Abstract
Patient‐specific instrumentation (PSI) technology has been developed to improve alignment when implanting total knee arthroplasty (TKA) and is a new focus in the orthopaedic community. Current controversial data concerning PSI are discussed. A systematic review to compare PSI with conventional instrumentation and assess the radiographic outcomes was performed. Electronic databases (including PubMed, Medline, Embase, the Cochrane Library and the Science Citation Index database) and conference proceedings from 1950 to 2014 in the English language were searched. Data, including relevant patient characteristics, sample size, radiographic method, PSI system manufacturer and outliers of implant positioning and alignment on radiography were independently extracted from all eligible studies by two of the authors. A total of 2739 TKAs were included (1410 performed with PSI and 1329 with conventional instrumentation). There were more TCA outliers (malalignment >3°) and tibial slope outliers (malalignment >3°) in the PSI group than in the conventional group. The other radiographic outcomes assessed, including coronal, sagittal or rotational alignment outliers did not differ between the two groups. With regard to radiographic outcomes, our findings indicate that PSI technology is not superior in reducing outliers of component alignment.
Keywords: Component alignment, Radiograph, Total knee arthroplasty
Introduction
Total knee arthroplasty (TKA) is a surgical intervention that provides pain relief, enhances mobility, and improves quality of life of patients with end‐stage knee arthritis. With increases in population size and longevity, the incidence of TKA is predicted to increase in the future1. With TKA, component alignment and position are important predictors of clinical outcome and longevity2, 3. The accuracy of conventional instrumentation has been questioned by many authors; the rate of implant malposition can be as high as 20%–40%4. Since the introduction of these systems in the 1970s, they have been modified repeatedly but without radical changes or substitutes. Technological advances aimed at improving limb alignment and component position include computer‐assisted surgery and patient‐specific instrumentation (PSI)5, 6, 7. Computer‐assisted surgery is rarely performed because of its drawbacks, which include difficulty in identifying landmarks accurately intraoperatively, increased set‐up and operative time, increased perioperative cost and a substantial learning curve5, 6.
Patient‐specific instrumentation is a new concept in which computer‐assisted preoperative planning and rapid prototyping technology provides patient‐specific instruments that can replace conventional instrumentation systems. Preoperative CT or MRI scans are acquired and imported to a special software system. Planning of sizing, alignment, and bone cutting and verification of optimal implantation and positioning is performed on a computer and two virtual templates designed and transformed into physical guides preoperatively. Information built into the guides can be transferred to the patient's knee: surgeons can use these guides or conventional cutting blocks. TKA can then be performed without using intra‐or extramedullary guides7.
Patient‐specific instrumentation can theoretically improve the accuracy of component position and alignment; however, the clinical results are currently controversial8, 9, 10, 11. We therefore performed a systematic review to compare the radiographic outcomes between PSI and conventional instrumentation.
Materials and Methods
A systematic search of the PubMed, Medline, Embase, Cochrane Library and Science Citation Index (SCI) databases was performed. The search terms were as follows: (“patient‐specific” or “patient specific” or custom*) AND (instrument* or template* or cutting or guide* or block*) AND (“total knee replacement” or “Total knee arthroplasty” or “Total knee arthroplasties”). The search was performed on 1 June 2014. Inclusion criteria were as follows: i) clinical research studies comparing PSI technology to conventional technology in TKA; ii) analysis of component alignment; iii) primary TKA; and iv) patients aged over 18 years. The titles of all studies were identified by the initial search and assessed by two authors. Review articles, technique descriptions, and editorials were excluded, as were clearly non‐relevant studies that failed to meet the inclusion criteria. Abstracts and full reports of the remaining studies were then independently assessed by two authors according to agreed inclusion and exclusion criteria. Disagreements were resolved by discussion until consensus was reached. When the two reviewers were unable to reach consensus, a third reviewer was asked for a final opinion, resulting in a group consensus. A flow diagram shows the search process (Fig. 1).
Figure 1.

Study flow diagram. A summary of the search process and study identification. 24 studies were included in the final analysis.
