Abstract
This study determines the extent of deposition in gadolinium-based contrast agent–exposed pediatric population and whether prior observations were related to age-dependent breakdown of the blood blood-brain barrier.
Approximately 40% of the 3 million annual pediatric magnetic resonance imaging (MRI) examinations in the United States are performed with intravenous administration of a gadolinium-based contrast agent (GBCA).1 Contrast-enhanced MRI provides critical clinical information that is often not apparent on unenhanced MRI or other imaging modalities. Notwithstanding their widespread use, studies demonstrating the unexpected accumulation of gadolinium within the neural tissues of adults following intravenous GBCA exposure have prompted ongoing investigations by the US Food and Drug Administration and European Medicines Agency regarding the safety and toxicity of these agents.2,3 Because these prior studies were limited to adults, the purpose of this study was to determine the extent of deposition in the GBCA-exposed population and whether prior observations were related to age-dependent breakdown of the blood-brain barrier through study of a cohort of pediatric patients who received gadodiamide-enhanced MRI examinations.
Methods
In this single-center retrospective case-control study approved by the Mayo Clinic institutional review board, postmortem brain tissues of pediatric patients (younger than age 18 years) who died between 2000 and 2015, received at least 4 gadodiamide-enhanced MRI examinations (Omniscan, 287 mg/mL, 0.1 mmol/kg; GE Healthcare, gadodiamide-exposed group), and who underwent autopsy following antemortem consent were compared with pediatric patients who did not receive any gadolinium-enhanced MRI examinations during their lifetime (control group). All clinical and imaging data were extracted from our medical record. Tissue samples were harvested from formalin-fixed whole-brain specimens at neuroanatomic locations (dentate nucleus, pons, globus pallidus, and thalamus) previously shown to be associated with increased levels of gadolinium deposition in adults.4 Hematoxylin-eosin–stained and neurofilament immunostained microscope slides were prepared using standard techniques. Gadolinium quantification and localization within tissue samples were carried out using inductively coupled plasma mass spectrometry and transmission electron microscopy with energy dispersive spectroscopy, respectively, as previously described.4
Results
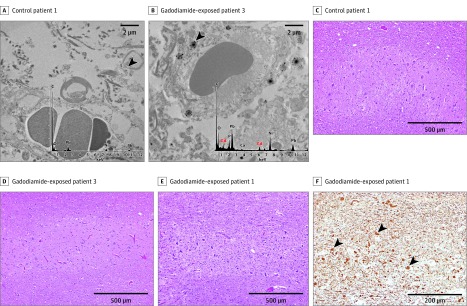
Three gadodiamide-exposed pediatric patients were compared with 3 GBCA-naive control pediatric patients (Table). Elemental gadolinium was detected in all 4 neuroanatomic regions among gadodiamide-exposed patients; the highest concentrations were detected in the dentate nucleus or pons. Transmission electron microscopy with energy dispersive spectroscopy revealed prominent clusters of gadolinium deposits within the endothelium and scattered smaller foci within the neural interstitium within these patients, indicating either direct or indirect transit across the blood-brain barrier (Figure A-B). No gadolinium was detected by inductively coupled plasma mass spectrometry or transmission electron microscopy in control patients. No pathologic changes were observed in dentate tissues of control or gadodiamide-exposed patients (Figure C-E). Dentate tissue from patients 1 and 2 who received the highest cumulative doses of gadodiamide had mildly to severely gliotic regions with prominent axonal spheroids (Figure F). It is unclear whether these pathologic changes are associated with prior external beam radiation therapy or with gadodiamide exposure.
