This post hoc cross-sectional analysis investigates the associations of diabetes and diabetic retinopathy severity with the likelihood of falling in participants from the multiethnic (Malay, Chinese, and Indian individuals) population-based Singapore Epidemiology of Eye Disease study.
Key Points
Question
What is the association between the severity of diabetic retinopathy and falls in Asian individuals with diabetes?
Findings
In a post hoc cross-sectional analysis of 9481 participants from a multiethnic (Malay, Chinese, and Indian individuals) population-based study, mild to moderate diabetic retinopathy was associated with a significantly increased likelihood of falling in persons with diabetes compared with persons having diabetes but not diabetic retinopathy.
Meaning
These results suggest that efforts to prevent falls need to be incorporated into the clinical care of individuals with diabetes and diabetic retinopathy, particularly during the early stage of the disease.
Abstract
Importance
The presence and severity of diabetic retinopathy (DR) may contribute to the risk of falling in persons with diabetes, but evidence is currently equivocal.
Objective
To investigate the associations of diabetes and DR severity with the likelihood of falls in a multiethnic Asian population.
Design, Setting, and Participants
Cross-sectional post hoc analysis of the Singapore Epidemiology of Eye Diseases study, a population-based study of participants from 3 ethnic groups (3280 Malay, 3400 Indian, and 3353 Chinese individuals) conducted from 2004 to 2011. Of these participants, 552 had data missing on diabetes, falls history, or other covariates or had ungradable fundus photographs and were excluded, leaving 9481 participants. These 9481 underwent a standardized clinical examination and responded to an interviewer-administered questionnaire that collected clinical and sociodemographic information. Multivariable logistic regression models adjusted for confounding fall risk factors assessed the associations of falls with diabetes, DR, and DR severity. A trend analysis was conducted in participants with diabetes to assess if risk of falling was associated with DR severity. Data were analyzed from January 1 through April 30, 2017.
Exposures
Diabetes was defined as a random glucose level of at least 200 mg/dL, hemoglobin A1c concentration of at least 6.5% of total hemoglobin, self-reported use of diabetic medication, or history of physician-diagnosed diabetes. Severity of DR was graded as none, minimal, mild, moderate, and vision threatening (VT).
Main Outcomes and Measures
A self-reported fall occurring in the previous 12 months, when the participant fell and landed on the ground.
Results
Of the 9481 participants with a mean (SD) age of 58.7 (10.3) years (4781 women [50.4%]), 6612 (69.7%) had no diabetes and 2869 (30.3%) had diabetes, of whom 857 (29.9%) had DR in at least 1 eye. A history of falls was reported in 872 (13.2%) without diabetes, 328 (16.3%) with no DR, 44 (14.2%) with minimal DR, 54 (26.2%) with mild DR, 34 (27.2%) with moderate DR, and 43 (19.9%) with VTDR (P for trend < .001). In multivariable models, those with DR were more likely to have fallen (odds ratio [OR], 1.31; 95% CI, 1.07-1.60; P = .008) compared with those with no diabetes; no associations were found for participants without DR compared with those with no diabetes. In addition, compared with participants with diabetes but without DR, those with mild (OR, 1.81; 95% CI, 1.23-2.67; P = .003) and moderate (OR, 1.89; 95% CI, 1.16-3.07; P = .01) nonproliferative DR were more likely to have fallen. Having VTDR was not independently associated with a higher likelihood of falling.
Conclusions and Relevance
The presence of mild to moderate nonproliferative DR was independently associated with an increased likelihood of falling in persons with diabetes compared with persons with diabetes but without DR. Management strategies for diabetes should incorporate fall education and prevention information, particularly in patients with early-stage DR. Longitudinal studies exploring the association between mild to moderate nonproliferative DR and falling will be required to confirm these findings.
