to comprehensively study a particular issue and create a good basis for evidence‐based decision‐making.
In this study, we collected published reports of cases of pelvic GCTs to construct a large sample to analyze and provide comprehensive information about pelvic GCTs for the first time. In this systematic analysis of pelvis GCTs, variables researched included age of onset, sex ratio, disease location, treatment, complications, recurrence rate, mortality and so on. We have particularly addressed the following three topics in this review: firstly, epidemiological characteristics and differences in location of pelvic GCTs; secondly, treatment‐related complications of pelvic GCTs; and thirdly, recurrence of pelvis GCTs after different treatments.
Materials and Methods
Using the terms “giant cell tumor” and “pelvis”, published reports were searched to identify patients who had been treated for GCT of the pelvis. Both English and non‐English language reports were searched for using Elseviver Science Direct, Springer Link, ProQuest Health and Medical Complete, John Wiley Interscience, EBSCO MEDLINE Complete, Chongqing VIP, CNKI and Chinese Medical Association and Chinese Medical Association journal full text database (1949−2012). Various types of article, including case reports, discussion of experience, case analyses, research and articles concerning the efficacy of surgery were identified. Articles that only included sacral GCTs were excluded after reading the full text. Duplicate reports in different articles were also eliminated.
Inclusion criteria included: (i) GCT of the pelvis; (ii) required information about treatment; (iii) required information about follow‐up; and (iv) follow‐up of at least 1 year. Exclusion criteria included: (i) GCT of the sacrum; (ii) duplicate report cases; and (iii) lack of information about follow‐up or follow‐up time less than 1 year.
Types of treatment included: (i) RT; (ii) S[IL]; (iii) surgery with intralesional margins and RT or cryosurgery (RT/Cryo + S[IL]); and (iv) S(W). If surgical resection margins were not reported, the treatment was classified as S(IL). Patients who had undergone chemotherapy, embolization and other rarely used treatments were not classified as an additional treatment group.
Aims of the study included assessment of: (i) age distribution; (ii) tumor location, where location information was available, locations were grouped by anatomical site; (iii) treatment outcomes, including complications, local recurrence and mortality; radiation‐induced sarcoma and malignant transformation were classified as local recurrence.
For search results matching the search criteria, the databases were initially screened for title and abstract content. Next, the full texts of articles thus identified were read and screened for the inclusion and exclusion criteria. All eligible patients' data were listed by research categories in an Excel spreadsheet. The epidemiological characteristics of pelvic GCTs were then derived from descriptive indicators whereas result‐oriented indicators were used to analyze differences between among different treatment groups.
In this study, SPSS16.0 statistical software was used for data analysis. Multiple comparisons between different treatment groups of result‐oriented indicators were performed. The method of χ2 segmentation was used for multiple comparisons and the differences were considered statistically significant when P < 0.05.
Results
Patient Variables
Thirty‐eight papers concerning pelvic GCTs were analyzed5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43. Publication dates ranged from 1949–2012. These papers included 165 patients, the largest series comprising 27 patients43. Numerous case reports were included.
Of the 165 patients, information about sex was provided for 119; this119 comprised 44 male (37.0%) and 75 female patients (63.0%). Information about age was provided for 130 patients, most of whom were in the third or fourth decade of life at first diagnosis (Fig. 1). The median age was 33.2 (14–73) years. The mean duration of follow‐up was 9.5 (1.5–35) years.
Figure 1.
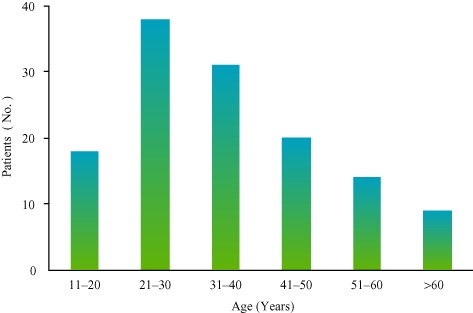
Bargraph showing the age distributions of the 130 patients who had GCTs of a pelvic bone; the peak age was the third and fourth decades.
Location
According to Enneking and Dunham's pelvic tumor resection classification44, for this review the locations of pelvic GCTs were classified as area A (iliac region), area B (acetabular region) and area C (pubis and ischium area). Definite disease locations were provided for 139/165 patients, area B being the most common location. There were 60 GCTs (43.2%) in area B, 48 (34.5%) in area A and 31 (22.3%) in area C (Fig. 2).