Eventually, 24 studies were eligible for this study (Table 1). Two authors independently extracted relevant data from all eligible studies, including patient characteristics, sample size, PSI system manufacturer, radiographic method and outcomes in terms of radiographically‐identified outliers. Radiographic measurements for coronal alignment were as follows: hip‐knee‐ankle angle (HKA), femorotibial angle (FTA), femoral coronal angle (FCA, defined as the medial angle between the femoral component and the mechanical axis of the femur) and tibia coronal angle (TCA, measured as the angle between the tibial component and the mechanical axis of the tibia). Radiographic measurements for sagittal alignment were the lateral femoral component angle (LFC angle, defined as the anterior angle between the femoral component and the anterior cortex of the femur), LTC angle (lateral tibia component angle, defined as the anterior angle between the tibial component and the posterior cortex of the tibia), femoral flexion angle and tibial slope angle. Radiographic measurements for rotational alignment were femoral rotational angle and tibial rotational angle. Two types of outliers with two different cutoff values (malalignment >3° or >2°) were recorded. Two authors independently identified the radiographic measurements of each eligible study and recorded the outliers.
Table 1.
Details of eligible studies
| Year | Journal | First author | Total | PSI | Con | PSI system | Study design | Measurement method |
|---|---|---|---|---|---|---|---|---|
| 2014 | Int Orthop (SICOT) | Kotela9 | 112 | 52 | 60 | Signature; Biomet | RCT | X‐ray |
| 2013 | Clin Orthop Relat Res | Roh10 | 100 | 50 | 50 | Signature; Biomet | RCT | X‐ray; CT |
| 2014 | J Bone Joint Surg Am | Woolson11 | 48 | 22 | 26 | TruMatch; DePuy | RCT | CT |
| 2014 | Knee Surg Sports Traumatol Arthrosc | Silva12 | 45 | 23 | 22 | Signature; Biomet | RCT | CT |
| 2014 | Knee | Chotanaphuti13 | 80 | 40 | 40 | TruMatch; DePuy | RCT | X‐ray; CT |
| 2013 | Knee Surg Sports Traumatol Arthrosc | Parratte14 | 40 | 20 | 20 | Unknown | RCT | X‐ray; CT |
| 2013 | Knee Surg Sports Traumatol Arthrosc | Boonen15 | 180 | 90 | 90 | Unknown | RCT | X‐ray |
| 2013 | J Arthroplasty | Hamilton16 | 52 | 26 | 26 | TruMatch; DePuy | RCT | X‐ray |
| 2013 | Knee Surg Sports Traumatol Arthrosc | Ng17 | 78 | 51 | 27 | PSI system; Zimmer | RCT | CT |
| 2013 | Bone Joint J | Chareancholvanich18 | 80 | 40 | 40 | PSI system; Zimmer | RCT | X‐ray |
| 2012 | Orthopedics | Dossett19 | 82 | 41 | 41 | Unknown | RCT | CT |
| 2014 | Clin Orthop Relat Res | Victor20 | 125 | 61 | 64 | Signature Biomet; TruMatch DePuy; Visionaire Smith & Nephew; PSI Zimmer | RCT | X‐ray; CT |
| 2012 | J Arthroplasty | Noble21 | 29 | 15 | 14 | Visionaire; Smith & Nephew | Quasi‐RCT | X‐ray |
| 2013 | J Arthroplasty | Vundelinckx22 | 62 | 31 | 31 | Visionaire; Smith & Nephew | Quasi‐RCT | X‐ray |
| 2012 | Clin Orthop Relat Res | Ng23 | 160 | 105 | 55 | Signature; Biomet | Retrospective cohort | X‐ray |
| 2014 | J Arthroplasty | Marimuthu24 | 300 | 115 | 185 | Visionaire; Smith & Nephew | Retrospective cohort | CT |
| 2014 | Orthop Traumatol Surg Res | Moubarak25 | 68 | 57 | 11 | Visionaire; Smith & Nephew | Prospective cohort | X‐ray |
| 2014 | J Arthroplasty | Stronach26 | 120 | 58 | 62 | Signature; Biomet | Retrospective cohort | X‐ray |
| 2014 | Int Orthop | Daniilidis27 | 340 | 170 | 170 | Visionaire; Smith & Nephew | Retrospective cohort | X‐ray |
| 2014 | Knee | Heyse28 | 94 | 46 | 48 | Visionaire; Smith & Nephew | Retrospective cohort | MRI |
| 2012 | J Bone Joint Surg Br | Barrack29 | 200 | 100 | 100 | Unknown | Prospective cohort | CT |
| 2012 | Acta Orthop | Boonen30 | 80 | 40 | 40 | Signature; Biomet | Retrospective Cohort | X‐ray |
| 2012 | Clin Orthop Relat Res | Nunley31 | 150 | 100 | 50 | OtisMed; Styker | Retrospective Cohort | X‐ray; CT |
| 2012 | Clin Orthop Relat Res | Nunley32 | 114 | 57 | 57 | Signature; Biomet | Retrospective Cohort | CT |
Con, conventional instrumentation; PSI, patient‐specific instrumentation; Quasi‐RCT, quasi‐random controlled trial; RCT, randomized controlled trial.