Introduction
Approximately 1 of 3 community-living elderly people fall each year, with approximately 1 in 10 falls resulting in trauma or fracture.1,2,3,4 Even nontraumatic falls are detrimental to the quality of life and can result in a reduction in social and physical activities, physical decline, disability, loss of independence, institutionalization, and mortality.5,6 The health care cost of falls is substantial, particularly as the frequency and severity of falls increase.7
Although falls have a multifactorial etiology,8 people with diabetes are at increased risk of falls compared with those without the disease.9,10,11,12,13,14 However, the reasons behind the association are unclear, and it can be speculated that diabetic retinopathy (DR), a common and potentially blinding microvascular complication of diabetes, is an underlying risk factor. Left untreated, the proliferative stages of DR, together with diabetic macular edema, which can develop at any stage of DR, cause substantial and irreversible vision loss.15 Several studies have found a clear association between glaucoma (which affects peripheral vision) or age-related macular degeneration (which affects central vision) and risk of falls.16,17 By contrast, previous investigations have found no significant association between DR and risk of falls, likely because of the limited number of DR cases in their population samples.18
To our knowledge, no study has explicitly evaluated the associations of diabetes and the severity of DR with falls in a large population-based sample. However, understanding this association is crucial to inform the systematic development of evidence-based interventions to reduce falls in individuals with diabetes and DR. The purpose of this study was to investigate the independent associations of diabetes and the severity of DR with the likelihood of falling in a population-based study conducted in Singapore.
Methods
Study Population
The Singapore Epidemiology Eye Diseases (SEED) study is a population-based cohort study comprising 3 major ethnic groups, Malay (recruited from the Singapore Malay Eye Study), Indian (recruited from the Singapore Indian Eye Study), and Chinese (recruited from the Singapore Chinese Eye Study) individuals, with baseline assessments conducted from 2004 to 2011. Details of the SEED study design and methodology have been reported elsewhere.19,20,21 In brief, the SEED study was conducted in the southwestern part of Singapore, using a standardized study protocol for all 3 ethnic groups. An age-stratified random sampling strategy was used in each ethnic group to select adults aged 40 to 80 years. Ethnicities were defined by the Singapore census and indicated on the National Registration Identity Card. A total of 4168 Malay, 4497 Indian, and 4605 Chinese individuals were identified and invited to participate in the study. At the baseline visits conducted from 2004 to 2011, 10 033 participants underwent the study protocol examinations, including 3280 Malay, 3400 Indian, and 3353 Chinese persons, with a response rate of 78.7%, 75.6%, and 72.8%, respectively. The study was approved by the SingHealth Institutional Review Board. All participants gave informed written consent, and the conduct of the study adhered to the tenets of the Declaration of Helsinki.22
Assessment of Falls
Data on falls occurring within the previous year were self-reported and were gathered using a questionnaire administered by trained interviewers fluent in English, Mandarin, Malay, or Tamil (dependent on participant choice). During the clinic visit, participants were asked the following question: “In the past 12 months, have you fallen and landed on the floor or ground?” A fall was defined as having occurred when an individual who had been sitting or standing was suddenly and unintentionally on the ground.
Assessment of Covariates
The study was conducted at the research clinic of the Singapore Eye Research Institute. The protocol included a comprehensive, standardized examination as well as a questionnaire administered by interviewers trained to collect clinical and sociodemographic data,19,23 including educational level, income level, occupation, lifestyle factors (eg, smoking, alcohol consumption), self-reported family and medical history (eg, diabetes, hypertension, stroke, and cardiovascular disease), current medication, and insulin use. All clinical and questionnaire data were collected from participants during a single visit.
Clinical covariates were obtained through the clinical examination conducted by trained and certified study optometrists (P.G. and R.M.). Presenting distance visual acuity (PVA) was determined monocularly using a logMAR vison chart at a distance of 4 m under standard lighting with the participant wearing refractive correction (if any). Visual impairment was defined as a logMAR score greater than 0.3 (a Snellen score <6/12) in the eye with the better vision. Measurements of systolic and diastolic blood pressures, obtained using an automatic blood pressure monitor (Dinamap Pro Series DP110X-RW; GE Medical Systems Information Technologies, Inc), height, weight, and body mass index (calculated as the weight in kilograms divided by the height in meters squared) were performed as per the SEED study protocol, which is described in detail elsewhere.19,20,24
Nonfasting venous blood samples were also collected to assess levels of glucose, hemoglobin A1c (HbA1c), serum creatinine, serum total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides. All samples were analyzed at the Singapore General Hospital Hematology Laboratory.