Figure 2.
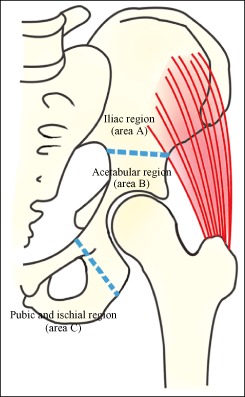
Tumor locations of pelvic GCTs were classified as area A (iliac region), area B (acetabular region) and area C (pubic and ischial area) in accordance with Enneking and Dunham's pelvic tumor resection classification44.
Treatment
Some treatment information was provided for all 165 patients; however, for three patients who had undergone surgery the surgical procedure was not specified. Of the remaining 162 patients, 20 (12.3%) had undergone RT; 72 patients (44.4%) S(IL); 16 patients (9.9%) RT/Cryo + S(IL); 51 patients (31.5%) S(W); and three patients (1.9%) selective arterial embolization without other treatment.
Complications
Information about complications was provided for 83 patients, 27 (32.5%) of whom had complications (Fig. 3). Of the 83 patients for whom information about complications was provided, local tissue reactions to RT had occurred in five of the 10 (50.0%) who had undergone RT; complications that included thrombogenesis, loosening of screws or bone cement and infections had occurred in five of the 35 (14.3%) who had undergone S(IL); complications that included delayed infection, poor wound healing and loosening of screws or bone cement had occurred in four of the 10 (40.0%) who had undergone RT/Cryo + S(IL); and complications that included infection, delayed infection, poor wound healing, nonunion, joint dislocation and fixation loosening had occurred in 13 of the 28 patients (46.4%) who had undergone S(W). Because of the relatively large number of patients in these treatment categories,, the incidence of complications in the S(IL) and S(W) groups could be compared. Complications occurred significantly less frequently in the S(IL) than in the S(W) group (P = 0.005, χ2 = 7.875).
Figure 3.
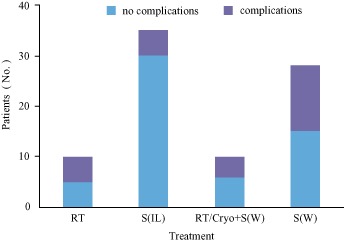
Incidence of complications according to treatment group. Significantly fewer complications occurred with S(IL) than with S(W) (P = 0.005, χ2 = 7.875).
Recurrence and Death
Information about recurrence and death was provided for 158 of the 165 patients. Five of these 158 patients (3.2%) had died of recurrence. Local recurrence had occurred in 39/158 patients (24.6%) comprising 24/72 (33.3%) who had undergone S(IL) only; 9/20 (45.0%) who had undergone RT only; 1/51 patients (2.0%) who had undergone S(W); and 5/14 (35.7%) who had undergone RT/Cryo + S(IL) (Fig. 4). There was no significant difference in the rate of local recurrence between S(IL) and RT/Cryo + S(IL) (P = 0.873 > 0.05, χ2 = 0.026) or between RT and S(IL) (P = 0.336 > 0.0125, χ2 = 0.926). There was a significant difference in local recurrence rate between RT and S(W) (P = 0.000 < 0.0125, χ2 = 21.992) and between S(IL) and S(W) (P = 0.000 < 0.0125, χ2 = 18.144). Recurrence rate was lower after S(W) than the other three treatments, whereas the recurrence rate was similar for RT, S(IL) and RT/Cryo + S(IL).
Figure 4.
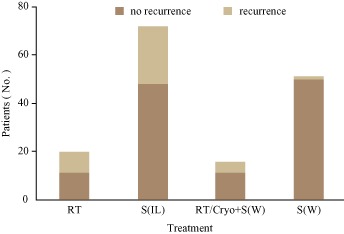
Recurrence rate according to treatment group. There was no significant difference in local recurrence rate between S(IL) and RT/Cryo + S (IL) (P = 0.873 > 0.05, χ2 = 0.026) or RT and S(IL) (P = 0.336 > 0.0125, χ2 = 0.926). There was a significant difference in local recurrence rate between RT andS(W) (P = 0.000 < 0.0125, χ2 = 21.992) and S(IL) and S(W) (P = 0.000 < 0.0125, χ2 = 18.144). The recurrence rate was lower after S(W) than after the other three treatments, whereas the recurrence rate was similar for RT, S(IL) and RT/Cryo + S (IL).