Table. Clinical Characteristics and Mass Spectrometry Results of Study Population.
| Group and Patient Identification | Age at Death, y | Examination Indication | MRI Examinations, No. | Total Gadolinium Dose, mL a | Scan Delay, db | eGFR, mL/min/1.73m2c | Tissue Gadolinium Detected via ICP-MS (μg Gadolinium/g Tissue) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Dentate | Pons | Globus Pallidus | Thalamus | |||||||
| Gadodiamide-exposed group | ||||||||||
| 1 | 8 | Neuroblastoma | 8 | 42 | 59-1140 | 131 (56-246) | 3.0 | 0.1 | 1.2 | 0.2 |
| 2 | 6 | Pontine glioma | 9 | 40 | 8-784 | 92 (64-226) | 2.2 | 0.2 | 1.6 | 0.9 |
| 3 | 13 | Pontine glioma | 4 | 57 | 52-266 | 85 (75-119) | 0.8 | 1.1 | 1.0 | 0.7 |
| Control group | ||||||||||
| 1 | 7 | NA | 0 | NA | NA | 121 (56-146) | 0 | 0 | 0 | 0 |
| 2 | 5 | NA | 0 | NA | NA | 141 (71-142) | 0 | 0 | 0 | 0 |
| 3 | 6 | NA | 0 | NA | NA | 118 (23-119) | 0 | 0 | 0 | 0 |
Abbreviations: eGFR, estimated glomerular filtration rate; ICP-MS, inductively coupled plasma mass spectrometry; MRI, magnetic resonance imaging,
Cumulative gadolinium dose from all intravenous gadolinium-enhanced MRI examinations (milliliters).
Scan delay: time range interval during which MRI examinations took place relative to time of death (day 0).
eGFR values (calculated using Bedside Schwartz isotope dilution mass spectrometry–traceable equation) reported as median (minimum-maximum) ranging from the first to the last date of gadolinium exposure in the contrast group and over the entire age range for the control group.
Figure. Tissue Localization and Cellular Response to Gadolinium Deposition.
Cellular localization of gadolinium using transmission electron microscopy (TEM) on tissue samples stained with 0.2% lead citrate are shown for the dentate nuclei of control patient 1 (A) and gadodiamide-exposed patient 3 (B) at 10 000-fold magnification. X-ray spectra of a selected electron dense foci (black arrowheads) are shown in the inset of each respective panel on a selected electron dense foci; gadolinium peaks in the spectra are indicated by red color overlay. Light microscopy images of hematoxylin-eosin–stained dentate nuclei samples (original magnification × 100) are shown for control patient 1 (C) and gadodiamide-exposed patients 3 and 1 (D and E). F, Neurofilament-immunostained dentate nuclei samples (original magnification × 200) for gadodiamide-exposed patient 1. Arrowheads indicate axonal spheroids. C indicates carbon; ca, calcium; gd, gadolinium; ni, nickel; o; oxygen; and pb, lead.
Discussion
Our findings confirm the presence of intracranial gadolinium deposits in pediatric patients with normal renal function following exposure to intravenous gadodiamide for MRI examinations. Although our data are limited by small sample size, the deposition appears to follow a dose-dependent trend with preferential accumulation in the dentate and deep gray nuclei, similar to what has been described in the adult population.4,5,6 Such confirmation indicates that this deposition occurs independently of patient age.
To our knowledge, there has been no observed adverse clinical symptom associated with gadolinium tissue deposition; yet, caution is warranted because free lanthanides are known to be neurotoxic and potentially mutagenic. Compared with adults, developing pediatric brains are more susceptible to the neurotoxic effects of heavy metal exposure, and it is possible that such susceptibility could also extend to the lanthanide-rare earth metals. For these reasons, ongoing research into the toxicology of GBCA exposure coupled with more judicious use of gadolinium contrast in the pediatric population is essential.
References
- 1.IMV MRI Benchmark Report 2015. http://www.imvinfo.com/index.aspx?sec=mri&sub=def. Accessed May 19, 2016.
- 2.European Medicines Agency PRAC concludes assessment of gadolinium agents used in body scans and recommends regulatory actions, including suspension for some marketing authorizations. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Gadolinium-containing_contrast_agents/human_referral_prac_000056.jsp&mid=WC0b01ac05805c516f Published April 7, 2017. Accessed April 18, 2017.
- 3.FDA Drug Safety Communication FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging. https://www.fda.gov/Drugs/DrugSafety/ucm455386.htm Published 2015. Accessed April 18, 2017.
- 4.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772-782. [DOI] [PubMed] [Google Scholar]
- 5.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276(1):228-232. [DOI] [PubMed] [Google Scholar]
- 6.Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51(7):447-453. [DOI] [PubMed] [Google Scholar]