Two-field fundus images were obtained with a nonmydriatic fundus camera (CR-DGI; Canon) and graded for severity of DR using data from the worse eye with the modified Airlie House classification system by trained graders at the Singapore Eye Research Institute using the Early Treatment of Diabetic Retinopathy Study scale scores of none (10-15), minimal nonproliferative DR (NPDR) (20), mild NPDR (35), moderate NPDR (43-47), severe NPDR (53-60), and proliferative DR (61-85).25 Vision-threatening diabetic retinopathy (VTDR) was defined using the Eye Diseases Prevalence Research Group definition as the presence of severe NPDR, proliferative DR, or clinically significant macular edema.26
Clinical Definitions of Ocular and Systemic Diseases
Glaucoma was defined using the scheme from the International Society of Geographic and Epidemiologic Ophthalmology27 based on findings from gonioscopy, optic disc characteristics, and visual field results. Signs of age-related macular degeneration were graded using fundus photographs with the Wisconsin age-related maculopathy grading system.28 Undercorrected refractive error was defined as the difference of at least 0.2 logMAR between PVA and the best-corrected visual acuity in either eye. The presence of a cataract, including nuclear, cortical, or posterior subcapsular cataract, in either eye was based on Lens Opacities Classification System III grading.29
Chronic kidney disease (CKD) was defined as having an estimated glomerular filtration rate less than 60 mL/min/1.73 m2.30,31 Cardiovascular disease (CVD) was defined as a participant self-reported history of myocardial infarction, unstable angina pectoris, stroke, or transient ischemic attack. Diabetes was defined as a random glucose level of at least 200 mg/dL (to convert glucose to millimoles per liter, multiply by 0.0555), HbA1c concentration of at least 6.5% of total hemoglobin (to convert HbA1c to a proportion of total hemoglobin, multiply by 0.01), self-reported use of diabetic medication, or history of physician-diagnosed diabetes.32 Hypertension was defined as having systolic blood pressure equal to or greater than 140 mm Hg, diastolic blood pressure equal to or greater than 90 mm Hg, or a self-reported history of hypertension or antihypertensive medication use. Polypharmacy was defined as taking 4 or more prescription medications.33
Statistical Analysis
All analyses were performed using Stata, version 14.1, for Windows (StataCorp) from January 1 through April 30, 2017. Characteristics of study participants who fell vs those who did not were compared using the τ2 statistic for proportions for categorical variables and 2-tailed unpaired t tests for continuous variables. The Mann-Whitney test was performed when the distribution of a continuous variable was observed to be highly skewed. Multivariable logistic regression analysis was performed to determine whether having diabetes but no DR, or diabetes with DR, with reference to individuals with no diabetes, was independently associated with the likelihood of falling. In participants with diabetes, a trend analysis was conducted by treating DR severity as an ordinal variable, assigning equally spaced numeric values to each category (ie, no DR = 0, minimal DR = 1, mild DR = 2, moderate DR = 3, and VTDR = 4). This enabled the assessment of whether there was a linear pattern of increasing risk of falls with increasing DR severity. The adjusted variables included those participant characteristics found to be substantively different by fall status and hitherto known risk factors of falls in people with diabetes, including age, sex, systolic blood pressure, PVA in the better-seeing eye, ethnicity, polypharmacy, and the presence of CVD, CKD, or any eye disease (including cataract, glaucoma, age-related macular degeneration, and undercorrected refractive error). Analyses in patients with diabetes only was additionally adjusted for insulin use and duration of disease because these are established risk factors for DR and were found to have substantial differences by fall status in the present study. Interactions between ethnicity and diabetes with DR or diabetes in the absence of DR were determined by including multiplicative terms between primary effects of these variables in each model. Odds ratios (ORs) were reported with 95% CIs and P values. Two-sided P values <.05 were considered statistically significant.
Results
Of the 10 033 participants in the SEED Study, 552 had missing data on diabetes, falls history, or other covariates or had ungradable fundus photographs and were thus excluded. A total of 9481 participants (3102 Malay, 3186 Indian, and 3193 Chinese individuals) were included in our analyses (mean age [SD], 58.7 [10.3] years; 4781 women [50.4%]). Of these, 6612 participants (69.7%) had no diabetes, whereas 2869 participants (30.3%) had diabetes, of whom 857 (29.8%) had diabetes with DR (minimal, 310 [10.8%]; mild, 206 [7.2%]; moderate, 125 [4.4%]; and VTDR, 216 [7.5%]) in at least 1 eye.
Among the 2012 participants having diabetes without DR, 328 (16.3%) reported falls, compared with 175 (20.4%) with diabetes and DR (minimal, 44 [14.2%]; mild 54 [26.2%]; moderate, 34 (27.2%); and VTDR 43 [19.9%]; P for trend < .001). Compared with those who did not have a history of falling, those who fell (1375 [14.5%]) were relatively older, were more likely to be women, had higher systolic blood pressure and HbA1c levels, had a longer duration of diabetes, and were taking a greater number of prescription medications (polypharmacy). Furthermore, they were more likely to have diabetes, poorer PVA in the better-seeing eye, and comorbid conditions, such as CVD, CKD, and eye disease (Table 1).