A meta‐analysis was performed with RevMan software (“Review Manager,” version 5.1, Nordic Cochrane Centre, Copenhagen, Denmark). A random effects meta‐analysis was generated using these dichotomous data to compare PSI with conventional instrumentation with regard to outliers of coronal alignment, sagittal alignment and rotational alignment. A P value of 0.05 was considered statistically significant.
Results
The final 24 studies included 2739 total knee arthroplasties (1410 performed with PSI and 1329 with conventional instrumentation). The details of these 24 studies are shown in Table 1. Twelve studies were randomized trials9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, two were quasi‐randomized trials21, 22 and ten were non‐randomized comparative trials23, 24, 25, 26, 27, 28, 29, 30, 31, 32. Five different PSI systems were utilized in the studies: Biomet Signature (Warsaw, IN, USA), Zimmer Patient Specific Instruments (Minneapolis, MN, USA), Smith & Nephew Visionaire (Memphis, TN, USA), DePuy TruMatch (Raynham, MA, USA) and Stryker OtisMed (Kalamazoo, MI, USA).
Coronal Alignment
Sixteen studies reported HKA outliers of malalignment >3°, the odds ratio for HKA outliers of malalignment >3° in the PSI group compared with the conventional group was 1.04 and the odds ratio for HKA outliers of malalignment >2° in the PSI group was 0.70. Four studies reported FTA outliers of malalignment >3°; the odds ratio for HKA outliers of malalignment >3° in the PSI group compared with the conventional group was 1.08. Both the incidence of FCA outliers of malalignment >3° and malalignment >2° were not statistically significant; the odds ratios were 0.72 (P = 0.16) and 0.69 (P = 0.23) respectively. The incidence of TCA outliers of malalignment >2° was also not significantly different (odds ratio: 1.01, P = 0.97); however, the odds ratio for outliers of malalignment >3° was 2.22 (P = 0.001); there were thus significantly fewer TCA outliers of malalignment >3° in the conventional group (Figs 2, 3, 4, 5).
Figure 2.

Forest plot for HKA outliers (malalignment >3°).
Figure 3.
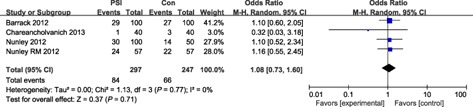
Forest plot for FTA outliers (malalignment >3°).
Figure 4.

Forest plot for FCA outliers (malalignment >3°).
Figure 5.
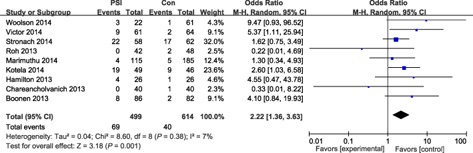
Forest plot for TCA outliers (malalignment >3°).
Sagittal Alignment
All femoral sagittal variables, including LFC outliers (malalignment >3°) and femoral flexion angle outliers (malalignment >3° or 2°) did not differ significantly between the two groups. LTC outliers of malalignment >3° were equivalent between the two groups (odds ratio, 1.46; P = 0.42). Thus, decreased tibial slope angle (outliers of malalignment >3°) occurred significantly less often with conventional instrumentation (odds ratio, 1.97; P = 0.001); however, outliers of malalignment >2° was not significantly different between the two groups (Figs 6, 7, 8, 9).
Figure 6.
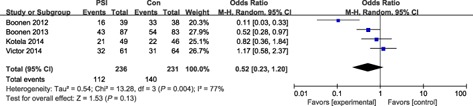
Forest plot for LFC outliers (malalignment >3°).
Figure 7.

Forest plot for femoral flexion outliers (malalignment >3°).
Figure 8.

Forest plot for LTC outliers (malalignment >3°).
Figure 9.
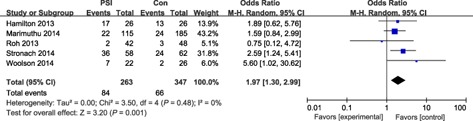
Forest plot for tibial slope outliers (malalignment >3°).
