Introduction
Deep venous thrombosis and pulmonary embolism are a major cause of morbidity and mortality after arthroplasty. Chemoprophylaxis has been effective in reducing the incidence of symptomatic deep venous thrombosis, but not of pulmonary embolism, after total hip arthroplasty (THA) and total knee arthroplasty (TKA)1. Rivaroxaban, an orally administered factor Xa inhibitor, has recently been introduced for reducing the incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) after hip and knee arthroplasty2, 3, 4, 5.
Factor Xa catalyzes the conversion of prothrombin to thrombin. Because a single molecule of Xa can generate thousands of thrombin molecules, factor Xa is an excellent drug target6. In many ways, rivaroxaban is an ideal drug, being orally administered, usually requiring no monitoring and having a faster onset of action than warfarin. However, like other effective thromboprophylactic agents, rivaroxaban has been associated with an increased incidence of bleeding from surgical wounds or other sites. Here we present a case of a patient who developed a symptomatic epidural hematoma after postoperative administration of rivaroxaban. The patient was informed that we wanted to submit this case for publication and gave written permission.
The purpose of this report was to describe an unanticipated, early complication of a new type of DVT chemoprophylaxis after total knee arthroplasty, thus educating patients and surgeons about the possible risks of chemoprophylaxis. This patient appeared to develop the complication while bridging from a different anticoagulant to rivaroxaban. We now recommend extreme caution re‐using rivaroxaban in the setting of recent spinal anesthesia after arthroplasty.
Case Report
History
The patient, 53‐year‐old woman, underwent a routine revision left total knee arthroplasty under spinal/epidural anesthetic, was discharged from hospital on postoperative day 2 and represented on postoperative day 7. She commenced 5 mg warfarin on hospital day 0 (day of surgery) and received 6.5 mg warfarin on hospital day 1 and 7 mg warfarin on postoperative day 2. She also received 40 mg enoxaparin on postoperative days 1 and 2. After an uncomplicated stay, she was discharged home on postoperative day 2 on warfarin and enoxaparin for DVT prevention. The international normalized ratio (INR) on postoperative day 2 was 1.0. She received 7.0 mg warfarin and 40 mg enoxaparin on postoperative day 3. The last doses of warfarin and enoxaparin were on postoperative day 3. On postoperative day 4, she was switched to rivaroxaban by an outpatient physician.
On postoperative day 6, she began to experience the atraumatic onset of progressively worsening burning pain down the right leg. By postoperative day 7, she had unbearable pain in her right buttock, thigh, posterolateral calf, and foot. She also reported numbness and paresthesias in both buttocks and the perineal region and several episodes of feeling that her bladder was not emptying completely; this progressed to frank urinary incontinence. She had a known history of lumbar stenosis for which she had undergone outpatient MRI, but she had no leg pain, weakness, numbness, or incontinence prior to starting the rivaroxaban. She had not undergone any spinal surgeries nor received any therapeutic epidural steroid injections. She had undergone a right total knee arthroplasty under spinal anesthetic six months previously with no complications. She had no known history of bleeding conditions or renal impairment.
Physical Examination
On examination on readmission (postoperative day 7), she had marked ecchymoses on her buttocks and right thigh. The strength of various muscles was as follows: both quadriceps 4/5, right tibialis anterior 2/5, right extensor hallucis longus 2/5, right gastrocsoleus 2/5, left tibialis anterior 4/5, left extensor hallucis longus 4/5, and left gastrocsoleus 4/5. She had decreased volitional rectal tone and decreased perineal sensation. A Foley catheter was inserted after she attempted voiding; her post‐void residual volume was 1.5 L. Her left knee was unremarkable. There was no wound drainage or wound hematoma. Her serum creatinine concentration was 0.9 mg/dL, INR 0.9, prothrombin time 10.0 s, partial thromboplastin time 21.7 s, hemoglobin 12.8 g/dL, and platelet count 262 × 109/L; all of these laboratory findings being within the normal range.
She underwent an emergencyt MRI that confirmed severe stenosis and a right L4–5 epidural hematoma (Fig. 1A and 1B). Specifically, the MRI showed a region of high signal intensity on T1 at L4–5, intermediate intensity on T2 that displaced the thecal sac and nerve rootlets to the left side of the spinal canal and contrast enhancement in the epidural space.