Table 1. Demographic and Clinical Characteristics of 1375 Participants With and 8106 Without Self-reported Falls.
| Characteristic | Without Falls (n = 8106) |
With Falls (n = 1375) |
P Valuea |
|---|---|---|---|
| Age, mean (SD), y | 58.3 (10.2) | 61.0 (10.8) | <.001 |
| Female, No. (%) | 3952 (48.8) | 829 (60.3) | <.001 |
| BMI, mean (SD) | 25.3 (4.6) | 26.0 (5.0) | <.001 |
| Systolic BP, mean (SD), mm Hg | 139.4 (21.5) | 141.9 (23.0) | <.001 |
| Diastolic BP, mean (SD), mm Hg | 78.5 (10.5) | 77.4 (10.5) | <.001 |
| HbA1c, mean (SD), % of total | 6.3 (1.3) | 6.4 (1.4) | <.001 |
| Duration of diabetes, mean (SD), y | 10.0 (8.9) | 12.0 (9.5) | <.001 |
| Polypharmacy, No. (%) | 1066 (13.2) | 288 (21.0) | <.001 |
| Treatment with insulin, No. (%) | 185 (11.8) | 57 (15.1) | .08 |
| CVD, No. (%) | 812 (10.0) | 205 (14.9) | <.001 |
| CKD, No. (%) | 941 (11.7) | 224 (16.6) | <.001 |
| Presence of eye diseases, No. (%)b | 4466 (55.1) | 897 (65.2) | <.001 |
| PVA in better-seeing eye, mean (SD), logMAR | 0.2 (0.2) | 0.2 (0.3) | <.001 |
| Alcohol consumption, No. (%) | 686 (8.5) | 119 (8.7) | .82 |
| Presence of diabetes, No. (%) | 2366 (29.2) | 503 (36.6) | <.001 |
| Presence of DR among those with diabetes, No. (%) | 682 (79.6) | 175 (20.4) | <.001 |
| No DR | 1684 (83.7) | 328 (16.3) | <.001 |
| Minimal DR | 266 (85.8) | 44 (14.2) | |
| Mild DR | 152 (73.8) | 54 (26.2) | |
| Moderate DR | 91 (72.8) | 34 (27.2) | |
| VTDR | 173 (80.1) | 43 (19.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular disease; DR, diabetic retinopathy; HbA1c, hemoglobin A1c; PVA, presenting visual acuity; VTDR, vision-threatening DR.
SI conversion factor: To convert HbA1c to a proportion of total hemoglobin, multiply by 0.01.
P < .05 considered statistically significant.
Eye disease includes glaucoma, age-related macular degeneration, cataract, and undercorrected refractive error.
In models adjusted for age and sex (Table 2, model 1) compared with participants with no diabetes, participants with diabetes alone (OR, 1.18; 95% CI, 1.02-1.35; P = .02) and those with diabetes and any severity of DR (OR, 1.54; 95% CI, 1.28-1.85; P < .001) had higher odds of falling. This association persisted in fully adjusted models for those with diabetes and any severity of DR (OR, 1.31; 95% CI, 1.07-1.60; P = .008). By contrast, participants with diabetes but without DR were not more likely than participants without diabetes to have fallen (OR, 1.06; 95% CI, 0.91-1.23; P = .42) (Table 2, model 2). When these 2 groups (ie, all individuals with diabetes, both with and without DR) were combined, no association was detected between the presence of diabetes and the odds of falling (OR, 1.13; 95% CI, 0.98-1.29; P = .07). There was no evidence that the observed associations differed depending on ethnicity (eTable in the Supplement).
Table 2. Multivariable-Adjusted Associations of Diabetes and DR With Risk of Falls.
| Variable | No. of Participants | Model 1a | Model 2b | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Valuec | OR (95% CI) | P Valuec | ||
| No diabetes | 6621 | 1 [Reference] | 1 [Reference] | ||
| Diabetes without DR | 2012 | 1.18 (1.02-1.35) | .02 | 1.06 (0.91-1.23) | .42 |
| Diabetes with DR | 857 | 1.54 (1.28-1.85) | <.001 | 1.31 (1.07-1.60) | .008 |
Abbreviations: DR, diabetic retinopathy; OR, odds ratio.
Model 1 includes age and sex.