Rotational Alignment
The incidence of femoral rotation outliers of malalignment >3° did not differ significantly between the two groups (odds ratio, 0.75; P = 0.38). The heterogeneity of the other two comparisons, namely femoral rotation outliers of malalignment >2° and tibial rotation outliers of malalignment >3° was unacceptable, so differences could not be accurately assessed (Fig. 10).
Figure 10.

Forest plot for femoral rotation outliers (malalignment >3°).
Discussion
This analysis showed that more TCA outliers of malalignment >3° and tibial slope outliers of malalignment >3°) in the PSI than in the conventional group. The other outliers of radiographic outcomes (HKA, FTA, FCA, LFC, LTC, femoral flexion angle and rotational angle) showed no differences between the two groups. These findings results are similar to those of previous studies33, 34, 35. Thus, there is still no evidence for routine use of PSI in TKA36.
These disappointing findings can be attributed to the following factors. First, PSI is a new technique for most surgeons, whereas conventional technology has been developed for several decades and surgeons are therefore more familiar with it. The different degree of familiarity may have influenced the outcomes. Second, the level of experience of orthopaedic surgeons influences the accuracy of operation; accordingly, the differences in radiographic outcome between the PSI group and conventional technology group were not statistically significant37. Third, the clinical use of PSI is at an exploratory stage and there are many problems in practice26, 38, 39. For example, some studies reported making intraoperative modifications. Stronach et al. made 161 changes in 66 cases in regard to thickness, angles and sizes (an average of 2.4 changes per knee)26. Issa et al. reported 29 intraoperative changes in 89 primary TKAs using PSI technology38. The above factors may account for the discrepancy between the theoretical advantages and the reality.
We also noted that some studies reported using PSI in some technically demanding cases in which the normal anatomical landmarks were hard to find and it was therefore difficult to achieve correct alignment. Thienpont et al. used TKA with the assistance of PSI to treat patients with knee arthritis and extra‐articular deformities from malunion or with retained femoral hardware40. Postoperatively, limb alignment was restored with a mean hip–knee–ankle angle of 179.3° ± 1.3° (P < 0.05). Maximum outliers were 177° to 181°. They concluded that mechanical alignment can easily be obtained with PSI technology by intra‐articular correction of deformities under 20°. Kerens et al. used PSI for revision of medial uni‐condylar knee arthroplasty to TKA41. Seven of 10 femoral prostheses were within the desired AP and sagittal angles of ±3°, all tibial components were within the desired AP angle of ±3° and 7 of 10 were within the desired sagittal angle. The hip–knee–ankle angle was within 0° ± 3° in 8 of 10 cases. The PSI technique provides a new and less demanding approach to preoperative planning and execution of the plan during surgery in some special situations.
This study has several limitations. One important limitation of this study is the intrinsic drawbacks of meta‐analysis. Although we assessed heterogeneity and bias in the randomized controlled trials, quasi‐ randomized controlled trials and retrospective cohort studies, the final conclusions should be interpreted with caution. Further studies with high evidence levels are necessary. An additional limitation is that the PSI systems of different manufacturers are based on MRI or CT, which provides a potential bias. There were too few data to perform subgroup analysis. We also did not analyze detailed data for each angle because postoperative radiographic methods differed between the studies and the measurements were not uniform. The variable of outliers is consistent and reliable; however, much potential information may be missing.
In conclusion, this study does not confirm the claimed benefit that PSI technology provides better accuracy of alignment than conventional technology.
Disclosure: All authors have no interests related to the subject of this article.