Research Focus in the Last Decade
Over the last decade, new progress has been made in bone tumor treatment technology and more detailed data and higher quality papers have been published than the past. Therefore, the 10 papers (75patients)10, 29, 30, 31, 32, 34, 36, 41, 42, 43 about pelvic GCT published from 2000 to 2012 years were analyzed in detail. Data on disease location, treatment and outcome of these 75 patients were assessed (Fig. 5). Area B has been the focus of attention in pelvic GCT research over the past decade, during which 56 patients with GCTs in area B were reported including one (1.8%) who had undergone RT; 27 (48.2%) who had undergone S(IL); and 28 (50.0 %) who had undergone S(W). In this decade, 14 patients with GCTs in area A were reported, including one (7.1%) who had undergone RT; eight (57.1%) who had undergone S(IL); and five (35.7%) who had undergone S(W). Five patients with GCTs in area C were reported, all of whom had undergone S(IL). The one patient with a GCT in area A (100%) who had undergone RT later developed a radiation‐induced sarcoma. Local recurrence occurred in 7/27 patients with GCTS in area B (25.9%) who had undergone S(IL) and in 1/28 (3.6%) who had undergone S(W). The remaining patients were disease‐free for the duration of reported follow‐up.
Figure 5.
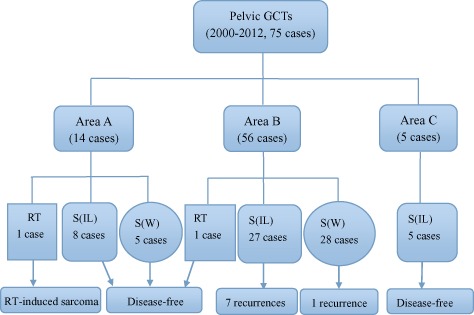
Treatments and outcomes of 75 patients with pelvic GCTs from 10 papers published from 2000 to 2012.
Discussion
Epidemiological Characteristics of Pelvis GCT
In this study, in the 119 patients for whom information about sex was available, male : female ratio was 1:1.7. This sex difference based on analysis of a large sample has not previously been reported. The mechanisms for this difference require further study. There was a higher prevalence of pelvic GCTs in the third (38/130, 29.2%) and fourth decades (31/130, 23.8%) of life. These age characteristics are consistent with those reported by Campanacci et al.45, Sanjay et al.5 and Balke et al.34.
Analysis of tumor location showed a clear difference between areas A, B and C. Area B was the most commonly involved site whereas area C was least often involved. In the 139 patients for whom tumor location was reported, the ratio of A : B : C was 1.5:2:1. This difference in the frequency at these sites has not been previously been reported. Areas A and B have abundant cancellous bone and blood supply, which may account for the difference in GCT locations. Area B is the most commonly involved pelvic GCT site, but also the most difficult region to treat. Tumors in area B often involve the acetabulum, which makes it difficult to balance the relative benefits of minimizing recurrence and good and postoperative hip function. Treatment of pelvic GCTs in area B is difficult and controversial; the optimal treatment option often depends on the region of tumor invasion and extent of acetabular involvement. Further study is needed to determine how to choose the appropriate treatment for pelvis GCTs in area B.
Recurrence and Mortality
Because of the complex anatomy of the pelvic region and the characteristic lack of early symptoms, the treatment of pelvic GCT's is much more difficult and the recurrence rate higher than for GCTs in other locations45, 46, 47, 48. In order to ensure good limb function and low recurrence after surgery, a variety of treatments have been proposed including RT; intralesional curettage; cryosurgery; microwave inactivation; high‐speed burr drill; selective arterial embolization and S(W)5, 7, 8, 9, 10. While the treatment of pelvic GCTs is currently controversial, it is undeniable that treatment is an important factor affecting recurrence. In this study, local recurrence occurred in 39 of 158 patients (24.6%) for whom recurrence data were available. The patients who had undergone RT had higher recurrence rates than the other treatment groups. Patients who had undergone S(IL) alone or RT/Cryo + S(IL) had similar recurrence rates (P = 0.873), indicating that the effect of adjuvant therapies aiming at reducing recurrence after S(IL) is uncertain for pelvic GCTs. Patients who had undergone S(W) had the lowest recurrence rates of all groups. Therefore, S(W) is recommended for those patients whose limb function would be minimally affected by surgery. In patients whose tumor is hard to expose for S(W) or limb function would be seriously affected after S(W), S(IL) is recommended.