Model 2 includes age, sex, systolic blood pressure, presenting visual acuity in the better-seeing eye, ethnicity, cardiovascular disease, chronic kidney disease, polypharmacy, and presence of eye disease (including glaucoma, age-related macular degeneration, cataract, and undercorrected refractive error).
P < .05 is considered statistically significant.
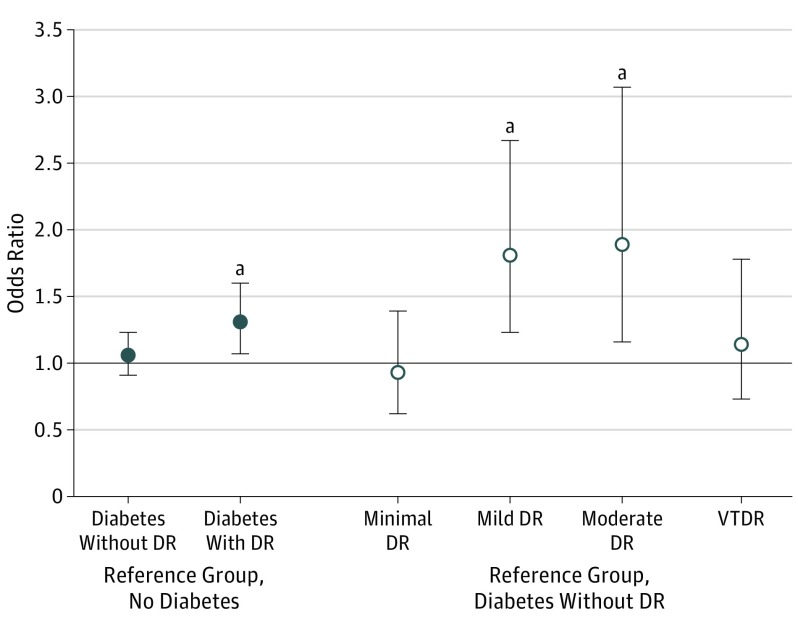
Table 3 gives the associations between DR severity and the odds of falling in participants with diabetes. In the fully adjusted model, among all DR severity levels, only participants with mild (OR, 1.81; 95% CI, 1.23-2.67; P = .003) and moderate (OR, 1.89; 95% CI, 1.16-3.07; P = .01) DR had higher odds of falling compared with participants with diabetes but without DR (model 2 and Figure). Although no association was observed between VTDR and falls, a trend analysis showed a linear pattern of increasing risk of falls with increasing severity of DR (OR per level increase in DR severity, 1.10; 95% CI, 1.00-1.21; P for trend = .04).
Table 3. Multivariable Logistic Regression Analysis for Associations Between Severity of DR and Risk of Falls in Participants With Diabetes.
| Variable | No. of Participants | Model 1a | Model 2b | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuec | P Value for Trend | OR (95% CI) | P Valuec | P Value for Trend | ||
| No DR | 2012 | 1 [Reference] | 1 [Reference] | ||||
| Minimal DR | 310 | 0.83 (0.59-1.17) | .29 | .002 | 0.93 (0.62-1.39) | .73 | .03 |
| Mild DR | 206 | 1.85 (1.32-2.59) | <.001 | 1.81 (1.23-2.67) | .003 | ||
| Moderate DR | 125 | 1.91 (1.26-2.90) | .002 | 1.89 (1.16-3.07) | .01 | ||
| VTDRd | 216 | 1.25 (0.87-1.78) | .22 | 1.14 (0.73-1.78) | .55 | ||
Abbreviations: DR, diabetic retinopathy; OR, odds ratio; VTDR, vision-threatening DR.
Model 1 includes age and sex.
Model 2 includes age, sex, systolic blood pressure, presenting visual acuity in the better-seeing eye, ethnicity, cardiovascular disease, chronic kidney disease, diabetes duration, insulin use, polypharmacy, and presence of eye disease, which includes glaucoma, age-related macular degeneration, cataract, and undercorrected refractive error.
P < .05 is considered statistically significant.
Includes severe nonproliferative DR, proliferative DR, or clinically significant macular edema.
Figure. Odds Ratios From Multivariable-Adjusted Logistic Regression for the Associations Between Risk of Falling and Diabetes Without Diabetic Retinopathy (DR), Diabetes With DR, and DR Severity Levels.
VTDR indicates vision-threatening DR; error bars, 95% CI. Plotted estimates are based on the fully adjusted models.
aP < .05 compared with the reference group.