References
- 1. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol, 2010, 11: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res, 2010, 468: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parratte S, Pagnano MW, Trousdale RT, Berry DJ. Effect of postoperative mechanical axis alignment on the fifteen‐year survival of modern, cemented total knee replacements. J Bone Joint Surg Am, 2010, 92: 2143–2149. [DOI] [PubMed] [Google Scholar]
- 4. Hetaimish BM, Khan MM, Simunovic N, Al‐Harbi HH, Bhandar M, Zalzal PK. Meta‐analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty, 2012, 27: 1177–1182. [DOI] [PubMed] [Google Scholar]
- 5. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta‐analysis of alignment outcomes in computer‐assisted total knee arthroplasty surgery. J Arthroplasty, 2007, 22: 1097–1106. [DOI] [PubMed] [Google Scholar]
- 6. Cheng T, Zhao S, Peng X, Zhang X. Does computer‐assisted surgery improve postoperative leg alignment and implant positioning following total knee arthroplasty? A meta‐analysis of randomized controlled trials? Knee Surg Sports Traumatol Arthrosc, 2012, 20: 1307–1322. [DOI] [PubMed] [Google Scholar]
- 7. Hafez MA, Chelule KL, Seedhom BB, Sherman KP. Computer‐assisted total knee arthroplasty using patient‐specific templating. Clin Orthop Relat Res, 2006, 444: 184–192. [DOI] [PubMed] [Google Scholar]
- 8. Russell R, Brown T, Huo M, Jones R. Patient‐specific instrumentation does not improve alignment in total knee arthroplasty. J Knee Surg, 2014, 27: 501–504. [DOI] [PubMed] [Google Scholar]
- 9. Kotela A, Kotela I. Patient‐specific computed tomography based instrumentation in total knee arthroplasty: a prospective randomized controlled study. Int Orthop, 2014, 38: 2099–2107. [DOI] [PubMed] [Google Scholar]
- 10. Roh YW, Kim TW, Lee S, Seong SC, Lee MC. Is TKA using patient‐specific instruments comparable to conventional TKA? A randomized controlled study of one system. Clin Orthop Relat Res, 2013, 471: 3988–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woolson ST, Harris AH, Wagner DW, Giori NJ. Component alignment during total knee arthroplasty with use of standard or custom instrumentation: a randomized clinical trial using computed tomography for postoperative alignment measurement. J Bone Joint Surg Am, 2014, 96: 366–372. [DOI] [PubMed] [Google Scholar]
- 12. Silva A, Sampaio R, Pinto E. Patient‐specific instrumentation improves tibial component rotation in TKA. Knee Surg Sports Traumatol Arthrosc, 2014, 22: 636–642. [DOI] [PubMed] [Google Scholar]
- 13. Chotanaphuti T, Wangwittayakul V, Khuangsirikul S, Foojareonyos T. The accuracy of component alignment in custom cutting blocks compared with conventional total knee arthroplasty instrumentation: prospective control trial. Knee, 2014, 21: 185–188. [DOI] [PubMed] [Google Scholar]
- 14. Parratte S, Blanc G, Boussemart T, Ollivier M, Le Corroller T, Argenson JN. Rotation in total knee arthroplasty: no difference between patient‐specific and conventional instrumentation. Knee Surg Sports Traumatol Arthrosc, 2013, 21: 2213–2219. [DOI] [PubMed] [Google Scholar]
- 15. Boonen B, Schotanus MG, Kerens B, van der Weegen W, van Drumpt RA, Kort NP. Intra‐operative results and radiological outcome of conventional and patient‐specific surgery in total knee arthroplasty: a multicentre, randomised controlled trial. Knee Surg Sports Traumatol Arthrosc, 2013, 21: 2206–2212. [DOI] [PubMed] [Google Scholar]
- 16. Hamilton WG, Parks NL, Saxena A. Patient‐specific instrumentation does not shorten surgical time: a prospective, randomized trial. J Arthroplasty, 2013, 28 (8 Suppl.): 96–100. [DOI] [PubMed] [Google Scholar]
- 17. Ng VY, Arnott L, Li J, et al Comparison of custom to standard TKA instrumentation with computed tomography. Knee Surg Sports Traumatol Arthrosc, 2014, 22: 1833–1842. [DOI] [PubMed] [Google Scholar]
- 18. Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient‐specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J, 2013, 95: 354–359. [DOI] [PubMed] [Google Scholar]
- 19. Dossett HG, Swartz GJ, Estrada NA, LeFevre GW, Kwasman BG. Kinematically versus mechanically aligned total knee arthroplasty. Orthopedics, 2012, 35: e160–e169. [DOI] [PubMed] [Google Scholar]
- 20. Victor J, Dujardin J, Vandenneucker H, Arnout N, Bellemans J. Patient‐specific guides do not improve accuracy in total knee arthroplasty: a prospective randomized controlled trial. Clin Orthop Relat Res, 2014, 472: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noble JW Jr, Moore CA, Liu N. The value of patient matched instrumentation in total knee arthroplasty. J Arthroplasty, 2012, 27: 153–155. [DOI] [PubMed] [Google Scholar]