Although S(IL) was associated with a higher recurrence rate than S(W), the low mortality rate of pelvic GCT (3.2%, 5/158 patients died in this study) means that there are further treatment opportunities for patient with recurrence. However, RT is not recommended for recurrence: in this study, all five patients who had died had undergone RT. We believe that RT is an important cause of death because of RT‐induced sarcomas.
The recurrence rate and mortality are partially reflect length of follow‐up; additional local recurrences may occur with longer follow‐up. Nevertheless, 70% of local recurrences occurred within 2 years41. In this study, 144 of 165 patients (87.3%) for whom follow‐up time was provided had a mean follow‐up of 9.5 (1.5–35) years.
Complications of Treatment of Pelvic GCT
Complications of treatment of pelvic GCT include wound infection; delayed infection; poor wound healing; loosening of screws or bone cement; nonunion and hip dislocation. Occurrence of complications correlates closely with treatment administered. Patients who undergo RT tend to have poor wound healing because RT causes vascular injury; patients who undergo S(W) are more likely to develop postoperative wound infection because of the large wound and the presence of implants; and loosening of screws and bone cement is more common in area B because area B participates in hip function and stress transduction. In the present study, although S(W) did significantly reduce the recurrence rate, it was associated with significantly more frequent complications than S(IL), this difference likely being attributable to the greater surgical exposure, use of implants and longer operative time with the former treatment.
Limb dysfunction is one of the commonest complications of treatment of pelvic GCTs. To retain or rebuild limb function after surgery is an important criterion for evaluating treatment of pelvic GCT. S(W) of tumors in areas A and C results in little functional compromise and low recurrence rate36. However, with GCTs in area B, postoperative functional evaluation is very important. In this review we were unable to assess postoperative function because the reported means of evaluating it differed markedly and could not be combined.
Limitations of this Review
In this study, some data was missing for many of the 165 patients, which would inevitably have resulted in some error. We look forward to a large study that provides reliable data for realistic assessment of the incidence and clinical treatment outcomes of pelvic GCT.
Disclosure: All the authors have no interests related to the subject of this article.
References
- 1. Karpik M. Giant Cell Tumor (tumor gigantocellularis, osteoclastoma)—epidemiology, diagnosis, treatment. Ortop Traumatol Rehabil, 2010, 12: 207–215. [PubMed] [Google Scholar]
- 2. Trieb K, Bitzan P, Lang S, Dominkus M, Kotz R. Recurrence of curetted and bone‐grafted giant‐cell tumours with and without adjuvant phenol therapy. Eur J Surg Oncol, 2001, 27: 200–202. [DOI] [PubMed] [Google Scholar]
- 3. Boons HW, Keijser LC, Schreuder HW, Pruszczynski M, Lemmens JA, Veth RP. Oncologic and functional results after treatment of giant cell tumors of bone. Arch Orthop Trauma Surg, 2002, 122: 17–23. [DOI] [PubMed] [Google Scholar]
- 4. Reid R, Banerjee SS, Sciot R. Giant cell tumour In: Fletcher CDM, Unni KK, Mertens F, eds. Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon: IARC Press, 2002; 309–313. [Google Scholar]
- 5. Sanjay BK, Frassica FJ, Frassica DA, Unni KK, McLeod RA, Sim FH. Treatment of giant‐cell tumor of the pelvis. J Bone Joint Surg Am, 1993, 75: 1466–1475. [DOI] [PubMed] [Google Scholar]
- 6. Schajowicz F. Giant cell tumor In: Schajowicz F, Sundaram M, Gitelis S, McDonald DJ, eds. Tumors and Tumorlike Lesions of Bone, 2nd edn New York: Springer‐Verlag, 1996; 257–295. [Google Scholar]