In a subgroup analysis of Malay participants who had data on peripheral nerve function, we found no change in the significance of the association between DR and falls when we analyzed the model with (OR, 0.78; 95% CI, 0.46-1.32; P = .37) or without (OR, 1.20; 95% CI: 0.85-1.71; P = .29) peripheral neuropathy, which may be explained by the small sample size of participants (175) with DR in the Malay population.
Discussion
In this post hoc cross-sectional analysis of a multiethnic population-based study, diabetes per se was not associated with a higher risk of falling; the risk of falling among participants with diabetes but without DR was no different than that in participants without diabetes (OR, 1.06; 95% CI, 0.91-1.23; P = .42). However, the presence of DR in participants with diabetes was significantly associated with falling. Among persons with diabetes, those with mild and moderate NPDR were independently more likely to have a history of falls than those without DR. These findings were independent of key fall risk factors, including PVA, blood pressure, systemic disease, duration of diabetes, insulin use, polypharmacy, and concurrent eye disease. Our findings suggest that strategies to prevent or delay the development of DR are likely to reduce the risk of falls in persons with diabetes, preventing fall-related decline in physical functioning, suboptimal quality of life, and lack of independence in adults with diabetes. Furthermore, our results underscore the importance of safety and mobility training even for people with mild to moderate DR who are not experiencing visual impairment.
Unlike previous studies that have reported that patients with diabetes are at higher risk of falling compared with those without diabetes,11,13,14,34,35 we found no significant association between the presence of diabetes and risk of falling when we analyzed the population as a whole, without differentiating between participants with and without DR. Our results suggest that previously demonstrated associations between diabetes and risk of falls may have been driven by the presence of DR. This is an important finding, but further research is needed to confirm it.
Our results suggested that adults with DR, particularly those with mild to moderate stages of the disease, had a higher risk of falling than those without DR, independent of PVA. Although falling has traditionally been linked with vision loss,18,36,37 studies have shown that a reduction in the components of the aging visual function system, such as contrast sensitivity, stereo acuity, and color perception, is more strongly associated with poor postural stability and a greater capacity to bump into objects, leading to a greater risk of falling.38,39,40 Given that components of aging visual function are compromised in DR,41,42 we speculate that this could explain the greater tendency to fall in patients with mild to moderate DR, independent of vision. Our results suggest that fall prevention efforts should be incorporated into the management of DR in patients, even those without existing visual impairment, to prevent serious injuries and loss of independence.
Patients with VTDR were not associated with a higher likelihood of falling. This may be because we did not have sufficient power to determine this association. However, this lack of association may also be because these patients were more likely to be older (mean age, 62.7 vs 61.0 years) and have comorbidities (a greater proportion of them had hypertension, CVD, and CKD), which likely decreased their mobility and life space over time. This hypothesis is supported by studies showing that patients with severe DR have less muscle mass,43 weaker muscle strength,43 lower physical activity,44 and increased fear of falling, mainly owing to balance and mobility impairment,45 resulting in activity restriction, which may in turn reduce the risk of falls despite increased fall risk factors. However, because we did not collect data on these variables and, to our knowledge, no other studies have investigated the association between DR severity and falls, future longitudinal studies are needed to confirm our findings.
Strengths and Limitations
The strengths of our study include a large study sample characterized using a standardized clinical testing protocol, a high number of individuals with diabetes representing the spectrum of DR severity, and the objective categorization of DR using fundus photographs. In addition, comprehensive measurements were available on risk factors for falls that are also associated with DR, including duration of diabetes, insulin use, polypharmacy, blood pressure, vision, and presence of systemic diseases such as CVD and CKD.
The main limitation of our study is that falls were self-reported, and, as a result, our outcome may be subject to recall bias.46 In addition, the cross-sectional nature of this study limited causal inferences. Further research using prospective and objective measures of falls, such as fall diaries, are required. Another limitation was the lack of information on the strength, balance, and gait components of mobility that have been linked to increased risk of falls.12,13 We did not account for physical inactivity or level of physical activity, although poor physical performance is associated with both decreased (due to activity restriction)47 and increased risk of falling (probably through frailty and muscle weakness).48 No data were captured on components of the aging visual system, such as contrast sensitivity, stereo acuity, and color perception, which may contribute to falls. Neither were data available on hypoglycemia, osteoporosis, hearing impairment, depression, or specific medications, such as antidepressants and β-blockers, all of which may contribute to falls or affect the recall of past falls. In addition, we did not adjust for peripheral neuropathy in our final model because these data were not captured for all 3 ethnic populations. Finally, because the study population comprised community-dwelling Asian persons with diabetes, our results may not apply to individuals who are institutionalized, belong to other ethnicities, or live in nonurbanized developing countries.