- 22. Vundelinckx BJ, Bruckers L, De Mulder K, De Schepper J, Van Esbroeck G. Functional and radiographic short‐term outcome evaluation of the Visionaire system, a patient‐matched instrumentation system for total knee arthroplasty. J Arthroplasty, 2013, 28: 964–970. [DOI] [PubMed] [Google Scholar]
- 23. Ng VY, DeClaire JH, Berend KR, Gulick BC, Lombardi AV Jr. Improved accuracy of alignment with patient‐specific positioning guides compared with manual instrumentation in TKA. Clin Orthop Relat Res, 2012, 470: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marimuthu K, Chen DB, Harris IA, Wheatley E, Bryant CJ, MacDessi SJ. A multi‐planar CT‐based comparative analysis of patient‐specific cutting guides with conventional instrumentation in total knee arthroplasty. J Arthroplasty, 2014, 29: 1138–1142. [DOI] [PubMed] [Google Scholar]
- 25. Moubarak H, Brilhault J. Contribution of patient‐specific cutting guides to lower limb alignment for total knee arthroplasty. Orthop Traumatol Surg Res, 2014, 100 (4 Suppl.): S239–S242. [DOI] [PubMed] [Google Scholar]
- 26. Stronach BM, Pelt CE, Erickson J, Peters CL. Patient‐specific total knee arthroplasty required frequent surgeon‐directed changes. Clin Orthop Relat Res, 2013, 471: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daniilidis K, Tibesku CO. Frontal plane alignment after total knee arthroplasty using patient‐specific instruments. Int Orthop, 2013, 37: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heyse TJ, Tibesku CO. Improved femoral component rotation in TKA using patient‐specific instrumentation. Knee, 2014, 21: 268–271. [DOI] [PubMed] [Google Scholar]
- 29. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br, 2012, 94 (11 Suppl. A): 95–99. [DOI] [PubMed] [Google Scholar]
- 30. Boonen B, Schotanus MG, Kort NP. Preliminary experience with the patient‐specific templating total knee arthroplasty. Acta Orthop, 2012, 83: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nunley RM, Ellison BS, Ruh EL, et al Are patient‐specific cutting blocks cost‐effective for total knee arthroplasty? Clin Orthop Relat Res, 2012, 470: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient‐specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res, 2012, 470: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stronach BM, Pelt CE, Erickson JA, Peters CL. Patient‐specific instrumentation in total knee arthroplasty provides no improvement in component alignment. J Arthroplasty, 2014, 29: 1705–1708. [DOI] [PubMed] [Google Scholar]
- 34. Fu H, Wang J, Zhou S, et al No difference in mechanical alignment and femoral component placement between patient‐specific instrumentation and conventional instrumentation in TKA. Knee Surg Sports Traumatol Arthrosc, 2014, June 11. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Thienpont E, Schwab PE, Fennema P. A systematic review and meta‐analysis of patient‐specific instrumentation for improving alignment of the components in total knee replacement. Bone Joint J, 2014, 96: 1052–1061. [DOI] [PubMed] [Google Scholar]
- 36. Voleti PB, Hamula MJ, Baldwin KD, Lee GC. Current data do not support routine use of patient‐specific instrumentation in total knee arthroplasty. J Arthroplasty, 2014, 29: 1709–1712. [DOI] [PubMed] [Google Scholar]
- 37. Plaskos C, Hodgson AJ, Inkpen K, McGraw RW. Bone cutting errors in total knee arthroplasty. J Arthroplasty, 2002, 17: 698–705. [DOI] [PubMed] [Google Scholar]
- 38. Issa K, Rifai A, McGrath MS, et al Reliability of templating with patient‐specific instrumentation in total knee arthroplasty. J Knee Surg, 2013, 26: 429–433. [DOI] [PubMed] [Google Scholar]
- 39. Scholes C, Sahni V, Lustig S, Parker DA, Coolican MR. Patient‐specific instrumentation for total knee arthroplasty does not match the pre‐operative plan as assessed by intra‐operative computer‐assisted navigation. Knee Surg Sports Traumatol Arthrosc, 2014, 22: 660–665. [DOI] [PubMed] [Google Scholar]
- 40. Thienpont E, Paternostre F, Pietsch M, Hafez M, Howell S. Total knee arthroplasty with patient‐specific instruments improves function and restores limb alignment in patients with extra‐articular deformity. Knee, 2013, 20: 407–411. [DOI] [PubMed] [Google Scholar]
- 41. Kerens B, Boonen B, Schotanus M, Kort N. Patient‐specific guide for revision of medial unicondylar knee arthroplasty to total knee arthroplasty: beneficial first results of a new operating technique performed on 10 patients. Acta Orthop, 2013, 84: 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]