- 7. Bell RS, Harwood AR, Goodman SB, Fornasier VL. Supervoltage radiotherapy in the treatment of difficult giant cell tumors of bone. Clin Orthop Relat Res, 1983, 174: 208–216. [PubMed] [Google Scholar]
- 8. Marcove RC, Weis LD, Vaghaiwalla MR, Pearson R. Cryosurgery in the treatment of giant cell tumors of bone: a report of 52 consecutive cases. Clin Orthop Relat Res, 1978, 134: 275–289. [PubMed] [Google Scholar]
- 9. Matsumoto K, Hukuda S, Ishizawa M, Chano T, Okabe H. Use of preoperative autologous blood donations and erythropoietin for treatment of giant cell tumor of the ischium. Clin Orthop Relat Res, 1996, 326: 246–249. [DOI] [PubMed] [Google Scholar]
- 10. Huang HC, Hu YC, Lun DX, et al The clinical application of femoral head exclusion after resection of pelvic tumors around acetabulum. Zhonghua Gu Ke Za Zhi, 2011, 31: 635–639 (in Chinese). [Google Scholar]
- 11. Chakravarti A, Spiro IJ, Hug EB, Mankin HJ, Efird JT, Suit HD. Megavoltage radiation therapy for axial and inoperable giant‐cell tumor of bone. J Bone Joint Surg Am, 1999, 81: 1566–1573. [DOI] [PubMed] [Google Scholar]
- 12. Eilers H, Habighorst LV, Albers P, Rebmann D. Radiation therapy of an inoperable giant cell tumor. Strahlentherapie, 1977, 153: 103–105. [PubMed] [Google Scholar]
- 13. Goldenberg RG, Campbell CJ, Bonfiglio M. Giant‐cell tumor of bone: an analysis of two hundred and eighteen cases. J Bone Joint Surg Am, 1970, 52: 619–664. [PubMed] [Google Scholar]
- 14. Johnson KA, Riley LH Jr. Giant cell tumor of bone: an evaluation of 24 cases treated at the Johns Hopkins Hospital between 1925 and 1955. Clin Orthop Relat Res, 1969, 62: 187–191. [PubMed] [Google Scholar]
- 15. Kattapuram AS, O'Donnell RJ, Huszar M, Rosenberg AE, Kattapuram SV, Mankin HJ. Surgical management of innominate giant cell tumor. Clin Orthop Relat Res, 1996, 329: 281–287. [DOI] [PubMed] [Google Scholar]
- 16. Marinò D, Grillo G, Fazioli F, Rosa D. Giant cell tumor of the ilium with involvement of the acetabulum: surgical treatment. Chir Organi Mov, 1986, 71: 407–410. [PubMed] [Google Scholar]
- 17. McGrath PJ. Giant‐cell tumour of bone: an analysis of fifty‐two cases. J Bone Joint Surg Br, 1972, 54: 216–229. [PubMed] [Google Scholar]
- 18. Mnaymneh WA, Dudley HR, Mnaymneh LG. Giant‐cell tumor of bone: an analysis and follow‐up of the forty‐one cases observed at the Massachusetts General Hospital between 1925 and 1960. J Bone Joint Surg Am, 1964, 46: 63–75. [PubMed] [Google Scholar]
- 19. Mnaymneh W, Mnaymneh LG. Giant cell tumor of the ischium: unusual site and outcome. South Med J, 1979, 72: 1012–1014. [DOI] [PubMed] [Google Scholar]
- 20. Osaka S, Toriyama S. Surgical treatment of giant cell tumors of the pelvis. Clin Orthop Relat Res, 1987, 222: 123–131. [PubMed] [Google Scholar]
- 21. Persson BM, Ekelund L, Lövdahl R, Gunterberg B. Favourable results of acrylic cementation for giant cell tumors. Acta Orthop Scand, 1984, 55: 209–214. [DOI] [PubMed] [Google Scholar]
- 22. Prossor TM. Treatment of giant‐cell tumours of bone, with a review of twenty‐five cases. J Bone Joint Surg Br, 1949, 31: 241–251. [PubMed] [Google Scholar]
- 23. Schrøder HA, Lindequist S, Thomsen PB, Damholt V. Giant‐cell tumor in the ischium treated with arterial embolism and resection. Ugeskr Laeger, 1986, 148: 962–963. [PubMed] [Google Scholar]
- 24. Schwartz LH, Okunieff PG, Rosenberg A, Suit HD. Radiation therapy in the treatment of difficult giant cell tumors. Int J Radiat Oncol Biol Phys, 1989, 17: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 25. Seider MJ, Rich TA, Ayala AG, et al Giant cell tumors of bone: treatment with radiation therapy. Radiology, 1986, 161: 537–540. [DOI] [PubMed] [Google Scholar]
- 26. Shankman S, Greenspan A, Klein MJ, Lewis MM. Giant cell tumor of the ischium: a report of two cases and review of the literature. Skeletal Radiol, 1988, 17: 46–51. [DOI] [PubMed] [Google Scholar]