Figure 1.
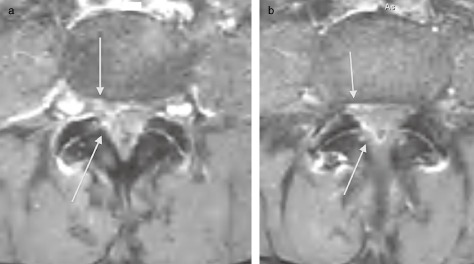
(a) Axial T 1 weighted post contrast image displaying increased enhancement adjacent to the ligamentum flavum of the L 4–5 level and on the right ventral epidural space (arrows). (b) Axial T 1 weighted post contrast image (1 mm caudal to Fig. 1a) displaying increased enhancement in the right epidural space both ventral and dorsal to the thecal sac (arrows).
Treatment
Based on these findings, she underwent an emergency L4,5 laminectomy and evacuation of a large, right, posterolateral, consolidated hematoma displacing the thecal sac, substantially freeing the nerve roots. No bleeding vessels were identified. A topical hemostatic agent was applied to the wound.
Outcome
Immediately postoperatively, she reported complete resolution of her leg pain. Her strength improved to baseline bilaterally. Because she had a neurogenic bladder, she was discharged home with a Foley catheter. She was able to complete knee arthroplasty rehabilitation. Because of her high thromboembolic disease risk (from the knee arthroplasty and cauda equina syndrome) and contraindications to anticoagulation (recent epidural hematoma and spinal surgery), an inferior vena cava filter was inserted. Anticoagulation with warfarin was initiated on day 3 following the spine decompression to reduce the risk of recurrent herniation.
At two‐year follow‐up, she was free of back and leg pain. She had residual peroneal anesthesia and neurogenic bladder with overflow incontinence but was able to control her bladder with a scheduled voiding regimen.
Discussion
We believe this is the first reported case of symptomatic spinal epidural hematoma after administration of an oral factor X inhibitor (rivaroxaban). We contend that this complication was uniquely attributable to the drug because it developed more than 72 hours after surgery coincident with commencement of this medication. We therefore recommend extreme caution in the use of rivaroxaban in patients who have undergone neuraxial anesthesia.
No published studies have established an significantly increased risk of bleeding with rivaroxaban. In the REgulation of Coagulation in ORthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism (RECORD) study, the major bleeding risks of rivaroxaban (starting 6–8 hours post‐operatively) and low molecular weight heparin (enoxaparin) were similar3, 5, 7, 8, 9. However, a recent meta‐analysis of pooled data from prospective trials found that rivaroxaban has a non‐significant trend towards an greater risk of hemorrhage than enoxaparin (RR 1.26; 95% CI 0.94–1.69, P = 0.13)10.
No intraspinal bleeding events were attributed to rivaroxaban administration in phase II or phase III prospective studies of rivaroxaban use after hip and knee arthroplasty3, 8, 11, 12, 13. Spinal bleeding episodes have been reported in rivaroxaban phase III clinical trials; however, these were all hemorrhagic spinal punctures prior to the administration of rivaroxaban. The bleeding episodes, which were asymptomatic, were therefore not attributed to drug use. One study specified that the patient had no signs or symptoms of neural compression7. Another study that reported a hemorrhagic spinal puncture prior to the administration of rivaroxaban did not describe any neurological compromise5.
We treated our patient according to the prescribing information, which specifies that epidural catheters should be placed or removed when the anticoagulant effect of the drug is low. The company (Janssen Pharmaceuticals, Titusville, NJ, USA) recommendation is “An epidural catheter should not be removed earlier than 18 hours after the last administration of XARELTO. The next XARELTO dose is not to be administered earlier than 6 hours after removal of the catheter. Delay the administration of XARELTOfor 24 hours if traumatic puncture occurs”. In our case, the epidural catheter was removed on postoperative day 1 and the patient discharged home on enoxaparin/warfarin in accordance with the anticoagulation protocol at our institution. An outside physician switched the patient to rivaroxaban on postoperative day 4 because she had not reached the therapeutic window on warfarin. The prescribing information advises against concurrent use of rivaroxaban with other anticoagulants. To the authors' knowledge, there are no studies describing the safe dosing or timing for bridging DVT prophylaxis from another agent to rivaroxaban after arthroplasty. However, the clinical scenario in which another anticoagulant is used to bridge patients to warfarin is relatively common. This case illustrates that the incidence of real‐world adverse outcomes associated with oral anticoagulants may be higher than that reported in clinical trials, because in non‐trial settings, deviations from established protocols occur. Such complications after systemic anticoagulant therapy are common and, overall, may contribute to a higher than recognized overall all‐cause mortality with anticoagulants14.