Conclusions
We found that diabetes per se was not a risk factor for falls; however, among individuals with diabetes, those with mild or moderate DR had a risk of falls nearly 2-fold as high as those without DR. Our findings suggest that efforts to prevent falls may need to be incorporated into the clinical care of individuals with diabetes, particularly those with early-stage DR. Subsequent longitudinal studies exploring the association between mild to moderate NPDR and falling are required to confirm our findings.
eTable. Estimates of the Association Between Diabetic Retinopathy (DR) and Falls Allowing for Interaction by Ethnicity
References
- 1.Tinetti ME, Kumar C. The patient who falls: “it’s always a trade-off”. JAMA. 2010;303(3):258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell AJ, Reinken J, Allan BC, Martinez GS. Falls in old age: a study of frequency and related clinical factors. Age Ageing. 1981;10(4):264-270. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701-1707. [DOI] [PubMed] [Google Scholar]
- 4.Prudham D, Evans JG. Factors associated with falls in the elderly: a community study. Age Ageing. 1981;10(3):141-146. [DOI] [PubMed] [Google Scholar]
- 5.Cumming RG, Kelsey JL, Nevitt MC. Methodologic issues in the study of frequent and recurrent health problems: falls in the elderly. Ann Epidemiol. 1990;1(1):49-56. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Powell L. Fear of falling and low self-efficacy: a case of dependence in elderly persons. J Gerontol. 1993;48(Spec No):35-38. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo JA, Friedkin R, Williams CS, Nabors J, Acampora D, Tinetti ME. Health care utilization and costs in a Medicare population by fall status. Med Care. 1998;36(8):1174-1188. [DOI] [PubMed] [Google Scholar]
- 8.Connell BR. Role of the environment in falls prevention. Clin Geriatr Med. 1996;12(4):859-880. [PubMed] [Google Scholar]
- 9.Crews RT, Yalla SV, Fleischer AE, Wu SC. A growing troubling triad: diabetes, aging, and falls. J Aging Res. 2013;2013:342650. doi: 10.1155/2013/342650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinik AI, Vinik EJ, Colberg SR, Morrison S. Falls risk in older adults with type 2 diabetes. Clin Geriatr Med. 2015;31(1):89-99. [DOI] [PubMed] [Google Scholar]
- 11.Pijpers E, Ferreira I, de Jongh RT, et al. Older individuals with diabetes have an increased risk of recurrent falls: analysis of potential mediating factors: the Longitudinal Ageing Study Amsterdam. Age Ageing. 2012;41(3):358-365. [DOI] [PubMed] [Google Scholar]
- 12.Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J Gerontol A Biol Sci Med Sci. 2005;60(9):1157-1162. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25(10):1749-1754. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. ; Health, Aging, and Body Composition Study . Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31(3):391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, IV: diabetic macular edema. Ophthalmology. 1984;91(12):1464-1474. [DOI] [PubMed] [Google Scholar]
- 16.Glynn RJ, Seddon JM, Krug JH Jr, Sahagian CR, Chiavelli ME, Campion EW. Falls in elderly patients with glaucoma. Arch Ophthalmol. 1991;109(2):205-210. [DOI] [PubMed] [Google Scholar]
- 17.Wood JM, Lacherez P, Black AA, Cole MH, Boon MY, Kerr GK. Risk of falls, injurious falls, and other injuries resulting from visual impairment among older adults with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(8):5088-5092. [DOI] [PubMed] [Google Scholar]
- 18.Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998;46(1):58-64. [DOI] [PubMed] [Google Scholar]
- 19.Foong AW, Saw SM, Loo JL, et al. Rationale and methodology for a population-based study of eye diseases in Malay people: the Singapore Malay Eye Study (SiMES). Ophthalmic Epidemiol. 2007;14(1):25-35. [DOI] [PubMed] [Google Scholar]
- 20.Lavanya R, Jeganathan VS, Zheng Y, et al. Methodology of the Singapore Indian Chinese Cohort (SICC) eye study: quantifying ethnic variations in the epidemiology of eye diseases in Asians. Ophthalmic Epidemiol. 2009;16(6):325-336. [DOI] [PubMed] [Google Scholar]
- 21.Chua J, Tham YC, Liao J, et al. Ethnic differences of intraocular pressure and central corneal thickness: the Singapore Epidemiology of Eye Diseases study. Ophthalmology. 2014;121(10):2013-2022. [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Luo N, Chew LH, Fong KY, et al. Validity and reliability of the EQ-5D self-report questionnaire in English-speaking Asian patients with rheumatic diseases in Singapore. Qual Life Res. 2003;12(1):87-92. [DOI] [PubMed] [Google Scholar]