- 27. Walter J. Giant‐cell lesions of bone: osteoclastoma and giant‐cell tumour variants: survey of a radiotherapeutic series. Clin Radiol, 1960, 11: 114–124. [DOI] [PubMed] [Google Scholar]
- 28. Di XY, Jin MS. Limb salvage: radical resection of a pelvic bone tumor: report of two cases. Orthopedics, 1987, 10: 1349–1352. [DOI] [PubMed] [Google Scholar]
- 29. Fu M, Shen JN, Huang G, Wang J, Fu QZ, Yang ZH. Reconstruction of the hemipelvis with saddle prosthesis after excision of malignant tumors around the pelvis and acetabulum: a report of 12 cases. Ai Zheng, 2007, 26: 1237–1242. [PubMed] [Google Scholar]
- 30. Knox K, Bitzos I, Granick M, Datiashvili R, Benevenia J, Patterson F. Immediate reconstruction of oncologic hemipelvectomy defects. Ann Plast Surg, 2006, 57: 184–189. [DOI] [PubMed] [Google Scholar]
- 31. Nishida J, Shiraishi H, Okada K, Ehara S, Shimamura T. Vascularized iliac bone graft for iliosacral bone defect after tumor excision. Clin Orthop Relat Res, 2006, 447: 145–151. [DOI] [PubMed] [Google Scholar]
- 32. Natarajan MV, Bose JC, Mazhavan V, Rajagopal TS, Selvam K. The saddle prosthesis in periacetabular tumours. Int Orthop, 2001, 25: 107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schutte HE, Taconis WK. Giant cell tumor in children and adolescents. Skeletal Radiol, 1993, 22: 173–176. [DOI] [PubMed] [Google Scholar]
- 34. Balke M, Streitbuerger A, Budny T, Henrichs M, Gosheger G, Hardes J. Treatment and outcome of giant cell tumors of the pelvis. Acta Orthop, 2009, 80: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malone S, O'Sullivan B, Catton C, Bell R, Fornasier V, Davis A. Long‐term follow‐up of efficacy and safety of megavoltage radiotherapy in high‐risk giant cell tumours of bone. Int J Radiat Oncol Biol Phys, 1995, 33: 689–694. [DOI] [PubMed] [Google Scholar]
- 36. Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res, 2004, 423: 196–207. [DOI] [PubMed] [Google Scholar]
- 37. Wanebo HJ, Koness RJ, Turk PS, Cohen SI. Composite resection of posterior pelvic malignancy. Ann Surg, 1992, 215: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wallace S, Granmayeh M, deSantos LA, et al Arterial occlusion of pelvic bone tumors. Cancer, 1979, 43: 322–328. [DOI] [PubMed] [Google Scholar]
- 39. Dawson GR. Giant cell tumor of the pelvis at the acetabulum, ilium, ischium, and pubis. J Bone Joint Surg Am, 1955, 37: 1278–1280. [PubMed] [Google Scholar]
- 40. Oda Y, Miura H, Tsuneyoshi M, Iwamoto Y. Giant cell tumor of bone: oncological and functional results of long‐term follow‐up. Jpn J Clin Oncol, 1998, 28: 323–328. [DOI] [PubMed] [Google Scholar]
- 41. Blake SM, Gie GA. Large pelvic giant cell tumor: a case report and a review of current treatment modalities. J Arthroplasty, 2004, 19: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 42. Donati D, Wafa H, Di Bella C, Colangeli M, Colangeli S, Bertoni F. Management of pelvic giant cell tumours involving the acetabular bone. Acta Orthop Belg, 2008, 74: 773–778. [PubMed] [Google Scholar]
- 43. Guo W, Sun X, Zang J, Qu H. Intralesional excision versus wide resection for giant cell tumor involving the acetabulum which is better? Clin Orthop Relat Res, 2012, 470: 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg, 1978, 60: 731–746. [PubMed] [Google Scholar]
- 45. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant‐cell tumor of bone. J Bone Joint Surg Am, 1987, 69: 106–114. [PubMed] [Google Scholar]
- 46. Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en bloc resection for giant‐cell tumors of bone. J Bone Joint Surg Am, 1993, 75: 1648–1655. [DOI] [PubMed] [Google Scholar]
- 47. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant‐cell tumor of bone. J Bone Joint Surg Am, 1986, 68: 235–242. [PubMed] [Google Scholar]
- 48. Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM. Giant‐cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am, 1982, 64: 755–761. [PubMed] [Google Scholar]