Other factors, which may have contributed to hematoma formation include the patient's preexisting congenital spinal stenosis and L4–5 disc herniation. However, she had no symptoms of spinal stenosis or radiculopathy prior to the knee arthroplasty and had successfully undergone a prior knee arthroplasty under spinal regional anesthesia without complications. Therefore, we do not believe that patient factors were sufficiently abnormal to provide the sole explanation for her adverse event in this case.
This is the first reported case of intraspinal hematoma causing permanent neurological deficit after administration of rivaroxaban. Based on this experience, we recommend extreme caution in patients in prescribing rivaroxaban to patients who have undergone neuraxial anesthesia. Furthermore, we recommend that no other agents be used prior to administration of rivaroxaban because information on the safety of rivaroxaban bridging is unavailable, which likely contributed to the complication in this case. Further study is necessary to determine if the bleeding risk is inherent to the drug.
Disclosure: The authors state that they have no actual or potential conflicts of interest.
References
- 1. Johanson NA, Lachiewicz PF, Lieberman JR, et al Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Am Acad Orthop Surg, 2009, 17: 183–196. [DOI] [PubMed] [Google Scholar]
- 2. EINSTEIN Investigators , Bauersachs R, Berkowitz SD, et al Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med, 2010, 363: 2499–2510. [DOI] [PubMed] [Google Scholar]
- 3. Eriksson BI, Borris LC, Friedman RJ, et al Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med, 2008, 358: 2765–2775. [DOI] [PubMed] [Google Scholar]
- 4. Landman GW, Gans RO. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med, 2011, 364: 1178. [DOI] [PubMed] [Google Scholar]
- 5. Lassen MR, Ageno W, Borris LC, et al Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med, 2008, 358: 2776–2786. [DOI] [PubMed] [Google Scholar]
- 6. Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med, 2005, 353: 1028–1040. [DOI] [PubMed] [Google Scholar]
- 7. Eriksson BI, Kakkar AK, Turpie AG, et al Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br, 2009, 91: 636–644. [DOI] [PubMed] [Google Scholar]
- 8. Kakkar AK, Brenner B, Dahl OE, Eriksson BI, et al Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomised controlled trial. Lancet, 2008, 372: 31–39. [DOI] [PubMed] [Google Scholar]
- 9. Turpie AG, Lassen MR, Davidson BL, et al Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet, 2009, 373: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 10. Loke YK, Kwok CS. Dabigatran and rivaroxaban for prevention of venous thromboembolism–systematic review and adjusted indirect comparison. J Clin Pharm Ther, 2011, 36: 111–124. [DOI] [PubMed] [Google Scholar]
- 11. Green L, Lawrie AS, Patel S, et al The impact of elective knee/hip replacement surgery and thromboprophylaxis with rivaroxaban or dalteparin on thrombin generation. Br J Haematol, 2010, 151: 469–476. [DOI] [PubMed] [Google Scholar]
- 12. Eriksson BI, Borris LC, Dahl OE, et al A once‐daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59‐7939), for thromboprophylaxis after total hip replacement. Circulation, 2006, 114: 2374–2381. [DOI] [PubMed] [Google Scholar]
- 13. Turpie AG, Fisher WD, Bauer KA, et al BAY 59‐7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose‐ranging study. J Thromb Haemost, 2005, 3: 2479–2486. [DOI] [PubMed] [Google Scholar]
- 14. Sharrock NE, Gonzalez Della Valle A, Go G, Lyman S, Salvati EA. Potent anticoagulants are associated with a higher all‐cause mortality rate after hip and knee arthroplasty. Clin Orthop Relat Res, 2008, 466: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]