- 24.Gupta P, Thakku SG, Sabanayagam C, et al. Characterisation of choroidal morphological and vascular features in diabetes and diabetic retinopathy. Br J Ophthalmol. 2017;101(8):1038-1044. [DOI] [PubMed] [Google Scholar]
- 25.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786-806. [PubMed] [Google Scholar]
- 26.Kempen JH, O’Colmain BJ, Leske MC, et al. ; Eye Diseases Prevalence Research Group . The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552-563. [DOI] [PubMed] [Google Scholar]
- 27.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128-1134. [DOI] [PubMed] [Google Scholar]
- 29.Chylack LT Jr, Wolfe JK, Singer DM, et al. ; Longitudinal Study of Cataract Study Group . The Lens Opacities Classification System III. Arch Ophthalmol. 1993;111(6):831-836. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim CC, Teo BW, Ong PG, et al. Chronic kidney disease, cardiovascular disease and mortality: a prospective cohort study in a multi-ethnic Asian population. Eur J Prev Cardiol. 2015;22(8):1018-1026. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care. 2015;38(suppl 1):S4. doi: 10.2337/dc15-S003 [DOI] [PubMed] [Google Scholar]
- 33.Huang ES, Karter AJ, Danielson KK, Warton EM, Ahmed AT. The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the Diabetes and Aging Study. J Gen Intern Med. 2010;25(2):141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yau RK, Strotmeyer ES, Resnick HE, et al. Diabetes and risk of hospitalized fall injury among older adults. Diabetes Care. 2013;36(12):3985-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewston P, Deshpande N. Falls and balance impairments in older adults with type 2 diabetes: thinking beyond diabetic peripheral neuropathy. Can J Diabetes. 2016;40(1):6-9. [DOI] [PubMed] [Google Scholar]
- 36.Lamoureux EL, Chong E, Wang JJ, et al. Visual impairment, causes of vision loss, and falls: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49(2):528-533. [DOI] [PubMed] [Google Scholar]
- 37.Lord SR. Visual risk factors for falls in older people. Age Ageing. 2006;35(suppl 2):ii42-ii45. [DOI] [PubMed] [Google Scholar]
- 38.Lord SR, Menz HB. Visual contributions to postural stability in older adults. Gerontology. 2000;46(6):306-310. [DOI] [PubMed] [Google Scholar]
- 39.Lord SR, Clark RD, Webster IW. Visual acuity and contrast sensitivity in relation to falls in an elderly population. Age Ageing. 1991;20(3):175-181. [DOI] [PubMed] [Google Scholar]
- 40.Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc. 1994;42(10):1110-1117. [DOI] [PubMed] [Google Scholar]
- 41.Shrestha GS, Kaiti R. Visual functions and disability in diabetic retinopathy patients. J Optom. 2014;7(1):37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol. 1985;103(1):51-54. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda T, Bouchi R, Takeuchi T, et al. Association of diabetic retinopathy with both sarcopenia and muscle quality in patients with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. 2017;5(1):e000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Praidou A, Harris M, Niakas D, Labiris G. Physical activity and its correlation to diabetic retinopathy. J Diabetes Complications. 2017;31(2):456-461. [DOI] [PubMed] [Google Scholar]
- 45.Coyne KS, Margolis MK, Kennedy-Martin T, et al. The impact of diabetic retinopathy: perspectives from patient focus groups. Fam Pract. 2004;21(4):447-453. [DOI] [PubMed] [Google Scholar]
- 46.Cummings SR, Nevitt MC, Kidd S. Forgetting falls: the limited accuracy of recall of falls in the elderly. J Am Geriatr Soc. 1988;36(7):613-616. [DOI] [PubMed] [Google Scholar]
- 47.Ford ES, Herman WH. Leisure-time physical activity patterns in the U.S. diabetic population: findings from the 1990 National Health Interview Survey—Health Promotion and Disease Prevention Supplement. Diabetes Care. 1995;18(1):27-33. [DOI] [PubMed] [Google Scholar]
- 48.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26(4):315-318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Estimates of the Association Between Diabetic Retinopathy (DR) and Falls Allowing for Interaction by Ethnicity